- 1Department of Cardiac Surgery, Ibn Albittar Cardiac Surgeon, Baghdad, Iraq
- 2Mosul Cardiac Center, Mousl, Iraq
- 3College of Medicine, University of Sulaimani, Sulaimani, Iraq
- 4Scientific Affairs, Smart Health Tower, Sulaimani, Iraq
- 5Sulaimani Centre for Heart Disease, Sulaimani, Iraq
- 6Kscien Organization, Sulaimani, Iraq
- 7Surgery Department, Shar Hospital, Sulaimani, Iraq
- 8Medical Laboratory Technology, Shaqlawa Technical College, Erbil Polytechnic University, Erbil, Iraq
- 9Department of Medical Analysis, Tishk International University, Erbil, Iraq
Background: Cardiac myxoma is a rare cardiac tumor that may be asymptomatic or can cause embolization or intracardiac obstruction, leading to heart failure, sudden cardiac death, and arrhythmia. This study aims to report an 11-year experience of a single center in the management of cardiac myxoma.
Method: This study is a single-center retrospective case series. Eighty cases of cardiac myxoma were collected in Ibn Albitar's specialized center for cardiac surgery. Transthoracic echocardiography was used to make the preoperative diagnosis in all patients. The surgeries were undertaken through the standard approach of a median sternotomy. All four cardiac chambers were thoroughly explored for additional myxomas. The major objective of the operations was complete tumor resection.
Result: The mean age of the patients was 46.3 years. Females (67.5%) were predominant over males (32.5%). Shortness of breath was the most common symptom (86.25%). The left atrium was the most affected site (83.75%), followed by the right atrium (13.75%). Coronary artery bypass grafting was required as the secondary or associated intervention in 19 (23.75%) cases. The recurrence rate was 11.25%, with a mortality rate of 3.75%.
Conclusion: Recurrence and tumor embolism are risks of surgical intervention for myxoma. Good preparation using transthoracic echocardiography as a diagnostic tool and standard median sternotomy to complete resection of the tumors can decrease the rate of recurrence, embolism, and even mortality.
Introduction
Primary cardiac tumors (PCT) are rare neoplasms that represent less than 0.2% of all tumors (1). Myxoma is a type of PCT that comprises 5%–10% of the cardiac and pericardial lesions. It most commonly affects females between the ages of 40 and 60 (2, 3). Generally, myxoma develops in the left atrium (LA), followed by the right atrium (RA) (4, 5). This tumor usually develops from the atrial septum near the fossa ovalis, but it can rarely develop from the posterior wall, anterior wall, or left atrial appendage (6). Based on the location, this neoplasm may be asymptomatic or can cause embolization or intracardiac obstruction, which results in heart failure, sudden cardiac death, and arrhythmia (4). However, surgical intervention has become the standard modality to manage cardiac myxoma, but intraoperative embolization and recurrence are the major complications (7). Therefore, surgical intervention is challenging and needs extensive care and experience to decrease morbidity and mortality (8, 9). This study aims to report an 11-year experience of a single center in the management of cardiac myxoma.
Method
Study design
This study is a single-center retrospective case series.
Setting
Eighty cases of cardiac myxoma were collected in Ibn Albitar's specialized center for cardiac surgery for 11 years, from 2010 to 2021. The Iraqi board of medical specialization granted the study ethical approval.
Inclusion criteria
This study enrolled those patients who underwent surgical excision of primary or recurrent intracardiac myxomas from 2010 to 2021.
Exclusion criteria
Patients with acute thromboembolic ischemia, acute renal failure (on dialysis), or who were over 80 years old were excluded from this study.
Data collection and analysis
All the required information on patients, including demographics, examination, medical summary, surgical indications, comorbidities, and follow-up, were recorded using an electronic registry database. The Statistical Package for the Social Sciences (SPSS) Version 25 was used to encode and perform descriptive analysis.
