
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 21 March 2023
Sec. Thoracic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1052932
This article is part of the Research Topic Advances in Esophageal Cancer Surgery with Neoadjuvant Therapies View all 13 articles
Objective: The aim of this study was to investigate the effect of preoperative radiotherapy (RT) on overall survival (OS) in patients with stage cTxN0M0 esophageal squamous cell carcinoma (ESCC).
Methods: A total of 467 patients with ESCC diagnosed as cTxN0M0 and undergoing esophagectomy between 2004 and 2016 were downloaded from the Surveillance, Epidemiology, and End Results (SEER) database. According to the presence or absence of preoperative RT, the patients were divided into preoperative RT group and non-preoperative RT group. Propensity score matching (PSM) was performed to equalize baseline levels between groups. Univariate and multivariate Cox regression analyses were used to compare the survival differences between the two groups.
Results: Using PSM, 162 pairs of patients were selected. Preoperative RT was not a prognostic factor for OS in all patients with cTx stage. After PSM, for patients with cT1–2 stage, univariate Cox regression analysis showed that preoperative RT was an influencing factor of OS, and multivariate Cox regression analysis confirmed that preoperative RT was an independent predictor of OS. Compared with non-preoperative RT, preoperative RT significantly decreased OS (HR = 1.556, 95%CI 1.008–2.464, p = 0.046). For patients with cT3–4, univariate Cox regression analysis showed that preoperative RT was an influencing factor for OS, and multivariate Cox regression analysis determined that preoperative RT was independent predictors of survival. Compared with non-preoperative RT, preoperative RT significantly improved the OS (HR = 0.479, 95%CI 0.272–0.841, p = 0.010).
Conclusion: For ESCC, preoperative RT can improve the OS of patients with cT3-4N0M0. However, preoperative RT is not suitable for patients with cT1-2N0M0.
The incidence of esophageal cancer is increasing year by year (1). At present, the radical treatment of esophageal cancer mainly adopts surgical resection, radiotherapy and chemotherapy. Clinical studies have shown that single treatment can not achieve the ideal treatment effect (2, 3). How to take the comprehensive treatment plan for esophageal cancer is the focus of clinical researchers.
Preoperative neoadjuvant therapy is associated with tumor downstaging and can improve the resection rate and long-term survival rate of esophageal cancer, so it is usually used in patients with locally advanced stage. Several clinical trials have demonstrated the efficacy of preoperative neoadjuvant therapy (4–7). However, given the frequent presence of locally advanced disease and frequent lymph node metastases in these clinical trials, it is difficult to conclude that patients with early stage or non-metastatic lymph nodes would benefit from preoperative neoadjuvant therapy. For these patients, the efficacy of preoperative neoadjuvant therapy remains to be verified.
This study aimed to explore the effect of preoperative radiotherapy (RT) on the prognosis of patients with cTxN0M0 esophageal squamous cell carcinoma (ESCC) through a propensity score matching (PSM) study based on the Surveillance, Epidemiology, and End Results (SEER) database.
The SEER database is a publicly available database that includes data from 18 cancer registries in the United States, representing approximately 29% of the U.S. population (8). Patients diagnosed with ESCC between 2004 and 2016 were downloaded from the SEER database. Inclusion criteria: (1) Patients undergoing preoperative radiotherapy combined with surgery. (2) Patients with definite preoperative cTNM staging. (3) Stage cN0 was diagnosed without distant metastasis. (4) Report follow-up data and survival status. Exclusion criteria: (1) Patients without surgery. (2) Patients without preoperative radiotherapy. (3) Patients receiving postoperative radiotherapy.
The characteristics analyzed in the current study included age at diagnosis (≤65, >65), sex (male, female), race (white, black and other/unkown), tumor site (upper, middle and lower third), tumor cT stage, histologic grade (high, moderate and poor), follow-up time, and survival status. OS was the end point of the study. OS was defined as the time from diagnosis to death from any reason.
