
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 21 February 2023
Sec. Orthopedic Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1047483
Background: Bone marrow stimulation (BMS) has been considered a well-established method for treating knee and ankle osteochondral lesions. Some studies have also shown that BMS can promote healing of the repaired tendon and enhance biomechanical properties during rotator cuff repair. Our purpose was to compare the clinical outcomes of arthroscopic repair rotator cuff (ARCR) with and without BMS.
Methods: A systematic review with meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). PubMed, Embase, Web of Science, Google scholar, ScienceDirect, and the Cochrane Library were searched from inception to March 20, 2022. Data on retear rates, shoulder functional outcomes, visual analog score and range of motion were pooled and analyzed. Dichotomous variables were presented as odds ratios (OR), and continuous variables were presented as mean differences (MD). Meta-analyses were conducted with Review Manager 5.3.
Results: Eight studies involving 674 patients were included, with mean follow-up period ranging from 12 to 36.8 months. Compared to ARCR alone, the intraoperative combination of the BMS resulted in lower retear rates (P < 0.0001), but showed similar results in Constant score (P = 0.10), University of California at Los Angeles (UCLA) score (P = 0.57), American Shoulder and Elbow Surgeons (ASES) score (P = 0.23), Disabilities of the Arm, Shoulder and Hand (DASH) score (P = 0.31), VAS (visual analog score) score (P = 0.34), and range of motion (ROM) (forward flexion, P = 0.42; external rotation, P = 0.21). After sensitivity analyses and subgroup analyses, no significant changes in statistical results were observed.
Conclusion: Compared to ARCR alone, the combination of intraoperative BMS can significantly reduce the retear rates, but showed similar short-term results in functional outcomes, ROM and pain. Better clinical outcomes are anticipated in the BMS group by improving structural integrity during long-term follow-up. Currently, BMS may be a viable option in ARCR based on its straightforward and cost-effective advantages.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022323379.
Rotator cuff tears are one of the most common causes of shoulder pain and impaired shoulder function (1). When conservative treatment fails, the patients are recommended for ARCR to restore the anatomy of the native rotator cuff tendon insertion. Although repair techniques have evolved from single-row repair to double-row repair to transosseous-equivalent/suture bridge repair, there are still considerable retear rates. Especially for large to massive tears, the retear rates range from 30% to 64% (2, 3). The primary factor for tendon retears is the disorganized scar tissue that formed during the healing process, which failed to restore biological structure and biomechanical strength (4).
Numerous initiatives have been launched to encourage tendon-bone mending in addition to ongoing advancements in surgical techniques. Among them, biological treatments for rotator cuff repair are attracting increasing attention (5). These biological strategies have promising avenues, but challenges remain at present. Some studies have reported that stem cells can significantly decrease retear rates (6, 7), but data on long-term impacts based on human studies are rare. Adverse events associated with stem cells cannot be ignored before clinical application, such as cell leakage, the growth of tumors and administration site reactions (8). PRP serves as a most common biologic agent for the treatment of musculoskeletal disorders. However, inconsistent efficacy claims and the unknown composition of PRP formulations have restricted further clinical use (9–11).
It has been extensively reported that the BMS technique produces satisfactory clinical results in osteochondral lesions of the knee and ankle (12–14). Proposed by Snyder in 2009 (15), BMS for rotator cuff repair is drawing increasing interest due to its safety and high cost-effectiveness. Bone marrow droplets containing mesenchymal stem cells, growth factors and other elements from the drilled hole are recruited onto the repaired tendon to promote tendon-bone healing. Nevertheless, conflicting results exist concerning the efficacy of the BMS in promoting healing (16–19).
Two reviews on this topic have been published (20, 21). However, the credibility of the conclusions is compromised by applying inappropriate inclusion criteria or recruiting overlapping patient populations. Besides, several high-quality and relevant articles have been published in recent years (19, 22, 23). This study aimed to assess whether the use of BMS in the ARCR could result in additional clinical benefits. We hypothesized that applying the BMS in the primary ARCR would lead to lower retear rates, better functional outcomes and ROM.
This study was reported according to the PRISMA guidelines (24). The protocol was registered at PROSPERO before starting this review (CRD42022304686).
We systematically searched electronic databases, including PubMed, Embase, Web of Science, Google scholar, ScienceDirect, and the Cochrane Library, on March 20, 2022, to identify potentially relevant studies. The literature search was performed using a search strategy with the combinations of the following items: [rotator cuff OR rotator cuff repair OR rotator cuff tear OR rotator injury OR rotator rupture] and [microfracture OR bone marrow stimulation OR marrow]. The gray literature and unpublished studies databases were also examined, as well as potentially eligible studies manually identified from the reference lists of included studies. There was no restriction on the publication date. Two reviewers independently performed literature searches, and any discrepancies were settled through discussion by the reviewers. On October 13, 2022, we repeated the search to update the search results, but no new qualifying publications were discovered.