Surgical intervention
The preoperative diagnosis was performed in all patients by transthoracic echocardiography (TTE). Coronary angiography was performed on patients over the age of 40. The operations were undertaken soon after the diagnosis of cardiac myxoma, through the standard surgical approach of a median sternotomy.
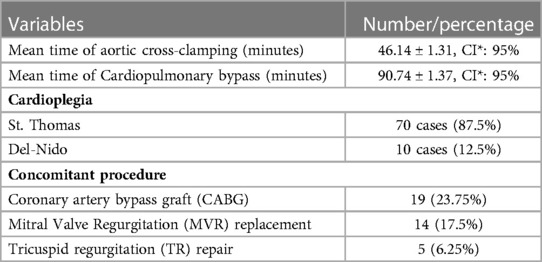
Cardiopulmonary bypass (CPB) with aorto-bicaval cannulation and mild hypothermia was done. Myocardial protection was achieved by cold St. Thomas and Del-Nido cardioplegia. The heart was not manipulated until the aorta had been cross-clamped to avoid systemic embolization. The surgical technique for LA myxomas was the right atrial transseptal or biatrial approaches. In order to decrease conduction problems, most of our operations were through the right atrium approach, so direct superior vena cava cannulation was an important technique, and the crisscross line suture connecting the right atrium to the inferior vena cava prevented the re-entry circuit.
The objectives of the operations were complete tumor resection with full-thickness removal of the tumor's attachment base and a cuff of the interatrial septum to prevent a recurrence. All four cardiac chambers were thoroughly explored for additional myxomas. The surgically defective sites were repaired with a Dacron patch because it is simple to handle and trim. Copious irrigation of the atria and ventricles with cold saline was done to eliminate any tumor fragments that might have been dislodged during the removal of the tumor. All the resected myxomas were subjected to routine histopathological examination. All the patients were followed up on an outpatient basis at regular intervals (3–6 months), and they underwent clinical examination, chest x-ray, electrocardiography, and echocardiography. To decrease the risk of atrial arrhythmias, a beta blocker was routinely used, and sometimes Cordarone was used pre- and post-operatively.
Result
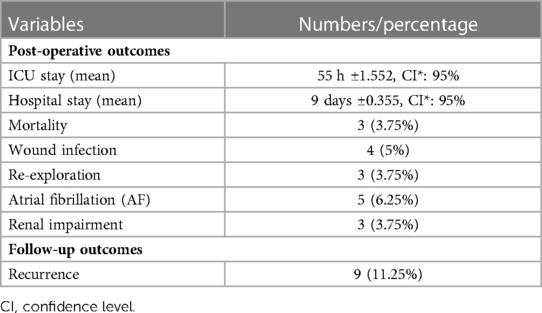
This study included 80 cases with either primary or recurrent intracardiac myxomas. The mean age of the patients was 46.3 years. Females (67.5%) were predominant over males (32.5%). Shortness of breath was the most common symptom (86.25%). The LA was the most affected site (83.75%), followed by the RA (13.75%) (Table 1). Coronary artery bypass grafting (CABG) was required as the secondary or associated intervention in 19 (23.75%) cases (Table 2). Atrial fibrillation (AF) was found in 6.25% of the cases. The mean time of intensive care unit (ICU) admission and hospital stays were 55 h and 9 days, respectively. Re-exploration (once) was done in 3.75% of the cases caused by bleeding. The recurrence and mortality rates were 11.25% and 3.75%, respectively (Table 3). Out of the recurrent cases, four (44.4%) were familial myxomas. The mortality in our cases was 30 days. One of them died after a concomitant procedure (mitral valve replacement) due to low cardiac output (right ventricular failure), and another one died after primary surgery caused by renal impairment. The third one died after reoperation due to a cerebral vascular accident (CVA). During the average 5 years of follow-up, an echocardiogram was done every three months for patients with a positive family history and every six months for patients with a negative family history.