The patients were divided into preoperative RT group and non-preoperative RT group according to the presence or absence of preoperative RT. Statistical analysis was performed using SPSS software. The chi-square test or Fisher exact test was used to compare categorical variables. PSM was used to balance baseline levels between groups (age, sex, race, tumor site, histologic grade) with a caliper value of 0.02. Univariate Cox regression analysis was used to screen the influencing factors of OS. Factors with p < 0.1 were included in multivariate Cox regression analysis to determine the independent predictors of OS. The hazard ratio (HR) and its 95% confidence intervals (95%CI) were calculated to assess the strength of association between different characteristics and OS. Subgroup analysis was performed by cT stage (cT1–2, cT3–4) to further determine the factors affecting OS. Kaplan-meier method was used to draw survival curves. A two-sided p-value of <0.05 was considered statistically significant.
A total of 2,161 patients diagnosed with ESCC who underwent surgery were downloaded from SEER database. 467 patients were enrolled in this study, including 206 patients who received preoperative RT and 261 patients who did not receive preoperative. The specific process of patient selection is shown in Figure 1. Baseline unadjusted comparisons of patient demographics and oncological outcomes by treatment group (preoperative RT vs. non-preoperative RT) are shown in Table 1. Among the patients with cT3–4, the patients with preoperative RT were more than those without preoperative RT. After PSM, 162 patients were enrolled in each group. The patient demographics and tumor outcomes comparisons between the two groups are shown in Table 2. Similarly, in patients with cT3–4, patients with preoperative RT are more than those without preoperative RT.
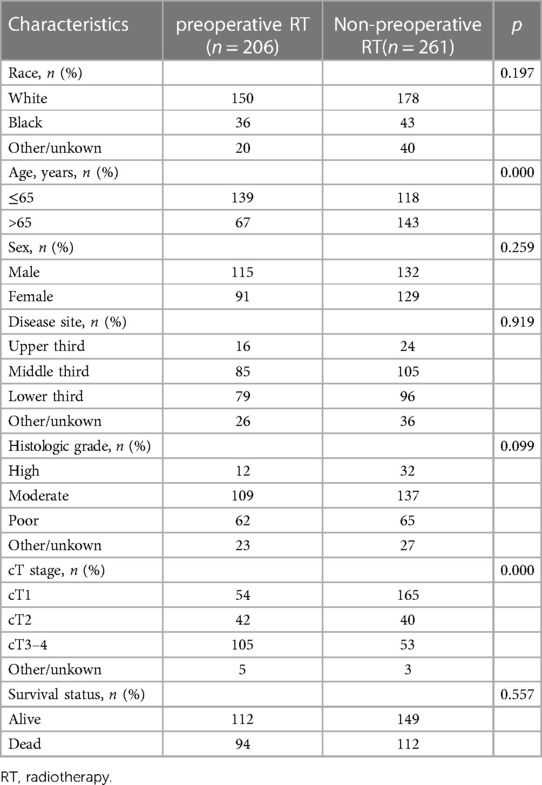
Table 1. Comparison of patient demographics and tumor characteristics for the clinical node-negative patients before propensity score matching.
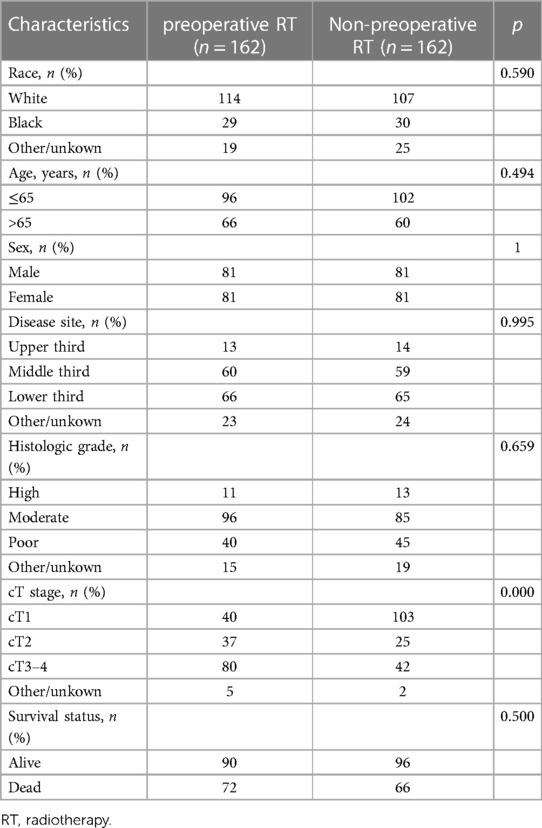
Table 2. Comparison of patient demographics and tumor characteristics for the clinical node-negative patients after propensity score matching.
