- 1Department of Gastrointestinal Surgery, The Third Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of Second Anorectal, Shijiazhuang Hospital of Traditional Chinese Medicine, Shijiazhuang, China
Background: Colorectal cancer (CRC) is the most common gastrointestinal malignancy and is generally thought to be caused by the transformation of colorectal polyps. It has been shown that early detection and removal of colorectal polyps may reduce the mortality and morbidity of colorectal cancer.
Objective: Based on the risk factors associated with colorectal polyps, an individualized clinical prediction model was built to predict and evaluate the possibility of developing colorectal polyp.
Methods: A case-control study was conducted. Clinical data were collected from 475 patients who underwent colonoscopy at the Third Hospital of Hebei Medical University from 2020 to 2021. All clinical data were then divided into training sets and validation sets by using R software (7:3). A multivariate logistic analysis was performed to identify the factors associated with colorectal polyps according to the training set, and a predictive nomogram was created by R software based on the multivariate analysis. The results were internally validated by receiver operating characteristic (ROC) curves, calibration curves, and externally validated by validation sets.
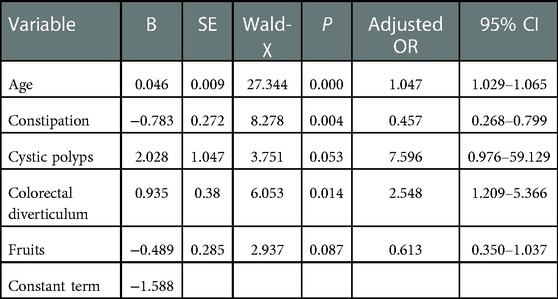
Results: Multivariate logistic regression analysis showed that age (OR = 1.047, 95% CI = 1.029–1.065), history of cystic polyp (OR = 7.596, 95% CI = 0.976–59.129), and history of colorectal diverticulums (OR = 2.548, 95% CI = 1.209–5.366) were independent risk factors for colorectal polyps. History of constipation (OR = 0.457, 95% CI = 0.268–0.799) and fruit consumption (OR = 0.613, 95% CI 0.350–1.037) were protective factors for colorectal polyps. The nomogram demonstrated good accuracy for predicting colorectal polyps, with both C index and AUC being 0.747 (95% CI = 0.692–0.801). The calibration curves showed good agreement between the predicted risk by the nomogram and real outcomes. Both internal and external validation of the model showed good results.
Conclusion: In our study, the nomogram prediction model is reliable and accurate, which can help early clinical screening of patients with high-risk colorectal polyps, improve polyp detection rate, and reduce the incidence of colorectal cancer (CRC).
Introduction
Colorectal polyps are a general term for all neoplasm that protrudes from the intestine. The CP has a high incidence worldwide, with a detection rate of 10%–20% through colonoscopy (1, 2). According to the well-known adenoma-to-carcinoma sequence; colorectal adenomatous polyps may develope into colorectal cancer owing to a series of genetic and epigenetic abnormalities (3).The majority of CRCs originate from adenomatous polyps (adenomas), which usually take more than a decade to become malignant(4, 5). Colorectal cancer (CRC) is the third most common cancer in the world, but it is also the second most common cause of death among the diseases (6). This poses a serious threat to human health. For this reason, the early identification and treatment of colorectal polyps have become increasingly important.
In most cases, colorectal polyps are asymptomatic Most of the patients seek medical attention due to the corresponding symptoms or abnormal physical examination results (such as fecal occult blood), and polyps are later revealed by colonoscopy. Colonoscopy is the most effective method for detecting colorectal cancers and polyps (7). In addition to detecting colorectal polyps, colonoscopy can also perform therapeutic polypectomy, making it the preferred method to identify and treat polyps. It would be very helpful if we encourage high-risk patients to have colonoscopy screenings in order to detect and treat colorectal polyps early. Several studies have shown that colonoscopy can significantly reduce colorectal cancer incidence and mortality in a variety of countries (8, 9). By intervening in the early stages of colorectal disease, adenomatous polyps can be prevented from transforming into colorectal cancer. Colonoscopy screening for CRC and adenoma, on the other hand, is rather expensive and unavailable in rural areas with limited resources in China. What's more, the rate of utilization and compliance of colonoscopies have remained relatively low in China due to their invasive nature and cumbersome preparation for insertion (10, 11). Moreover, the incidence of colorectal polyps and CRC has been on the rise in the past few years, with a trend of younger patients (12). However, colonoscopy is not a screening procedure, and young people are less inclined to undergo it.