Inclusion criteria for studies were as follows: (1) All comparative studies [randomized controlled trials (RCTs) or observational studies] of human patients undergoing primary ARCR; (2) The control group was treated by ARCR alone. The BMS group was treated by arthroscopic repair with BMS, including multiple channeling, microfractures, Crimson Duvet procedure, etc.; (3) Studies with a minimum 1-year follow-up; (4) At least one of the following outcomes was reported (retear rates, the Constant score, the UCLA score, the ASES score, the DASH score, the VAS score, ROM). Exclusion criteria were as follows: (1) Combined BMS and any augmentation for ARCR; (2) Applying BMS prior to arthroscopic surgery; (3) Nonclinical studies (e.g., cadaveric or animal model); (4) Studies with the smallest cohort or shortest follow-up (different studies focusing on the same group of patients); (5) Case reports, case series, comments, ongoing trials; (6) Studies published in languages other than English.
For RCTs, 2 reviewers (L.Z. and Y.Z.) independently assessed the methodological quality of the included RCTs using the Cochrane Collaboration's risk of bias tool (25). Each RCT was evaluated based on the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. The risk of bias for each item was rated as high, low, or unclear.
For non-RCTs, the same 2 reviewers independently assessed the risk of study bias and methodological quality using the methodological index for non-randomized studies (MINORS) (26). A MINORS item scored 0 if not reported, 1 if reported but not adequate and 2 if reported and adequate. Twelve items with a maximum possible score of 24 points. Comparative studies with a MINORS score of 17 or higher were considered high quality, otherwise low quality (27). Any disagreements in the quality assessment were resolved by discussion with a third reviewer (W.F.).
Two reviewers independently extracted data from eligible studies according to predefined criteria, including publication information (first author, year of publication, study design, level of evidence), patient information (sample size, age, sex), surgical procedure (method of fixation, BMS protocol), rehabilitation program and surgical outcomes (retear rates, functional outcomes, VAS score, ROM). Functional outcomes included the Constant score, the UCLA score, the ASES score, and the DASH score. If necessary, we will contact the corresponding authors of the included studies to obtain the original data.
This study was conducted according to the Cochrane Reviewer's Handbook, and statistical analyses were performed using Review Manager (RevMan for Macintosh version 5.3; The Cochrane Collaboration). For continuous outcomes, a generic inverse-variance method was used to calculated mean differences (MD) and 95% confidence intervals (CI). For dichotomous outcomes, a Mantel-Haenszel method was used to calculated odds ratios (OR) and 95% CI. Heterogeneity between studies was quantified by I2. I2 < 25%, 25%–50%, and >75% indicated low, medium and high heterogeneity, respectively. When I2 < 50%, the fixed-effects model was applied; otherwise, the random-effects model was used. P < .05 was considered to be statistically significant. Sensitivity analyses were performed by sequentially removing included studies to assess the impact of individual studies on the pooled results. Subgroup analyses according to RCT design or non-RCT design were conducted for available outcomes.
A total of 781 records were retrieved through the literature search. After removing 67 duplicate studies, we further excluded 714 based on title and abstract screening, resulting in 26 studies for full-text review. Two studies (18, 28) by Jo et al. focused on the same group of patients, and the study with shorter follow-up (18) was excluded. The study by Yoon et al. (29) was excluded because it combined BMS and patch augmentation. The study by Lapner et al. (30) was excluded because the surgeon performed the BMS technique 5 to 7 days prior to surgery, rather than during arthroscopic surgery. Ultimately, 8 articles (19, 22, 23, 28, 31–34) were included in the meta-analysis, including 4 RCTs (19, 28, 31, 32) and 4 retrospective cohort studies (RCSs) (22, 23, 33, 34). The PRISMA diagram of the article search and selection process is shown in Figure 1.