Preoperative RT was not a significant factor in OS for all patients, regardless of whether PSM was performed for covariates. Table 3 shows the effects of pre-PSM and post-PSM covariates on postoperative OS.
After PSM, 205 patients were in cT1–2 stage, and 77 of them received preoperative RT. Baseline comparisons of patient demographics and oncological outcomes by treatment group are shown in Supplementary Table S1. Baseline characteristics were not significantly unbalanced between the two groups. Univariate Cox regression analysis showed that preoperative RT and histologic grade were the influencing factors of OS, and preoperative RT was associated with increased risk of death (HR = 1.585, 95%CI 1.027–2.446, p = 0.037). Multivariate Cox regression analysis also showed that preoperative radiotherapy was an independent risk factor for OS in patients with stage cT1–2 ESCC (HR = 1.556, 95%CI 1.008–2.464, p = 0.046). The specific results of Cox analysis are shown in Table 4.
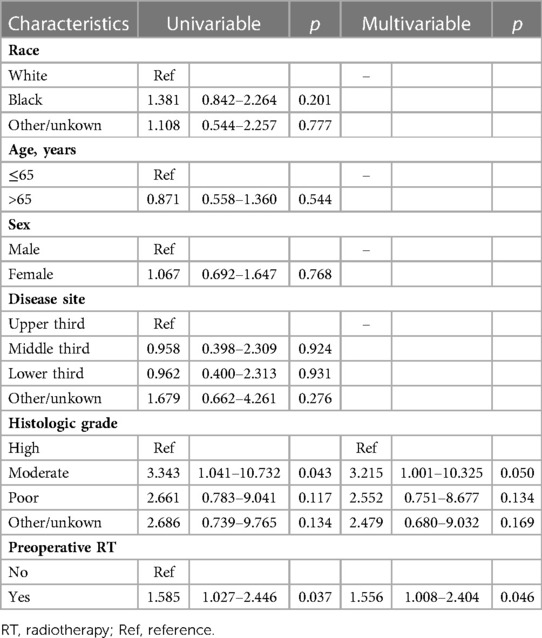
Table 4. Univariable and multivariable cox analysis of the influence of each characteristic on overall survival for cT1-2 patients after propensity score matching.
The survival curve drawn according to the Kaplan-Meier method is shown in Figure 2A. Preoperative RT increased the overall risk of death in patients with cT1–2. There was no significant difference in 1-year (76.62 vs. 86.72, chi-square = 3.461, p = 0.063) and 3-year (62.34 vs. 71.88, chi-square = 2.020, p = 0.155) survival between the two groups, but preoperative radiotherapy was associated with a significant reduction in 5-year survival (50.65 vs. 67.19%, chi-square = 5.526, p = 0.019).