Thus, it is necessary to correctly identify the high-risk population of colorectal polyps and promote colonoscopy in a wider range of potential patients, which can improve screening efficiency, reduce medical costs and save medical resources. In our study, risk factors for colorectal polyp incidence would be analyzed in order to establish a nomogram that can evaluate the risk of colorectal polyp incidence. With this model, we manage to assess the patients' risk of colon polyps objectively, screen for colorectal polyps in high-risk populations, with an individual and intuitive solution, as well as educate the public about colorectal polyps.
Materials and methods
Study population
We conducted a case-control study in this research. Clinical data of patients who underwent colonoscopy at the Gastrointestinal Surgery Department of the Third Hospital of Hebei Medical University from January 2020 to September 2021 were collected as subjects. The data collected from clinical studies were retrospectively analyzed. The following criteria were used to collect clinical case information: inclusion criteria: (1) Patient undergoing colorectal polypectomy and electronic colonoscopy at the Third Hospital of Hebei Medical University. (2) Colonoscopy can access to the ileocaecal region. (3)Intestinal preparation was perfect, and mucosal observation was unaffected after endoscopy. (4) Patients who had an indication for a colonoscopy. (5) Patients who were able to receive telephone follow-up were included. On the other hand, the exclusion criteria were as follows: (1) with symptoms of mental illness. (2) Patients who were not compliant or refused to participate in the study were excluded. (3) Patients with pathologically confirmed or previous colorectal cancer. (4) Patients who had previously undergone colorectal resection. This study adhered to the principles and ethical requirements of the Helsinki Declaration. Due to the retrospective nature of the study, the requirement for informed patient consent was waived.
Data collection
Based on the criteria above, data were collected from 475 patients, in which including 298 patients with colorectal polyps (CP group) and 177 patients with normal endoscopic and inflammatory lesions (control group). Polyploidy lesions were found during endoscopic surgery, and the pathology was non-neoplastic lesions, while in the control group, there were normal or inflammatory lesions. Data included family history, relative diseases, personal diet habits, and pathological results were collected.
Results of evaluation
Colonoscopy was performed by 2 experienced gastroenterologists who performed at least 1000 colonoscopies per year and were blinded to the bowel preparation regimens the patients received. Endoscopic diagnosis of colorectal polyps would be based on Classification and Digestive Neoplasm (2019 edition) (13). The pathological diagnosis of the colorectal polyp was made by two pathologists. If the diagnosis was disputed, further discussion would be held, or a third pathologist experienced in explaining the results will be invited. Still, if there was disagreement, the seriousness of the disease would prevail.
Statistical analysis
The data was processed and statistical analyses were conducted using SPSS 25.0 (SPSS, Inc., Chicago, IL, United States), and R software (version 4.0.1) with the ‘rms’ package. The quantitative data that conform to the normal distribution were expressed as means ± standard deviation, and the difference between groups was checked by an independent sample t-test. Comparisons between groups that did not obey the normal distribution were done using the nonparametric test. Data from the enumeration process were expressed as percentages and cases, and the Chi-square test was used to determine whether groups differed.
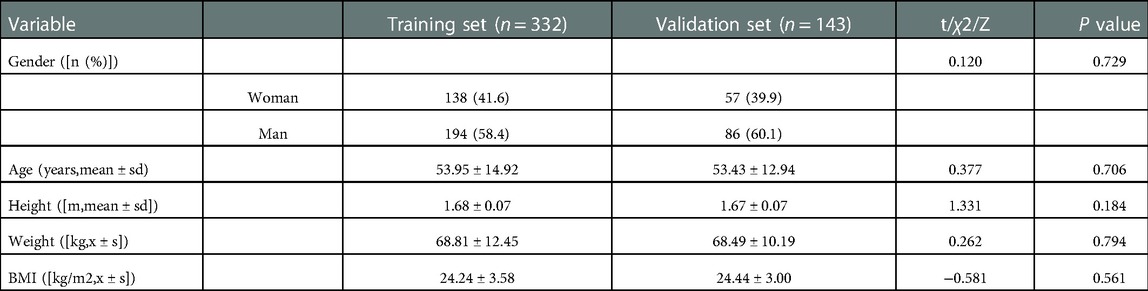
Randomly, we divided the 475 patients into training sets and validation sets according to the proportions of 7:3. The training set included 332 people, while the verification set included 143 people. Based on a single factor logistic regression analysis of the training data, the potential risk factors for colorectal polyps were identified. In univariate analysis, exposure factors with P ≤ 0.2 were selected (14–16). In order to examine the independent risk factors for colorectal polyp development, these potential risk factors were included in a multivariate logistic regression analysis.