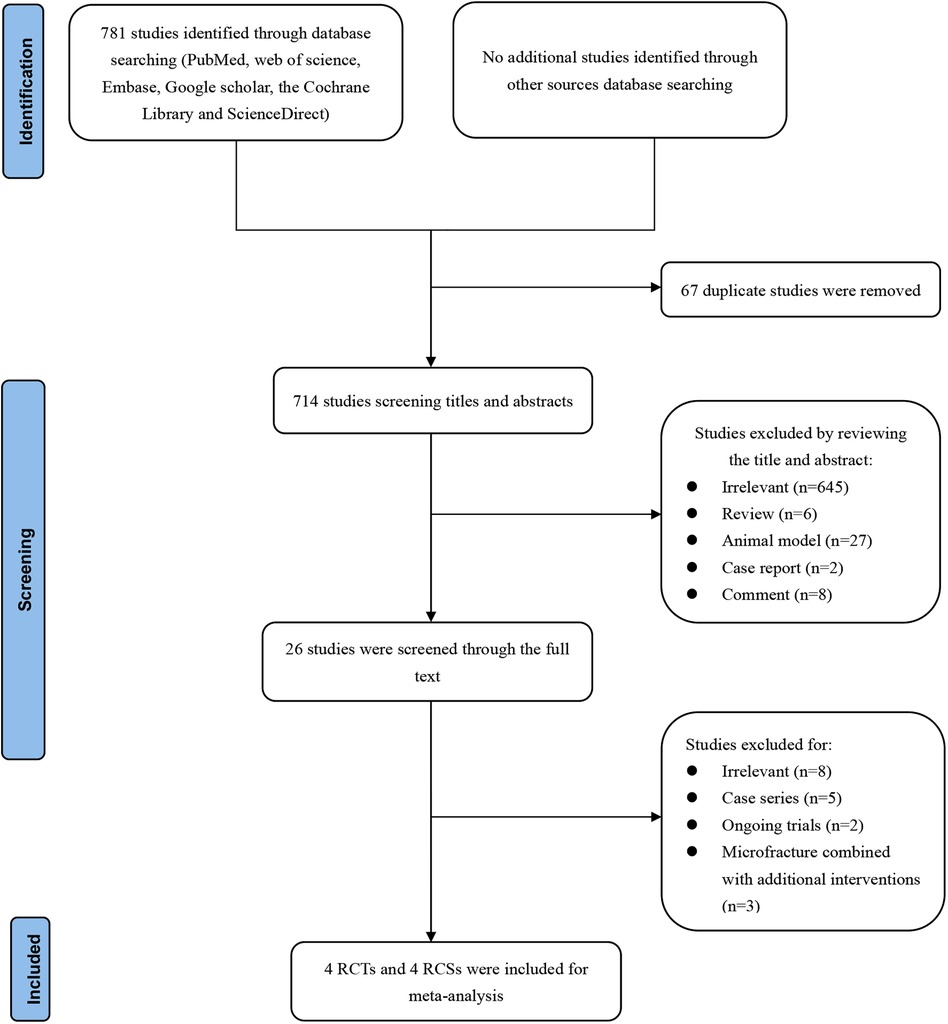
Figure 1. Preferred reporting items for systematic reviews and meta-analyses diagram on article selection for systematic review.
A total of 674 patients (354 male, 320 female) were included in this meta-analysis. Of those, 336 patients underwent ARCR combined with BMS and 338 patients received ARCR only. The mean age of patients ranged from 57.8 to 64.3 years, and the mean follow-up period ranged from 12 to 36.8 months. Six studies (22, 28, 31–34) enrolled patients with full-thickness rotator cuff tears. One study (19) included patients with large to massive rotator cuff tears. One study (23) included patients with supraspinatus tears smaller than 3 cm. As for the technique of ARCR, 4 studies (19, 31, 33, 34) used a single-row technique, 2 studies (23, 28) used double row or transosseous equivalent repair, and 1 study (32) used the surface-holding method. One study (22) selected surgical techniques based on tear size; for tears less than 1 cm, the single row technique was used, and the double row technique was preferred for tears greater than 1 cm. As for other procedures combined intraoperatively, 5 studies (22, 23, 28, 31, 33) performed biceps tenotomy or tenodesis according to the age and preoperative findings of biceps tendon integrity, except Osti et al. (34). They performed a long head of the biceps tenotomy in all instances. In addition, 4 studies (22, 23, 28, 31, 32) reported that acromioplasty was performed when necessary. We found that 1 study (31) compared three techniques, and to reduce heterogeneity, data were extracted only from the BMS group and its control group. The basic characteristics of these studies are shown in Table 1. The BMS technique and rehabilitation program are shown in Table 2.
Two authors independently assessed the quality of the included studies based on the study design. The results of the risk of bias assessment on included RCTs were summarized in Figure 2. Only 1 study (22) did not clearly report the procedure of randomization and was rated as unclear risk of bias. Two studies (22, 34) did not adequately report allocation concealment and were rated as unclear risk of bias. All RCTs reported the blinding of outcome assessments and were rated low risk of bias. The results of the risk of bias assessment included non-RCTs were summarized in Table 3. The MINORS score ranged from 17 to 19, with a total score indicating good quality.
The retear was determined by postoperative computed tomography arthrography (CTA), ultrasound (US), magnetic resonance imaging (MRI) or magnetic resonance arthrography (MRA). A retear is defined when the following conditions are matched: Sugaya type IV or V appearance, modified Boileau grading system (types of retears and new tears), French Society of Arthroscopy (stage 3 and 4) or any lack of continuity in the repaired rotator cuff at follow-up. Retear rates were reported in all included studies. The retear rates for the BMS group were 18.15% (61/336), compared to 31.07% (105/338) for the control group. The pooled results from 674 patients indicated significantly lower retear rates in patients with BMS techniques than in conventional repair (OR, 0.45; 95% CI, 0.31–0.65; P < 0.0001; I2 = 0%) (Figure 3).
A total of 6 studies with 502 patients reported the postoperative Constant score. The mean Constant score in the BMS group was 86.51, and the mean Constant score in the control group was 83.87. The pooled results showed no significant difference between the 2 groups (MD, 1.66; 95% CI, −0.29–3.61; P = 0.10; I2 = 14%) (Figure 4).
A total of 3 studies with 279 patients reported the postoperative UCLA score. The mean UCLA score in the BMS group was 30.64, and the mean UCLA score in the control group was 30.33. The pooled results showed no significant difference between the 2 groups (MD, 0.37; 95% CI, −0.90–1.65; P = 0.57; I2 = 0%) (Figure 5).