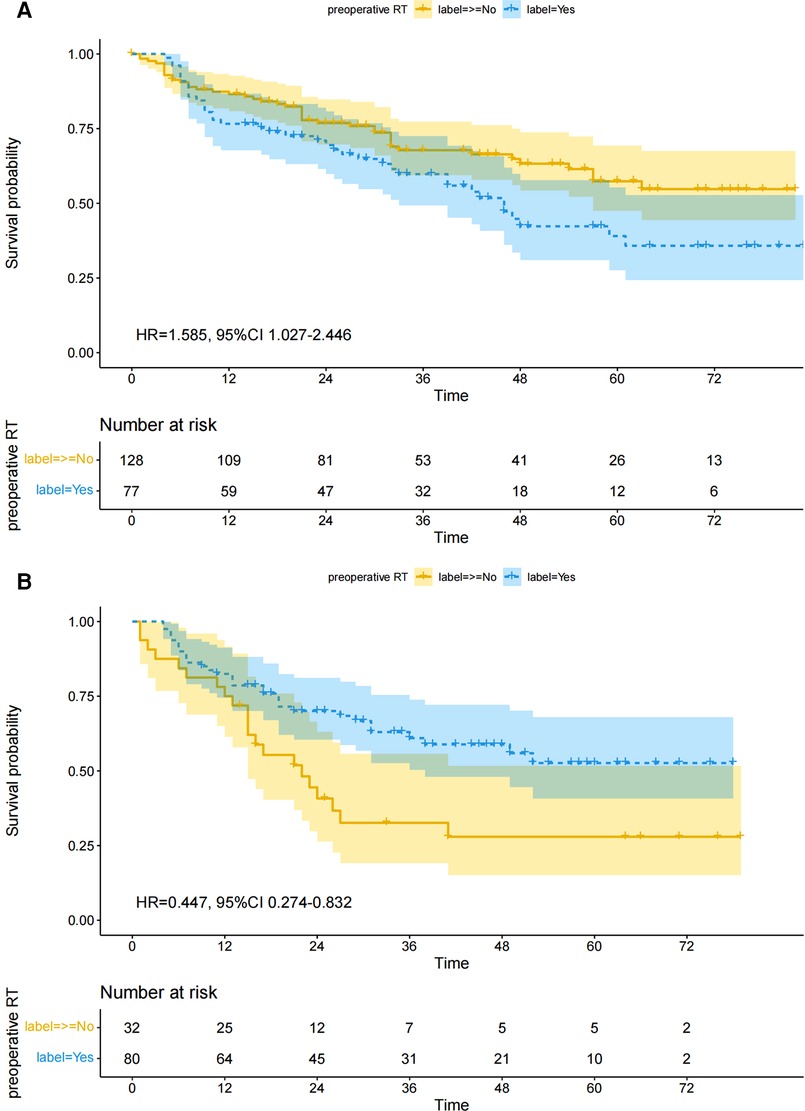
Figure 2. Overall survival between preoperative RT and non-preoperative RT groups after matching. (A) cT1–2, (B) cT3–4.
After PSM, 112 patients were in cT3–4 stage, and 80 of them received preoperative RT. Baseline comparisons of patient demographics and oncological outcomes by treatment group are shown in Supplementary Table S2. Baseline characteristics were not significantly unbalanced between the two groups. Univariate Cox regression analysis showed that preoperative RT and race were the influencing factors of OS, and preoperative RT was associated with a reduced risk of death (HR = 0.477, 95%CI 0.274–0.832, p = 0.009). Multivariate Cox regression analysis also showed that preoperative RT was an independent predictor of OS in patients with cT3–4 ESCC, and preoperative RT significantly reduced the overall risk of death (HR = 0.479, 95%CI 0.272–0.841, p = 0.010). The specific results of Cox analysis are shown in Table 5.
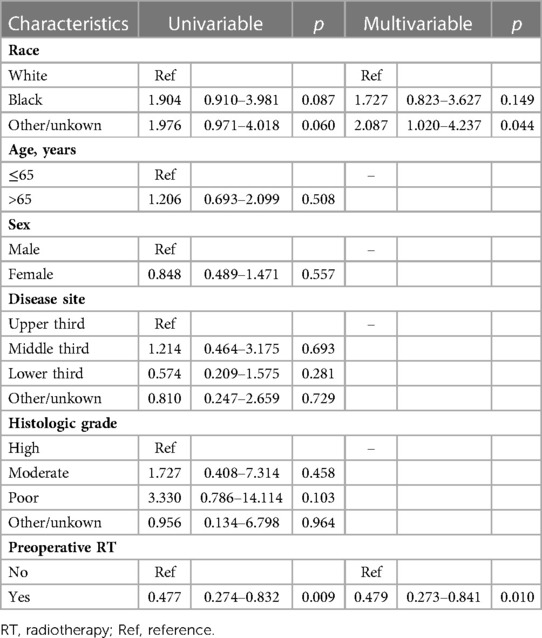
Table 5. Univariable and multivariable cox analysis of the influence of each characteristic on postoperative survival for cT3-4 patients after propensity score matching.