The independent risk factors were incorporated into R software and created the rosette map using the ‘rms’ program package to predict colorectal polyp risk. Bootstrapping was applied to repeat the sampling 1000 times to conduct internal verification of the nomogram, and validation set data was applied for external verification. The c-index and area under Receiver Operating Characteristic (AUC) curves were used to measure the discrimination of the nomogram, and the calibration curve between the predicted and observed probabilities was used to evaluate the calibration. Last but not least, the validation set data was applied to validate the model externally. A nomogram chart's clinical application value was assessed by evaluating its sensitivity, specificity, predictive value, and likelihood ratio of the optimal cut-off. The optimal cut-off value was determined by the Youden index. Statistically significant differences were considered to exist when the p-value is below 0.05 in all tests.
Results
Study baseline characteristics
In the training set and the validation set, there were no significant differences found in baseline characters (P > 0.05). The detailed results were shown in Table 1. According to colonoscopy, 204 (61.4%) and 94 (65.7%) patients were detected in the case and control groups, respectively.
Univariate logistic regression model analysis results of colorectal polyp occurrence
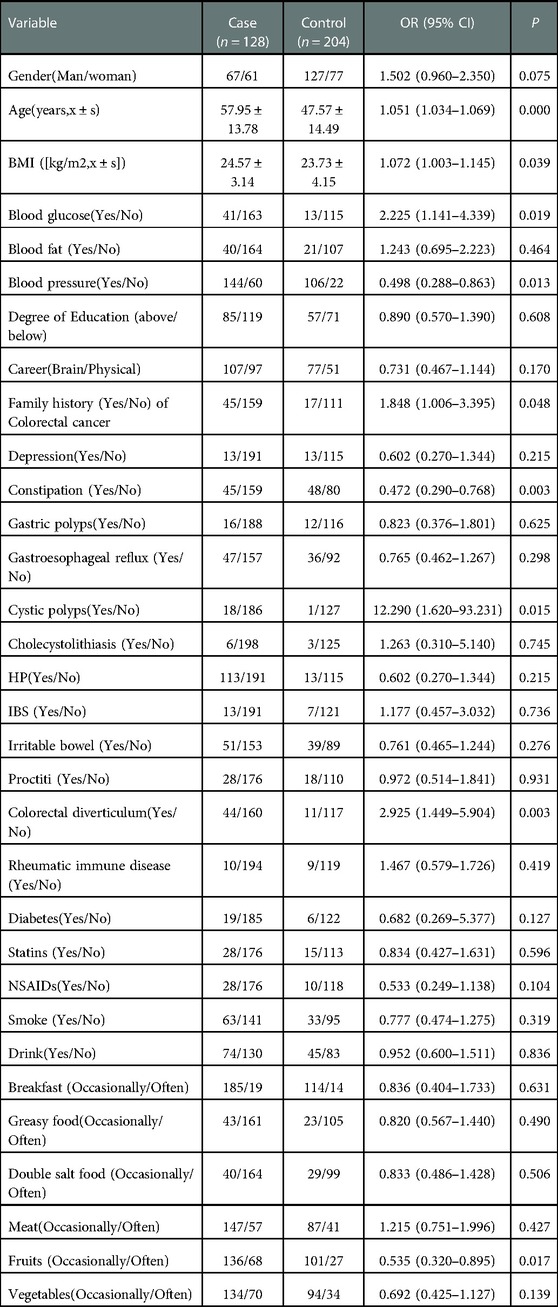
As part of the training set, 332 subjects were classified into the case (n = 204) and control (n = 128) groups Univariate logistic regression analysis showed that the occurrence of colorectal polyps may be correlated with gender, age, BMI, blood glucose, blood pressure, occupational habits (Brain/Physical), family genetic history, history of depression, history of cystic polyps, history of the colorectal diverticulum, history of diabetes, history of Non-Steroidal Anti-inflammatory Drugs (NSAIDs) drugs, and consumption of fruits and vegetables (P ≤ 0.2). The results were shown in Table 2.
Colorectal polyps: multivariate logistic regression model analysis, independent risk factors
Due to the desire to analyze the factors related to the occurrence of colorectal polyps as much as possible, the index P ≤ 0.2 was analyzed by multiple factors. Multivariate logistic regression analysis showed that age (OR = 1.047, 95% CI = 1.029–1.065), history of cystic polyp (OR = 7.596, 95% CI = 0.976–59.129) and history of the colorectal diverticulum (OR = 2.548, 95% CI = 1.209–5.366, P < 0.05) were independent risk factors for colorectal polyps. History of constipation (OR = 0.457, 95% CI = 0.268–0.799) and fruit consumption (OR = 0.613, 95% CI = 0.350–1.037) were a protective factor for colorectal polyps. The detailed results were shown in Table 3. In addition, the Hosmer-Lemeshow goodness of fit test and the Omnibus test of model coefficients confirmed the reliability of the regression model.