A total of 3 studies that included 317 patients reported postoperative ASES score. The mean ASES score in the BMS group was 90.68, and the mean ASES score in the control group was 89.11. The pooled results showed no significant difference between the 2 groups (MD, 1.30; 95% CI, −0.83–3.43; P = 0.23; I2 = 0%) (Figure 6).
A total of 2 studies with 197 patients reported the postoperative DASH score. The mean DASH score in the BMS group was 17.07, and the mean DASH score in the control group was 20.5. The pooled results showed no significant difference between the 2 groups (MD, −2.57; 95% CI, −7.50–2.35; P = 0.31; I2 = 0%) (Figure 7).
A total of 3 studies with 306 patients reported the postoperative VAS score. The mean VAS score in the BMS group was 1.43, and the mean VAS score in the control group was 1.76. The pooled results showed no significant difference between the 2 groups (MD, −0.30; 95% CI, −0.91–0.31; P = 0.34; I2 = 68%) (Figure 8). The heterogeneity among the studies was high, and sensitivity analyses were performed by sequentially removing the included studies. Heterogeneity was dramatically reduced after removing the studies by Pulatkan et al. (31) (MD, 0; 95% CI, −0.39–0.40; P = 1.00; I2 = 0%). Based on the investigation of study characteristics, it was speculated that the main source of heterogeneity might be the difference in the depth and diameter of the holes. The diameter and depth of the holes drilled by Pulatkan et al. were significantly lower than in the other two studies, which may account for the significantly lower VAS score of the patients after surgery than the other groups.
A total of 4 studies with 374 patients reported postoperative ROM. Based on the available data, we conducted statistical analyses of the 2 directions of the ROM: forward flexion and external rotation. Our results showed that there were no significant differences in forward flexion (MD, 1.51; 95% CI, −1.62–3.91; P = 0.42; I2= 0%) or external rotation (MD, 1.54; 95% CI, −0.86–3.94; P = 0.21; I2 = 0%) between the 2 groups (Figure 9).
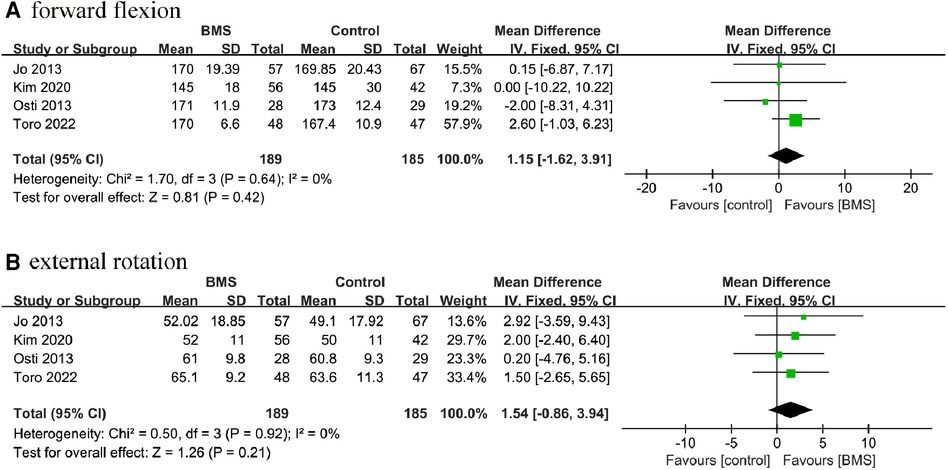
Figure 9. Forest plot comparing postoperative ROM between the BMS and control groups. (A) forward flexion; (B) external rotation.
According to sensitivity analysis, all results remained robust after sequentially excluding individual study except for the Constant score. After excluding the study by Osti et al. (34) heterogeneity decreased markedly and significant difference in Constant score between the 2 groups was observed (MD, 2.65; 95% CI, 0.40–4.91; P = 0.02; I2 = 0%). But given that the minimal clinically important difference (MCID) of the Constant score is set to at least 10 points (35, 36), the difference between the 2 groups in this score was not clinically significant.
Although a meta-analysis of well-designed non-RCTs of surgical procedures is probably as accurate as that of RCTs (27), mixing RCTs and observational studies may skew the results. Therefore, we performed subgroup analyses for available outcomes based on study design (RCT or non-RCT), including retear rates, Constant score, UCLA score, ASES score, VAS score and ROM. The statistical results were stable and supported our conclusion favorably (Table 4). Besides, we performed subgroups analyses based on follow-up time (≤24 months and >24 months), depth and diameter of holes, and repair technique. The details of the results are summarized in Table 5.
We performed a publication bias analysis for the primary outcome-retear rate. The funnel plot for studies reporting re-tear rate data was symmetric, suggesting a low risk of publication bias (Figure 10).
The critical finding of this study was that the combination of the BMS technique in the primary arthroscopic repair of the rotator cuff significantly reduced the retear rates. At a mean follow-up of 23.2 months, the VAS score, ROM, and functional outcomes—including the Constant score, UCLA score, ASES score, and DASH score—did not, however, show statistically significant differences between the 2 groups. These findings are similar to some studies on the effects of PRP in rotator cuff repair (10, 37, 38). The results are also in line with previous systematic reviews by Ajrawat et al. (21) and Li et al. (20). Nevertheless, we included more recent studies (19, 22, 23, 31) and excluded certain low-quality studies to improve the credibility of the results. Furthermore, we did not take the study (29) assessing concomitant BMS and patch augmentation into account.