Discussion
Myxoma is the most common type of benign cardiac tumor that originates from primitive stromal cells and differentiates along the endothelial lines (3, 10). This tumor usually develops in the LA; however, its occurrence in other sites like the RA, left ventricle, mitral, and tricuspid valves has been reported (10, 11). Females between the ages of 40 and 60 are more frequently affected by myxoma, except for familial myxoma, which is commonly seen in young-aged individuals (3, 10). In the current series, females were the dominant group, and the mean age of the patients was 46.3 years. The LA was found to be the affected site in 83.75% of the cases, followed by the RA (13.75%). There was only one case of biatrial myxoma and one of right ventricular myxoma.
Alongside the hemodynamic abnormalities owing to the embolism or tumor obstruction, several constitutional symptoms may be observed in patients with myxoma, including dyspnea, palpitation, fever, and weight loss. The clinical manifestations of the tumor are determined by its size, location, surface, and mobility (1, 3). Dyspnea (70%) and palpitation (35%) have been reported to be the most familiar symptoms (4, 12). An embolism caused by myxoma may result in myocardial infarction (13). Therefore, symptomatic myxomas should be surgically excised immediately after diagnosis to prevent pulmonary embolism and other complications (1). The incidence of pulmonary embolism and systemic embolization from left atrial myxomas has been reported to be less than 10% and 25%–50%, respectively. Another study reported cerebrovascular accidents (CVA) in 22% of the cases (3, 14). In this study, most of the cases (86.25%) presented with shortness of breath (dyspnea), but a small number of patients (8.8%) had palpitations. CVA was found to be 6.3%, and none of the cases were associated with systemic or pulmonary embolism.
Different techniques have been proposed to diagnose cardiac myxoma. Cardiac catheterization and angiocardiography were originally used to diagnose atrial myxoma, but this traditional method is an invasive procedure that can cause peripheral embolism and may provide false-positive or false-negative results (7). Echocardiography is a noninvasive and accurate procedure to detect intracardiac tumors. This modality can determine the character, location, and mobility of cardiac myxoma, with the capability to consider the presence of biatrial tumors and multiple carcinomas. TTE has a sensitivity of 95% in detecting myxoma (4, 7). In open-heart surgical interventions, ischemic cardiac arrest by cardioplegic solutions and cardiac hypothermia have been encouraged, especially in the excision of atrial myxoma. This application provides a relaxed field for operation and decreases the risk of tumor embolism (7). The recent surgical method in the treatment of atrial myxomas is the standard median sternotomy with CPB, mild hypothermia, and cardioplegic solutions (2, 15). In the present study, TTE was conducted in all patients preoperatively, and coronary angiography was also performed in patients older than 40 years. CPB and mild hypothermia were used, and myocardial protection was achieved by cold St. Thomas and Del-Nido cardioplegia solutions. All surgical operations were conducted through the standard median sternotomy.
Selkane et al. in their case series reported six concomitant mitral valve procedures with three cases (7.5%) of secondary replacement of the mitral valve (16). Furthermore, Kabbani et al. conducted four (16.6%) mitral valve replacements in the resection of 24 myxoma tumors. In a study by Semb et al., tricuspid valve replacement was performed in 18.18% of the cases (17, 18). In this study, CABG was performed in 23.75% of the cases, and mitral valve replacement and tricuspid valve repair were conducted in 17.5% and 6.25% of the cases, respectively.