Building a predictive nomogram model
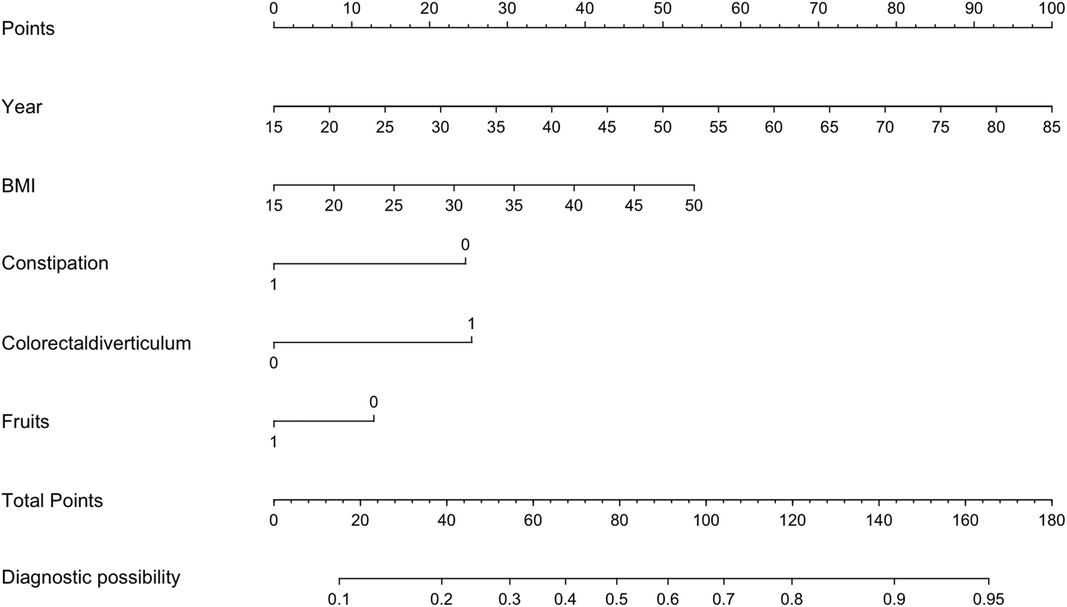
Based on the 5 independent predictors examined by multivariate logistic regression analysis, a risk nomogram for colorectal polyps was built (Figure 1): Points correspond to the upper rating scale for each independent predictor, and the total Points for each subject are the sum of the scores of each independent predictor. Colorectal polyp risk is determined by the value of the total score on the risk axis of colorectal polyps. The higher the total score, the higher the risk of colorectal polyps.
The validation of a model includes both external and internal checks
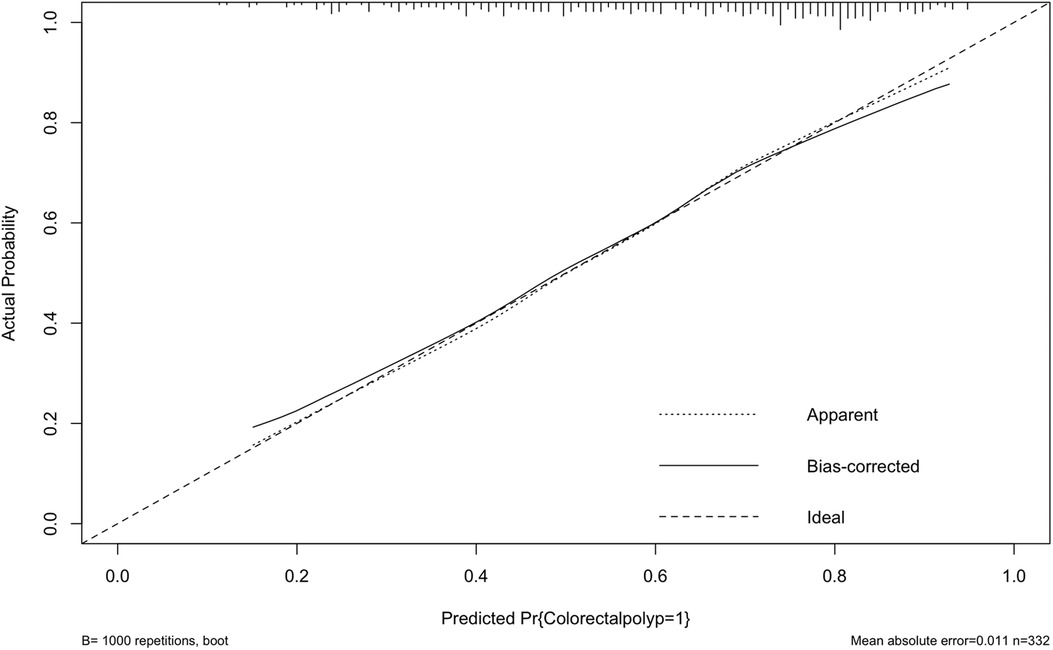
Internally, the nomogram was verified by repeating sampling 1000 times using the Bootstrap method in R software, and verification sets from external sources served as the data for external verification. The nomogram had good segmentation and calibration in predicting colorectal polyps, with C index and AUC both of which was 0.747 (95% CI = 0.692 −0.801) (Figure 2). There was a good agreement between the nomogram prediction model and the colonoscopy detection of real wind risks in the calibration curve (Figure 3).