Retear rate is one of the most important indicators to assess the success of rotator cuff tear repair and an influential point for patient satisfaction. Although previous studies have demonstrated that functional outcomes are unrelated to the structural integrity of rotator cuff repair, they have the limitation of a relatively short mean follow-up period, with the longest not exceeding 30.1 months (39–41). Recent studies with long-term follow-up have revealed the opposite results (42, 43). Jeong et al. (44) conducted a retrospective study of 201 patients with rotator cuff repair at a mean follow-up of 8.6 years. They demonstrated that functional outcomes in retear patients deteriorated over time but were unrecognized at the 2-year postoperative follow-up. At the final follow-up (>5 years postoperatively), the functional outcome of the retear group was significantly worse than the intact rotator cuff group (P < .001). Retears of the rotator cuff disrupt the dynamic stability of the shoulder and accelerate the progression of glenohumeral osteoarthritis, leading to worse functional outcomes. It will be a relatively long time before shoulder function deteriorates.
The mean follow-up time for our included studies was only 23.2 months, which may explain why our statistics show no significant differences in clinical outcomes between the 2 groups. Notably, most rotator cuff retears occur within 6 months of surgery, and radiographic evaluation of the repaired tendon at 6 months postoperatively is sufficient to be a reliable predictor of retear rates at long-term follow-up (45–47). Each of the four imaging modalities—MRI, MRA, CTA, and US—has been shown to be equally accurate and reliable in determining the condition of the rotator cuff (48). Therefore, it can be hypothesized that additional BMS procedures are conductive to maintaining the structural integrity of the repaired tendon and cause better clinical outcomes in long-term follow-up. But more studies with long-term follow-up are warranted to demonstrate this effectiveness.
BMS has been proven to be a well-established treatment for osteochondral lesions of the knee (49) and ankle (50). When evaluating the clinical outcomes of different knee cartilage restoration techniques, microfracture are often used as a control group to compare with other techniques, including autologous chondrocyte implantation (ACI) and osteochondral autograft transfer (OAT). Several studies (51–53) have shown that these techniques provide similar clinical benefits as microfracture. A meta-analysis by Gou et al. (51) involving 659 patients with knee cartilage lesions found no significant differences in functional outcomes of ACI compared to microfracture at 1 to 5 years of follow-up. Another meta-analysis by Mundi et al. (52) also suggested that there were no significant differences between microfracture, ACI, and OAT in improving function and pain at intermediate-term follow-up.
BMS, also described as “microfracture,” “multiple channeling,” and “Crimson Duvet,” has gained increasing attention for its utility in enhancing rotator cuff repair. The rationale for BMS is to induce multiple fractures of the greater tuberosity of the proximal humerus, which leads to the release of bone marrow mesenchymal stem cells (BMSCs), growth factors and the formation of blood clots (54, 55). Since the shoulder cartilage is not as thick as the knee joint, it is more difficult for the blood clot to stay in situ. Less weight bearing, however, may aid to achieve the optimal healing effect (56). Jo et al. (28) reported that the proximal humeral greater tuberosity contains typical characteristic BMSCs. In addition, Kida et al. (57) showed the efficacy of BMS here using bone marrow chimeric rats specifically expressing the green fluorescent protein in bone marrow-derived cells. They demonstrated that bone marrow-derived cells passed through holes drilled into the greater tuberosity, were recruited to the surface of the footprint and promoted rotator cuff healing. Experiments with rabbit models have shown that microfractures in rotator cuff repair promoted tendon healing and significantly increased tendon biomechanical properties with thicker collagen bundles (16). Microfracture in isolation is also an optional treatment for glenohumeral osteoarthritis or cartilage defects. Considering that the incidence of these 2 diseases in patients with rotator cuff tears ranges from 12.5% to as high as 28% (58), concurrent operations can be beneficial for a substantial portion of these patients.
However, there is a lack of a standard protocol for BMS application in the arthroscopic repair of rotator cuff patients. The diameter and depth of the drill hole may affect the clinical benefit. Sun et al. (59) investigated the impact of microfractures with various sizes on repair in rabbit rotator cuff tear models. They found that the control group without microfractures showed superior biomechanical properties compared to the large microfracture (1 mm) group, but inferior biomechanical properties compared to the small microfracture (0.5 mm) group. The large-diameter microfractures lead to subchondral collapse or failure of remodeling and worsen the healing process. This result is consistent with previous studies of cartilage defect treatment (60, 61). It is crucial to maintain a balance between promoting tendon healing and the risk of anchor loosening and damaging the vascular supply of the greater tuberosity. Among the 8 studies we included, the BMS group did not lead to inferior outcomes or complications. Theoretically, narrow and deep holes reflecting the physiological subchondral trabecular distance are sufficient to stimulate bone marrow release while preventing anchor failure (62). By microstructural analysis of the humeral tuberosity in patients with rotator cuff tears, Sakamoto et al. found that the average minimum distance between the trabecular separation was 0.7 mm (63). Therefore, based on similar studies on the knee, we speculate that small holes with a diameter of 0.7 mm are a better option for ARCR. However, there are no studies comparing the effect of different diameter holes on rotator cuff repair in humans. High-quality RCTs must be conducted to explore specifics of the BMS method. Furthermore, the BMSCs induced by microfractures are not completely retained on the surface of the tendon-bone and are partially lost to the surrounding tissue, which would compromise the effectiveness of the BMS. Yoon et al. (29) designed a novel repair technique that combined BMS and patch augmentation to enrich BMSCs and improve initial mechanical properties. Their results showed that this concomitant procedure significantly reduced retear and medial-row failure rates in the arthroscopic repair of massive rotator cuff tears.