To reduce the risk of recurrence, the myxoma must be completely removed by resection of the tumor's base and a portion of the surrounding interatrial septum (1). The recurrence rate of atrial myxoma has been reported to be 5% to over 14% (6). Several factors, like family history of myxoma, young age, multifocal tumors, and interleukin-6, play a significant role in the recurrence of myxoma (6, 16). Postoperatively, the recurrence in patients with familial myxoma is 21%–67% (19, 20). In previous studies, the mortality rate due to the excision of myxoma has been quite variable. In a study by Sutton et al., the mortality rate was 2.7%, and 5% mortality has also been reported (2, 21). A high mortality rate of about 25% was reported in a series of 17 cases (22). Symbas et al. recorded a high mortality rate in their study due to embolization and obstruction of intracardiac blood flow in the time interval between diagnosis and operation (23). In the current study, total resection of the tumors was conducted. Irrigation of the atria and ventricles with cold saline was done to eliminate any tumor fragments and decrease the risk of recurrence. However, the recurrence rate in this series was 11.25%, and nearly half of them were familial myxomas; this may be an explanation for this rate of recurrence in this study. The mortality rate was 3.75%, which is a fair outcome in comparison to the previously mentioned studies.
Conclusion
Recurrence and tumor embolism are major risks of surgical intervention for myxoma. Good preparation using TTE as a diagnostic tool and conducting a standard median sternotomy by experienced surgeons to complete resection of the tumors can decrease the rate of recurrence, embolism, and even mortality rate.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Iraqi board of medical specialization. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AA and OA: The surgeons performed operation, revision and final approval of manuscript. FK: Writing manuscripts, literature review, final approval of the manuscript. DM-S, SA, ZZ, RA, SK, DO, MM: literature review, final approval of the manuscript. BA, SM, HA: collected of the data, final approval of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Choi J, de Costa A, Sabetai MM. Surgical management of a giant right atrial myxoma. J Surg Case Rep. (2018) 2018(10):rjy288. doi: 10.1093/jscr/rjy288
2. Turkyilmaz S, Kavala AA. Management of cardiac myxoma; tertiary academic center experience. Med J Bakirkoy. (2018) 14(1):98–103. doi: 10.5350/BTDMJB.20171230024937
3. Ipek G, Erentug V, Bozbuga N, Polat A, Guler M, Kirali K, et al. Surgical management of cardiac myxoma. J Card Surg. (2005) 20(3):300–4. doi: 10.1111/j.1540-8191.2005.200415.x
4. Kawall J, Seecheran R, Seecheran V, Persad S, Maharaj S, Seecheran NA. Medical management of a suspected atrial myxoma in a nonagenarian. SAGE Open Med Case Rep. (2020) 8:2050313X20933484. doi: 10.1177/2050313X20933484
5. MacGowan SW, Sidhu P, Aherne T, Luke D, Wood AE, Neligan MC, et al. Atrial myxoma: national incidence, diagnosis and surgical management. Ir J Med Sci. (1993) 162(6):223–6. doi: 10.1007/BF02945200
6. Lone RA, Ahanger AG, Singh S, Mehmood W, Shah S, Lone GN, et al. Atrial myxoma: trends in management. Int J Health Sci. (2008) 2(2):141.
7. Donahoo JS, Weiss JL, Gardner TJ, Fortuin NJ, Brawley RK. Current management of atrial myxoma with emphasis on a new diagnostic technique. Ann Surg. (1979) 189(6):763. doi: 10.1097/00000658-197906000-00013
8. Disesa VJ, Collins JJ Jr, Cohn LH. Considerations in the surgical management of left atrial myxoma. J Card Surg. (1988) 3(1):15–22. doi: 10.1111/j.1540-8191.1988.tb00213.x