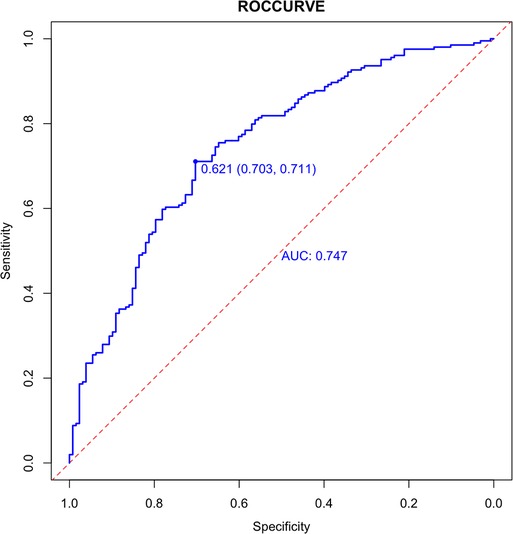
Figure 2. Internal validation of the column diagram: ROC. Of note: AUC: 0.747; 95% CI: 0.692-0.801; p < 0.001.
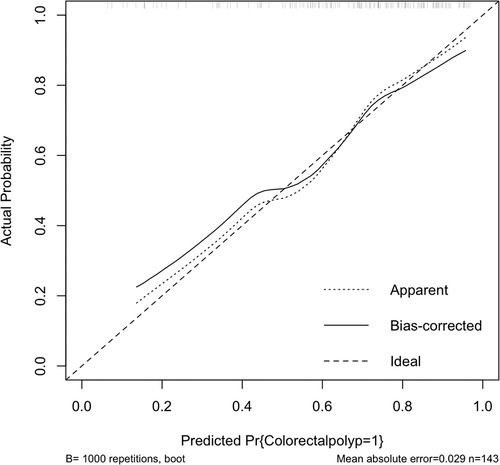
In the validation set, external validation of the model was conducted. In the validation set, the graph also performed well in agreement and calibration, both C index and AUC were 0.782 (95% CI = 0.703–0.861) (Figure 4). A good calibration curve between the predicted and actual wind hazards can also be seen in (Figure 5).
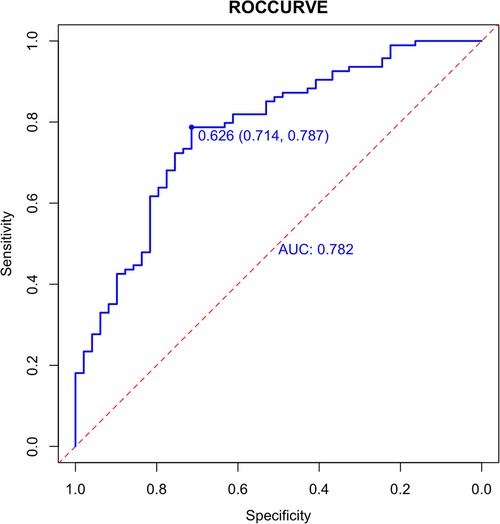
Figure 4. External validation of the column diagram: ROC. Of note: AUC: 0.782; 95% CI: 0.703-0.861; p < 0.001.
An analysis of the clinical effectiveness of the nomogram model
The optimal cut-off value of the training set's nomogram's total score was calculated based on the Youden index as about 93.8 points in order to determine the clinical significance of the model. Those whose overall score exceeded or equaled 93.8 were considered high-risk subjects, and those whose score was less than 93.8 were considered low-risk subjects. Under the cut-off value, sensitivity and specificity were 70.3% and 71.1% in the training set and 71.4% and 78.7% in the verification set.
Disscussion
A colorectal polyp is an abnormal growth that sticks out from the colorectal surface. Generally, CRC develops from colorectal polyps (CP) when an adenoma-carcinoma sequence is initiated (3, 17). A colorectal polyp detected early and treated effectively can improve the outcome of colorectal cancer prevention and treatment. Colorectal cancer screening reports have shown that it reduces the risk of mortality; but patient adherence to screening recommendations remains low. The selection of CRC screening modality depends not only on the validity of the modality in the target population but also on the feasibility, affordability, compliance, and clinical capacity of screening, particularly in resource-limited settings (18). Furthermore, most participants with CRC do not have any clinical symptom, which complicates the process of finding intestinal polyps. In our study, we analyzed the risk factors for colorectal polyp and built an individualized clinical prediction model to help improve the efficiency of colorectal polyp screening and reduce the incidence of colorectal polyps.