Overall, BMS is a straightforward and safe technique that can promote rotator cuff healing and slow the progression of osteoarthritis. It does not require additional costs or particular instruments. Even for massive tears, it can be completed in approximately 10 min (18). Currently, BMS is a viable and effective method for promoting tendon healing as compared to alternative biological repair techniques, which are expensive or have undetermined side effects.
There were some noted limitations of this review. First, half of the 8 studies included were non-RCTs, possibly compromising the credibility due to selection bias. Although the subgroup analyses based on the study design demonstrated the robustness of the results, the findings should be interpreted with caution. Second, some baseline characteristics including fixation method, tear size, rehabilitation protocol, parameters of the BMS technique, varied across studies, and these factors could affect clinical outcomes. Thirdly, analyses of long-term clinical outcomes were not possible due to the short average follow-up period of the included trials. Fourth, some risk factors affecting postoperative outcomes, including smoking, body mass index and diabetes, were not documented in the included studies and may contribute to confounding bias.
Compared to ARCR alone, the combination of intraoperative BMS technique can significantly reduce the retear rates, but showed similar short-term results in functional outcomes, ROM and pain. Better clinical outcomes are anticipated in the BMS group by improving structural integrity during long-term follow-up. Future studies are also encouraged to investigate standard parameters of BMS, such as depth, diameter and drilling method, which may affect repair outcomes. The current results show that the smaller diameter of the hole can achieve the desired effect without negatively affecting the function. BMS may be a viable option in ARCR based on its straightforward and cost-effective advantages.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
WF and LZ conceived, designed, and planned the study. LZ and YZ conducted the database searching and data extraction. TX and LZ analyzed the data. FW was responsible to explain the results. Zhang wrote the manuscript while WF revised it. And all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. (2010) 19(1):116–20. doi: 10.1016/j.jse.2009.04.006
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am Vol. (2004) 86(2):219–24. doi: 10.2106/00004623-200402000-00002
3. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am Vol. (2005) 87(6):1229–40. doi: 10.2106/jbjs.D.02035
4. Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. (1998) 7(6):599–605. doi: 10.1016/s1058-2746(98)90007-6
5. Zhang C, Wu J, Li X, Wang Z, Lu WW, Wong TM. Current biological strategies to enhance surgical treatment for rotator cuff repair. Front Bioeng Biotechnol. (2021) 9:657584. doi: 10.3389/fbioe.2021.657584
6. Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med. (2017) 45(9):2010–8. doi: 10.1177/0363546517702863
7. Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. (2014) 38(9):1811–8. doi: 10.1007/s00264-014-2391-1
8. Fda Warns About Stem Cell Therapies: Some Patients May Be Vulnerable to Stem Cell Treatments That Are Illegal and Potentially Harmful. (2019). Available at: https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies
9. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Corrigendum. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. (2016) 44(1):Np3. doi: 10.1177/0363546515621880
10. Cai YZ, Zhang C, Lin XJ. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elbow Surg. (2015) 24(12):1852–9. doi: 10.1016/j.jse.2015.07.035
11. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. (2015) 31(2):306–20. doi: 10.1016/j.arthro.2014.09.007
12. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. (2009) 37(10):2053–63. doi: 10.1177/0363546508328414
13. Park JH, Park KH, Cho JY, Han SH, Lee JW. Bone marrow stimulation for osteochondral lesions of the talus: are clinical outcomes maintained 10 years later? Am J Sports Med. (2021) 49(5):1220–6. doi: 10.1177/0363546521992471
14. Orth P, Gao L, Madry H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg Sports Traumatol Arthrosc. (2020) 28(3):670–706. doi: 10.1007/s00167-019-05359-9
15. Snyder SJ. Rotator cuff healing and the bone marrow “Crimson Duvet” from clinical observations to science. Tech Shoulder Elbow Surg. (2009) 10(4):130–7. doi: 10.1097/BTE.0b013e3181c2a940