The survival curve drawn according to the Kaplan-Meier method is shown in Figure 2B. Preoperative RT reduced the overall risk of death in patients with cT3–4. There was no significant difference in 1-year (82.50 vs. 75.00, chi-square = 0.815, p = 0.367) survival between the two groups, but preoperative radiotherapy was significantly associated with improved 3-year (65.00 vs. 37.50, chi-square = 7.058, p = 0.008) and 5-year (61.25 vs. 34.38, chi-square = 6.637, p = 0.008) survival.
In this propensity score matching study, we found that preoperative RT was associated with OS in patients undergoing surgery for ESCC with preoperative diagnosis of stage cTxN0M0. Especially in patients with cT3–4 stage, preoperative RT produced a significant effect. But for low stage patients (cT1–2), preoperative RT had a negative impact.
Preoperative neoadjuvant therapy has been widely used as a supplement to surgery for esophageal cancer (9, 10). The CROSS trial and the 5010 trial confirmed that preoperative chemoradiotherapy could significantly improve the long-term survival of patients with locally advanced esophageal cancer, and the 5010 trial alone targeted esophageal squamous cell carcinoma (4, 5). A study by the JCOG group in Japan has shown that preoperative chemotherapy also has a good survival effect (6).
For preoperative RT, there were also clinical studies reporting results. For example, the study by Dong et al. showed that preoperative RT improved long-term survival in locally advanced ESCC (11). However, the study of Gao et al. showed that preoperative radiotherapy is only suitable in certain populations (12). This difference means that preoperative radiotherapy is not suitable for all patients. In addition, most of the patients included in the previous clinical trials were locally advanced with lymph node metastasis. Therefore, for patients without lymph node metastasis, the effect of preoperative neoadjuvant therapy is still unclear (13–15). We designed a propensity matching study specifically for patients with ESCC who were preoperatively diagnosed as stage cTxN0M0. Considering that the number of patients diagnosed with stage cN0M0 and receiving preoperative neoadjuvant therapy in a single medical center is small, it is difficult to formulate a valid analysis. Therefore, we downloaded the data of such patients from the SEER database. Due to its population-based nature, there are significant advantages to using the SEER database: the database collects data from 18 registries in 14 U.S. states, representing nearly 30% of the U.S. population, equivalent to a large multicenter database. In addition, treatment decisions for esophageal cancer must be made according to stage, so we also stratified patients according to cT stage to further study the efficacy of preoperative radiotherapy. Theoretically, preoperative treatment can help to shrink the tumor and shrink the lymph node, thereby increasing the radical resection rate and improving the long-term survival rate. However, in practice, comprehensive treatment is very complicated. Preoperative radiotherapy is associated with additional treatment-related adverse effects compared with surgery alone, adversely affecting quality of life in some patients, and potentially increasing postoperative mortality. Our study showed that preoperative RT was not appropriate for all patients with cTxN0M0. Preoperative RT was suitable for patients with stage cT3–4 ESCC, while patients with stage cT1–2 could not benefit from preoperative RT, and preoperative RT had a negative effect on patients with low cT stage. It's not hard to understand. For patients with cT1–2, the probability of occult lymph node metastasis and the depth of tumor invasion is low, and R0 resection is easy to be achieved by surgery. As a result, preoperative radiotherapy cannot bring significant survival effect, and the patients bear potential radiotherapy related adverse reactions. A meta-analysis showed that preoperative neoadjuvant therapy could reduce the tumor stage of cT2N0 stage esophageal cancer, but did not improve patient survival (16). Two multi-center retrospective studies in Taiwan and Europe showed that neoadjuvant therapy provided significant survival benefits for cT3N0 esophageal cancer (17, 18). Similarly, a large retrospective study by Gao et al. showed that although neoadjuvant therapy helped to improve postoperative survival in esophageal cancer patients with cN0 on the whole, neoadjuvant therapy was associated with decreased survival for early-staged true node-negative patients (12).