Age and history of colorectal diverticulums were independent risk factors for colorectal cancer development in the study. Furthermore, constipation (Dehydrated stool and reduced frequency of stools) and vegetable consumption (eat vegetables regularly) were protective factors for colorectal polyps. In the Asia Pacific Colorectal Screening Scoring System (19) and its revisions (20), people at high risk for CRC and advanced adenomas are screened based on their age. At the same time, studies have found that colorectal adenoma incidence increases with age (21). In our study, this was corroborated.
In our study, a history of gallbladder polyps' disease was shown as an independent risk factor for developing CP. In a previous study performed in a Japanese case-control study, the prevalence of CP in patients with biliary tract disease was significantly higher than that of controls. The presence of biliary tract disease is an independent risk factor for colon polyps (OR = 1.57,95% CI = 1.14–2.18) (22). One possible explanation is that gallbladder disease produces more deoxygenated bile acids and this secondary bile acid can be formed by promoting the formation of intestinal adenoma (23).
Our data showed that the history of colorectal diverticulums was an independent risk factor for colorectal polyps, in addition to age and gallbladder polyps' disease, which is consistent with a previous study by Liu et.al (OR = 2.548, 95% CI = 1.209–5.366, P < 0.05) (24). Nevertheless, some researchers disagreed. Their argument was that there was controversy surrounding the link between colorectal diverticular disease and colorectal adenoma (25).
Further, our study found that the consumption of fruit and a history of constipation were protective factors for CPs. Previous studies have shown that smoking, alcohol consumption, high-fat diet, red meat, and low-fiber diets increase the risk of colorectal polyps (25–28). Smoking has also been shown to promote the development of cancer of the colon originating from colorectal polyps (29). The results of our study, however, suggested that fruit consumption alone was protective against colorectal polyps. In another aspect, constipation was associated with colorectal cancer (30, 31). There has not been a detailed analysis of the relationship between them and the mechanism of their mutual influence.
Many researches focuses on the development of clinical models of disease risk, and many related risk models are available, including those for coronary heart disease and colorectal cancer (32, 33). Currently, most colorectal disease predictive models are based on colorectal cancer (34, 35). Few colorectal polyp risk prediction models exist. A small number of studies developed predictive models was established based on postoperative pathological features combined with artificial intelligence (36, 37). While these studies differ from the research concept of this study, the findings of our study are more consistent with the concept of early diagnosis and early treatment in some way.
A nomogram was constructed based on the predictors screened by statistical analysis. In this study, a nomogram could predict risk with 0.703 accuracies, and 0.711 accuracies for the validation set. This nomogram can provide a certain reference value for medical workers to intuitively analyze individual risk, as well as for patients at high risk of colorectal polyps to be screened. Depending on the predicted risk, a reasonable evil screening procedure can be designed.
There are still some limitations in this study. The enrolled population selected in this study was a group of people who visited our hospital for colorectal examination at one time, and the bias of admission rate was inevitable in the selection of the population. Due to the different medical conditions of the population and the uneven understanding of colorectal polyps, the proportion of patients in the positive group may be too high. And the study sample was relatively small; it was only conducted retrospectively over one year at one hospital. In addition, the established model had a weaker generalization ability and transferability than the space test. It is still necessary to carry out large-scale research with multiple centers in order to verify this. Also screen the high-risk population for colorectal polyps, so as to achieve the goal of early detection, prevention, and treatment of colorectal polyps, as well as to serve as a reference for further prevention and treatment.