16. Bilsel K, Yildiz F, Kapicioglu M, Uzer G, Elmadag M, Pulatkan A, et al. Efficacy of bone marrow-stimulating technique in rotator cuff repair. J Shoulder Elbow Surg. (2017) 26(8):1360–6. doi: 10.1016/j.jse.2017.02.014
17. Lacheta L, Braun S. Limited evidence for biological treatment measures for cartilage and tendon injuries of the shoulder. Knee Surg Sports Traumatol Arthrosc. (2022) 30(4):1132–7. doi: 10.1007/s00167-021-06499-7
18. Jo CH, Yoon KS, Lee JH, Kang SB, Lee JH, Han HS, et al. The effect of multiple channeling on the structural integrity of repaired rotator cuff. Knee Surg Sports Traumatol Arthrosc. (2011) 19(12):2098–107. doi: 10.1007/s00167-011-1520-2
19. Kim C, Lee YJ, Kim SJ, Yoon TH, Chun YM. Bone marrow stimulation in arthroscopic repair for large to massive rotator cuff tears with incomplete footprint coverage. Am J Sports Med. (2020) 48(13):3322–7. doi: 10.1177/0363546520959314
20. Li Z, Zhang Y. Efficacy of bone marrow stimulation in arthroscopic repair of full thickness rotator cuff tears: a meta-analysis. J Orthop Surg Res. (2019) 14(1):36. doi: 10.1186/s13018-019-1072-6
21. Ajrawat P, Dwyer T, Almasri M, Veillette C, Romeo A, Leroux T, et al. Bone marrow stimulation decreases retear rates after primary arthroscopic rotator cuff repair: a systematic review and meta-analysis. J Shoulder Elbow Surg. (2019) 28(4):782–91. doi: 10.1016/j.jse.2018.11.049
22. Toro F, Pinochet F, Ruiz F, Moraga C, Pozo R, Oliva JP, et al. Functional and radiological results of the crimson duvet procedure in rotator cuff treatment. a randomize controlled clinical trial. J Shoulder Elbow Surg. (2022) 31(6):1200–7. doi: 10.1016/j.jse.2021.12.004
23. Ruiz Ibán MA, Sanchez Alepuz E, Diaz Heredia J, Hachem AI, Ezagüi Bentolila L, Calvo A, et al. Footprint preparation with nanofractures in a supraspinatus repair cuts in half the retear rate at 1-year follow-up. A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. (2021) 29(7):2249–56. doi: 10.1007/s00167-020-06073-7
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
27. Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. (2010) 63(3):238–45. doi: 10.1016/j.jclinepi.2009.04.005
28. Jo CH, Shin JS, Park IW, Kim H, Lee SY. Multiple channeling improves the structural integrity of rotator cuff repair. Am J Sports Med. (2013) 41(11):2650–7. doi: 10.1177/0363546513499138
29. Yoon JP, Chung SW, Kim JY, Lee BJ, Kim HS, Kim JE, et al. Outcomes of combined bone marrow stimulation and patch augmentation for massive rotator cuff tears. Am J Sports Med. (2016) 44(4):963–71. doi: 10.1177/0363546515625044
30. Lapner P, Pollock JW, Laneuville O, Uhthoff HK, Zhang T, Sheikh A, et al. Preoperative bone marrow stimulation does not improve functional outcomes in arthroscopic cuff repair: a prospective randomized controlled trial. Bone Joint Jl. (2021) 103-B(1):123–30. doi: 10.1302/0301-620X.103B1.BJJ-2020-0011.R2
31. Pulatkan A, Anwar W, Tokdemir S, Akpinar S, Bilsel K. The clinical and radiologic outcome of microfracture on arthroscopic repair for full-thickness rotator cuff tear. J Shoulder Elbow Surg. (2020) 29(2):252–7. doi: 10.1016/j.jse.2019.07.010
32. Taniguchi N, Suenaga N, Oizumi N, Miyoshi N, Yamaguchi H, Inoue K, et al. Bone marrow stimulation at the footprint of arthroscopic surface-holding repair advances cuff repair integrity. J Shoulder Elbow Surg. (2015) 24(6):860–6. doi: 10.1016/j.jse.2014.09.031
33. Milano G, Saccomanno MF, Careri S, Taccardo G, De Vitis R, Fabbriciani C. Efficacy of marrow-stimulating technique in arthroscopic rotator cuff repair: a prospective randomized study. Arthroscopy. (2013) 29(5):802–10. doi: 10.1016/j.arthro.2013.01.019
34. Osti L, Del Buono A, Maffulli N. Microfractures at the rotator cuff footprint: a randomised controlled study. Int Orthop. (2013) 37(11):2165–71. doi: 10.1007/s00264-013-1952-z
35. Louwerens JKG, van den Bekerom MPJ, van Royen BJ, Eygendaal D, van Noort A, Sierevelt IN. Quantifying the minimal and substantial clinical benefit of the constant-murley score and the disabilities of the arm, shoulder and hand score in patients with calcific tendinitis of the rotator cuff. JSES Int. (2020) 4(3):606–11. doi: 10.1016/j.jseint.2020.05.001
36. Copay AG, Eyberg B, Chung AS, Zurcher KS, Chutkan N, Spangehl MJ. Minimum clinically important difference: current trends in the orthopaedic literature, part ii: lower extremity: a systematic review. JBJS Rev. (2018) 6(9):e2. doi: 10.2106/jbjs.Rvw.17.00160