9. Taksaudom N, Traisrisilp K, Kanjanavanit R. Left atrial myxoma in pregnancy: management strategy using minimally invasive surgical approach. Case Rep Cardiol. (2017) 2017:8510160. doi: 10.1155/2017/8510160
10. John AS, Connolly HM, Schaff HV, Klarich K. Management of cardiac myxoma during pregnancy: a case series and review of the literature. Int J Cardiol. (2012) 155(2):177–80. doi: 10.1016/j.ijcard.2011.05.069
11. Pero J, Summers D, Krasnow N. The distribution patterns of biatrial myxomas. Ann Thorac Surg. (1980) 29(5):469–73. doi: 10.1016/S0003-4975(10)61682-7
12. Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol. (2011) 57(21):e371. doi: 10.1016/j.jacc.2010.09.085
13. Taşdemir K, Andaç MH, Ceyran H, Yasim A. Myxomas causing coronary emboli resulting in acute myocardial infarction. Asian Cardiovasc Thorac Ann. (1999) 7(2):150–2. doi: 10.1177/021849239900700220
14. Goodwin JF. Introduction the spectrum of cardiac tumors. Am J Cardiol. (1968) 3(21):307–14. doi: 10.1016/0002-9149(68)90135-5
15. Marvasti MA, Obeid AI, Potts JL, Parker FB. Approach in the management or atrial myxoma with long-term follow-up. Ann Thorac Surg. (1984) 38(1):53–8. doi: 10.1016/S0003-4975(10)62186-8
16. Selkane C, Amahzoune B, Chavanis N, Raisky O, Robin J, Ninet J, et al. Changing management of cardiac myxoma based on a series of 40 cases with long-term follow-up. Ann Thorac Surg. (2003) 76(6):1935–8. doi: 10.1016/S0003-4975(03)01245-1
17. Kabbani SS, Jokhadar M, Meada R, Jamil H, Abdun F, Sandouk A, et al. Atrial myxoma: report of 24 operations using the biatrial approach. Ann Thorac Surg. (1994) 58(2):483–7. doi: 10.1016/0003-4975(94)92234-9
18. Semb BK. Surgical considerations in the treatment of cardiac myxoma. J Thorac Cardiovasc Surg. (1984) 87(2):251–9. doi: 10.1016/S0022-5223(19)37419-7
19. Lee VH, Connolly HM, Brown RD. Central nervous system manifestations of cardiac myxoma. Arch Neurol. (2007) 64(8):1115–20. doi: 10.1001/archneur.64.8.1115
20. Van Gelder HM, O’Brien DJ, Staples ED, Alexander JA. Familial cardiac myxoma. Ann Thorac Surg. (1992) 53(3):419–24. doi: 10.1016/0003-4975(92)90261-2
21. St John Sutton MG, Mercier LA, Giuliani ER, Lie JT. Atrial myxomas: a review of clinical experience in 40 patients. Mayo Clin Proc. (1980) 55(6):371–6.7382545
22. Boyacioglu K, Donmez AA, Aksut M, Akdemir I, Ketenciler S, Adademir T, et al. Surgical management of cardiac myxomas in elderly patients. BMB. (2016) 1(1):1–5. doi: 10.5350/BMB2016010101
Keywords: cardiac myxoma, cardiac tumor, cardiac surgery, case series, myxomas
Citation: Alhasso AA, Ahmed OF, Mohammed-Saeed DH, Kakamad FH, Almodhaffer SS, Zaid ZA, Abdullah HO, Ali RK, Kakamad SH, Omar DA, Abdalla BA, Mohammed SH and Mustafa MQ (2023) Operative management and outcomes in patients with myxomas: A single-center experience. Front. Surg. 10:1084447. doi: 10.3389/fsurg.2023.1084447
Received: 30 October 2022; Accepted: 15 March 2023;
Published: 19 April 2023.
Edited by:
Jacob Bergsland, Oslo University Hospital, NorwayReviewed by:
Hratch Karamanoukian, Vein Treatment Center, United StatesGeorge Samanidis, Onassis Cardiac Surgery Center, Greece
© 2023 Alhasso, Ahmed, Mohammed-Saeed, Kakamad, Almodhaffer, Zaid, Abdullah, Ali, Kakamad, Omar, Abdalla, Mohammed and Mustafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahmi H. Kakamad ZmFobWkuaHVzc2VpbkB1bml2c3VsLmVkdS5pcQ==
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
 Ahmed Abdulfattah Alhasso1
Ahmed Abdulfattah Alhasso1 Fahmi H. Kakamad
Fahmi H. Kakamad