Conclusions
In our study, the nomogram predictive model is reliable and accurate, which helps early clinical screening of patients with high-risk colorectal polyps, improves polyp detection rate, and reduces the incidence of colorectal cancer (CRC).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH, HL, SY, and GW contributed to the conception and design of the study. YL, XY, TZ, PZ, YH, and YY acquired and analyzed the data. YL and YH drafted and revised a significant portion of the manuscript or figures. SL, XY, and YH conducted the statistical analysis. YH, YL, and XY wrote the paper. All authors read and approved the present version of the manuscript to be published. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Hebei Provincial Natural Science Foundation precision medicine joint project (H2020206485) and Hebei Provincial Department of science and technology key project (206Z7705G).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Koessler T, Bichard P, Lepilliez V, Puppa G, Ris F, Roth A. [Epidemiology, treatment and follow-up of colorectal polyps]. Rev Med Suisse. (2016) 12(519):982–8. PMID: 2742442527424425
2. Roperch J-P, Incitti R, Forbin S, Bard F, Mansour H, Mesli F, et al. Aberrant methylation of NPY, PENK, and WIF1 as a promising marker for blood-based diagnosis of colorectal cancer. BMC Cancer. (2013) 13:566. doi: 10.1186/1471-2407-13-566
3. Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. (1988) 319(9):525–32. doi: 10.1056/NEJM198809013190901
4. Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. (1987) 93(5):1009–13. doi: 10.1016/0016-5085(87)90563-4
5. Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. (2002) 12(1):1–9. doi: 10.1016/S1052-5157(03)00053-9
6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
7. Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. (2014) 80(1):133–43. doi: 10.1016/j.gie.2014.01.014
8. Cross AJ, Robbins EC, Pack K, Stenson I, Kirby PL, Patel B, et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut. (2020) 69(9):1645–58. doi: 10.1136/gutjnl-2019-320036
9. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69(1):7–34. doi: 10.3322/caac.21551
10. Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. (2019) 68(8):1450–7. doi: 10.1136/gutjnl-2018-317124
11. Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. (2018) 30(1):1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01
12. Wang Q, He R, Tan T, Li J, Hu Z, Luo W, et al. A novel long non-coding RNA-KAT7 is low expressed in colorectal cancer and acts as a tumor suppressor. Cancer Cell Int. (2019) 19:40. doi: 10.1186/s12935-019-0760-y
13. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. (2020) 76(2):182–8. doi: 10.1111/his.13975
14. Dagnew M, Million Y, Gizachew M, Eshetie S, Yitayew G, Asrade L, et al. Hepatitis B and C Viruses’ infection and associated factors among pregnant women attending antenatal care in hospitals in the amhara national regional state, Ethiopia. Int J Microbiol. (2020) 2020:8848561. doi: 10.1155/2020/8848561
15. Semunigus T, Tessema B, Eshetie S, Moges F. Smear positive pulmonary tuberculosis and associated factors among homeless individuals in dessie and debre birhan towns, northeast Ethiopia. Ann Clin Microbiol Antimicrob. (2016) 15(1):50. doi: 10.1186/s12941-016-0165-x
16. Storms AD, Chen J, Jackson LA, Nordin JD, Naleway AL, Glanz JM, et al. Rates and risk factors associated with hospitalization for pneumonia with ICU admission among adults. BMC Pulm Med. (2017) 17(1):208. doi: 10.1186/s12890-017-0552-x
17. Cho KR, Vogelstein B. Genetic alterations in the adenoma–carcinoma sequence. Cancer. (1992) 70(6 Suppl):1727–31. doi: 10.1002/1097-0142(19920915)70:4+%3C1727::aid-cncr2820701613%3E3.0.co;2-p
18. Bénard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average-risk adults: summarizing the current global recommendations. World J Gastroenterol. (2018) 24(1):124–38. doi: 10.3748/wjg.v24.i1.124
19. Yeoh K-G, Ho K-Y, Chiu H-M, Zhu F, Ching JYL, Wu D-C, et al. The Asia-pacific colorectal screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut. (2011) 60(9):1236–41. doi: 10.1136/gut.2010.221168
20. Wong MCS, Lam TYT, Tsoi KKF, Hirai HW, Chan VCW, Ching JYL, et al. A validated tool to predict colorectal neoplasia and inform screening choice for asymptomatic subjects. Gut. (2014) 63(7):1130–6. doi: 10.1136/gutjnl-2013-305639
21. Wang J-Y, Li Z-T, Zhu Y-M, Wang W-C, Ma Y, Liu Y-L. Utility of the Asia-pacific colorectal screening scoring system and the presence of metabolic syndrome components in screening for sporadic colorectal cancer. World J Gastroenterol. (2014) 20(32):11394–9. doi: 10.3748/wjg.v20.i32.11394