Of course, there are some limitations in this study. First, our results were based on a retrospective study. We grouped patients according to treatment mode and were therefore not random, which could lead to selection bias. Second, although propensity matching was used to avoid the imbalance between groups as much as possible, due to the limitations of the database itself, other data that might affect survival (such as comorbidities, physical status, etc.) were not available. Moreover, we do not have the exact treatment data of the patients, such as the specific dose and regimen of radiotherapy. Radiotherapy regimens and methods have been rapidly developed in the past decades, such as 3-dimensional conformal radiation therapy (3D-CRT) and intensity modulated radiation therapy (IMRT), and their efficacy has been proven (19–21). In addition, due to limited access to the database, it was not possible to know whether patients receiving preoperative radiation therapy included some patients receiving salvage surgery after radiotherapy. Patient survival depends on the treatment techniques and regimens used, and further research is needed in this aspect. And, due to the limitation of the number of people diagnosed with cN0 stage in the SEER database from 2004 to 2016, the number of patients included in this article was not large. As the database data is constantly updated, we will dig deeper.
For ESCC, preoperative RT can improve the OS of patients with cT3-4N0M0, which is worthy of clinical application. However, preoperative RT is not suitable for patients with cT1-2N0M0. The role of preoperative RT should be further investigated in prospective studies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Because the SEER database is publicly accessible worldwide, therefore, we did not provide the approval of an institutional review board in the current study.
ZJ and BZ: had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JS and BZ. Acquisition, analysis, or interpretation of data: ZJ, JS and JZ. Drafting of the manuscript: ZJ, JS and JZ. Critical revision of the manuscript for important intellectual content: ZJ, JS and BZ. Statistical analysis: ZJ, JS and JZ. Study supervision: JS and BZ. All authors contributed to the article and approved the submitted version.
The authors are grateful for the research platform provided by Key Laboratory of Minimally Invasive Techniques & Rapid Rehabilitation of Digestive System Tumor of Zhejiang Province, Taizhou Hospital of Zhejiang Province and Director Chengchu Zhu. The authors are very thankful to the SEER Program for providing the opening and high-quality resources for researchers.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1052932/full#supplementary-material.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. (2017) 390(10110):2383–96. doi: 10.1016/S0140-6736(17)31462-9
3. Borggreve AS, Kingma BF, Domrachev SA, Koshkin MA, Ruurda JP, van Hillegersberg R, et al. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci. (2018) 1434(1):192–209. doi: 10.1111/nyas.13677
4. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. (2021) 156(8):721–9. doi: 10.1001/jamasurg.2021.2373
5. Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39(18):1995–2004. doi: 10.1200/JCO.20.03614
6. Nakamura K, Kato K, Igaki H, Ito Y, Mizusawa J, Ando N, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. (2013) 43(7):752–5. doi: 10.1093/jjco/hyt061
7. Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. (2010) 16(13):1649–54. doi: 10.3748/wjg.v16.i13.1649
8. Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: surveillance, epidemiology, and end results (SEER) database. JAMA Surg. (2018) 153(6):588–9. doi: 10.1001/jamasurg.2018.0501