22. Yamaji Y, Okamoto M, Yoshida H, Kawabe T, Wada R, Mitsushima T, et al. Cholelithiasis is a risk factor for colorectal adenoma. Am J Gastroenterol. (2008) 103(11):2847–52. doi: 10.1111/j.1572-0241.2008.02069.x
23. Liu Y-L, Wu J-S, Yang Y-C, Lu F-H, Lee C-T, Lin W-J, et al. Gallbladder stones and gallbladder polyps associated with increased risk of colorectal adenoma in men. J Gastroenterol Hepatol. (2018) 33(4):800–6. doi: 10.1111/jgh.14006
24. Kim Y-J, Kim DB, Chung WC, Lee JM. Does colonic diverticulosis raise the risk of colorectal adenoma in patients with colorectal cancer? Gastroenterol Res Pract. (2019) 2019:8901026. doi: 10.1155/2019/8901026
25. Wang F-W, Chuang H-Y, Tu M-S, King T-M, Wang J-H, Hsu C-W, et al. Prevalence and risk factors of asymptomatic colorectal diverticulosis in Taiwan. BMC Gastroenterol. (2015) 15:40. doi: 10.1186/s12876-015-0267-5
26. Mühl H, Paulukat J, Höfler S, Hellmuth M, Franzen R, Pfeilschifter J. The HIV protease inhibitor ritonavir synergizes with butyrate for induction of apoptotic cell death and mediates expression of heme oxygenase-1 in DLD-1 colon carcinoma cells. Br J Pharmacol. (2004) 143(7):890–8. doi: 10.1038/sj.bjp.0706023
27. Pan J, Cen L, Xu L, Miao M, Li Y, Yu C, et al. Prevalence and risk factors for colorectal polyps in a Chinese population: a retrospective study. Sci Rep. (2020) 10(1):6974. doi: 10.1038/s41598-020-63827-6
28. Sharara AI, El Mokahal A, Harb AH, Khalaf N, Sarkis FS, El-Halabi M M, et al. Risk prediction rule for advanced neoplasia on screening colonoscopy for average-risk individuals. World J Gastroenterol. (2020) 26(37):5705–17. doi: 10.3748/wjg.v26.i37.5705
29. Kishida Y, Hotta K, Imai K, Ito S, Yoshida M, Kawata N, et al. Risk analysis of colorectal post-polypectomy bleeding due to antithrombotic agent. Digestion. (2019) 99(2):148–56. doi: 10.1159/000490791
30. Liu B. [Correlation between chronic constipation and colorectal neoplasms]. Zhonghua Wei Chang Wai Ke Za Zhi. (2017) 20(3):255–7. PMID: 2833815528338155
31. Shemerovskiĭ KA. [Constipation–a risk factor for colorectal cancer]. Klin Med (Mosk. (2005) 83(12):60–4. PMID: 16502728
32. Guan H, Dai G-H, Gao W-L, Zhao X, Cai Z-H, Zhang J-Z, et al. A 5-year survival prediction model for chronic heart failure patients induced by coronary heart disease with traditional Chinese medicine intervention. Evid Based Complement Alternat Med. (2021) 2021:4381256. doi: 10.1155/2021/4381256
33. Wang S, Xie H, Gong Y, Kuang J, Yan L, Ruan G, et al. The value of L3 skeletal muscle index in evaluating preoperative nutritional risk and long-term prognosis in colorectal cancer patients. Sci Rep. (2020) 10(1):8153. doi: 10.1038/s41598-020-65091-0
34. Huang X, Cai W, Yuan W, Peng S. Identification of key lncRNAs as prognostic prediction models for colorectal cancer based on LASSO. Int J Clin Exp Pathol. (2020) 13(4):675–84. PMID: 3235551532355515
35. Reidy E, Leonard NA, Treacy O, Ryan AE. A 3D view of colorectal cancer models in predicting therapeutic responses and resistance. Cancers (Basel). (2021) 13(2):227. doi: 10.3390/cancers13020227
36. Byrne MF, Chapados N, Soudan F, Oertel C, Linares Pérez M, Kelly R, et al. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. (2019) 68(1):94–100. doi: 10.1136/gutjnl-2017-314547
Keywords: intestinal polyps, influencing factors, intestinal tumor, nomogram, prevention
Citation: Huang Y, Liu Y, Yin X, Zhang T, Hao Y, Zhang P, Yang Y, Gao Z, Liu S, Yu S, Li H and Wang G (2023) Establishment of clinical predictive model based on the study of influence factors in patients with colorectal polyps. Front. Surg. 10:1077175. doi: 10.3389/fsurg.2023.1077175
Received: 22 October 2022; Accepted: 9 January 2023;
Published: 23 February 2023.
Edited by:
Stefano Pontone, Sapienza University of Rome, ItalyReviewed by:
Filippo Carannante, Campus Bio-Medico University, ItalySergey Achkasov, Ryzhikh National Medical Research Centre of Coloproctology, Russia
Shenghui Huang, Fujian Medical University Union Hospital, China
© 2023 Huang, Liu, Yin, Zhang, Hao, Zhang, Yang, Gao, Liu, Yu, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suyang Yu eXN5cXR0QDE2My5jb20= Hongyan Li d2luZGhieWRAMTI2LmNvbQ== Guiying Wang d2FuZ2d1aXlpbmdAaGVibXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Yu Huang
Yu Huang Yating Liu
Yating Liu Xu Yin1
Xu Yin1 Yang Yang
Yang Yang Suyang Yu
Suyang Yu Guiying Wang
Guiying Wang