9. Xiao X, Hong HG, Zeng X, Yang YS, Luan SY, Li Y, et al. The efficacy of neoadjuvant versus adjuvant therapy for resectable esophageal cancer patients: a systematic review and meta-analysis. World J Surg. (2020) 44(12):4161–74. doi: 10.1007/s00268-020-05721-w
10. Yuan M, Bao Y, Ma Z, Men Y, Wang Y, Hui Z. The optimal treatment for resectable esophageal cancer: a network meta-analysis of 6168 patients. Front Oncol. (2021) 11:628706. doi: 10.3389/fonc.2021.628706
11. Dong J, Shen W, Du X, Zhu S. Effects of preoperative radiotherapy on survival of patients with stage II and III esophageal squamous cell carcinoma: a population-based study. Medicine. (2021) 100(41):e27345. doi: 10.1097/MD.0000000000027345
12. Gao HJ, Wei YC, Gong L, Ge N, Han B, Shi GD, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for clinical node-negative esophageal carcinoma. Thorac Cancer. (2020) 11(9):2618–29. doi: 10.1111/1759-7714.13586
13. Chen WH, Chao YK, Chang HK, Tseng CK, Wu YC, Liu YH, et al. Long-term outcomes following neoadjuvant chemoradiotherapy in patients with clinical T2N0 esophageal squamous cell carcinoma. Dis Esophagus. (2012) 25(3):250–5. doi: 10.1111/j.1442-2050.2011.01243.x
14. Gabriel E, Attwood K, Du W, Tuttle R, Alnaji RM, Nurkin S, et al. Association between clinically staged node-negative esophageal adenocarcinoma and overall survival benefit from neoadjuvant chemoradiation. JAMA Surg. (2016) 151(3):234–45. doi: 10.1001/jamasurg.2015.4068
15. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. (2014) 32(23):2416–22. doi: 10.1200/JCO.2013.53.6532
16. Lv HW, Xing WQ, Shen SN, Cheng JW. Induction therapy for clinical stage T2N0M0 esophageal cancer: a systematic review and meta-analysis. Medicine. (2018) 97(40):e12651. doi: 10.1097/MD.0000000000012651
17. Chao YK, Ku HY, Chen CY, Liu TW. Induction therapy before surgery improves survival in patients with clinical T3N0 esophageal cancer: a nationwide study in Taiwan. Dis Esophagus. (2017) 30(12):1–7. doi: 10.1093/dote/dox103
18. Mantziari S, Gronnier C, Renaud F, Duhamel A, Théreaux J, Brigand C, et al. Survival benefit of neoadjuvant treatment in clinical T3N0M0 esophageal cancer: results from a retrospective multicenter European study. Ann Surg. (2017) 266(5):805–13. doi: 10.1097/SLA.0000000000002402
19. Sun J, Huang W, Chen J, Zhang Y. Association of 3D-CRT and IMRT accelerated hyperfractionated radiotherapy with local control rate and 5-year survival in esophageal squamous cell carcinoma patients. Br J Radiol. (2022) 95(1133):20211195. doi: 10.1259/bjr.20211195
20. Chen NB, Qiu B, Zhang J, Qiang MY, Zhu YJ, Wang B, et al. Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy in definitive chemoradiotherapy for cervical esophageal squamous cell carcinoma: comparison of survival outcomes and toxicities. Cancer Res Treat. (2020) 52(1):31–40. doi: 10.4143/crt.2018.624
21. Innocente R, Navarria F, Petri R, Palazzari E, Vecchiato M, Polesel J, et al. Feasibility and oncological outcome of preoperative chemoradiation with IMRT dose intensification for locally advanced esophageal and gastroesophageal cancer. Front Oncol. (2021) 11:626275. doi: 10.3389/fonc.2021.626275
Keywords: ESCC, cN0, PSM, OS, radiotherapy
Citation: Jin Z, Sun J, Zhang J, Shen J and Zhang B (2023) Effect of preoperative radiotherapy on the prognosis of patients with stage cTxN0M0 esophageal squamous cell carcinoma: propensity score matching analysis based on SEER database. Front. Surg. 10:1052932. doi: 10.3389/fsurg.2023.1052932
Received: 24 September 2022; Accepted: 6 March 2023;
Published: 21 March 2023.
Edited by:
Mingqiang Kang, Fujian Medical University Union Hospital, ChinaReviewed by:
Mingqiu Chen, Fujian Medical University, China© 2023 Jin, Sun, Zhang, Shen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfei Shen amlhbmZlaTA1MUAxNjMuY29t Bo Zhang emhib0BlbnplbWVkLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.