37. Zhao D, Han YH, Pan JK, Yang WY, Zeng LF, Liang GH, et al. The clinical efficacy of leukocyte-poor platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. J Shoulder Elbow Surg. (2021) 30(4):918–28. doi: 10.1016/j.jse.2020.10.014
38. Saltzman BM, Jain A, Campbell KA, Mascarenhas R, Romeo AA, Verma NN, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A Systematic Review of Meta-Analyses. Arthroscopy. (2016) 32(5):906–18. doi: 10.1016/j.arthro.2015.10.007
39. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am Vol. (2014) 96(4):265–71. doi: 10.2106/jbjs.M.00265
40. Millett PJ, Warth RJ, Dornan GJ, Lee JT, Spiegl UJ. Clinical and structural outcomes after arthroscopic single-row versus double-row rotator cuff repair: a systematic review and meta-analysis of level I randomized clinical trials. J Shoulder Elbow Surg. (2014) 23(4):586–97. doi: 10.1016/j.jse.2013.10.006
41. Holtedahl R, Bøe B, Brox JI. Better short-term outcomes after rotator cuff repair in studies with poorer mean shoulder scores and predominantly small to Medium-sized tears at baseline: a systematic review and meta-analysis. Arthroscopy. (2022) 38(3):967–79.e4. doi: 10.1016/j.arthro.2021.08.019
42. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am Vol. (2008) 90(11):2423–31. doi: 10.2106/jbjs.G.00677
43. Jost B, Zumstein M, Pfirrmann CW, Gerber C. Long-term outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am Vol. (2006) 88(3):472–9. doi: 10.2106/jbjs.E.00003
44. Jeong HJ, Nam KP, Yeo JH, Rhee SM, Oh JH. Retear after arthroscopic rotator cuff repair results in functional outcome deterioration over time. Arthroscopy. (2022) 38(8):2399–412. doi: 10.1016/j.arthro.2022.02.016
45. Kluger R, Bock P, Mittlböck M, Krampla W, Engel A. Long-term survivorship of rotator cuff repairs using ultrasound and magnetic resonance imaging analysis. Am J Sports Med. (2011) 39(10):2071–81. doi: 10.1177/0363546511406395
46. Baring TK, Cashman PP, Reilly P, Emery RJ, Amis AA. Rotator cuff repair failure in vivo: a radiostereometric measurement study. J Shoulder Elbow Surg. (2011) 20(8):1194–9. doi: 10.1016/j.jse.2011.04.010
47. Iannotti JP, Deutsch A, Green A, Rudicel S, Christensen J, Marraffino S, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am Vol. (2013) 95(11):965–71. doi: 10.2106/jbjs.L.00708
48. Roy JS, Braën C, Leblond J, Desmeules F, Dionne CE, MacDermid JC, et al. Diagnostic accuracy of ultrasonography, mri and mr arthrography in the characterisation of rotator cuff disorders: a systematic review and meta-analysis. Br J Sports Med. (2015) 49(20):1316–28. doi: 10.1136/bjsports-2014-094148
49. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. (2003) 19(5):477–84. doi: 10.1053/jars.2003.50112
50. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. (2008) 24(1):106–12. doi: 10.1016/j.arthro.2007.07.022
51. Gou GH, Tseng FJ, Wang SH, Chen PJ, Shyu JF, Weng CF, et al. Autologous chondrocyte implantation versus microfracture in the knee: a meta-analysis and systematic review. Arthroscopy. (2020) 36(1):289–303. doi: 10.1016/j.arthro.2019.06.033
52. Mundi R, Bedi A, Chow L, Crouch S, Simunovic N, Sibilsky Enselman E, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. (2016) 44(7):1888–95. doi: 10.1177/0363546515589167
53. Kraeutler MJ, Belk JW, Purcell JM, McCarty EC. Microfracture versus autologous chondrocyte implantation for articular cartilage lesions in the knee: a systematic review of 5-year outcomes. Am J Sports Med. (2018) 46(4):995–9. doi: 10.1177/0363546517701912
54. van Eekeren IC, Reilingh ML, van Dijk CN. Rehabilitation and return-to-sports activity after debridement and bone marrow stimulation of osteochondral talar defects. Sports Med (Auckland, NZ). (2012) 42(10):857–70. doi: 10.1007/bf03262299
55. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med (Auckland, NZ). (2003) 33(5):381–94. doi: 10.2165/00007256-200333050-00004
56. Lacheta L, Braun S. Limited evidence for biological treatment measures for cartilage and tendon injuries of the shoulder. Knee Surg Sports Traumatol Arthrosc. (2022) 30(4):1132–7. doi: 10.1007/s00167-021-06499-7
57. Kida Y, Morihara T, Matsuda K, Kajikawa Y, Tachiiri H, Iwata Y, et al. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elbow Surg. (2013) 22(2):197–205. doi: 10.1016/j.jse.2012.02.007
58. Gartsman GM, Taverna E. The incidence of glenohumeral joint abnormalities associated with full-thickness, reparable rotator cuff tears. Arthroscopy. (1997) 13(4):450–5. doi: 10.1016/s0749-8063(97)90123-7
59. Sun Y, Kwak JM, Kholinne E, Zhou Y, Tan J, Koh KH, et al. Small subchondral drill holes improve marrow stimulation of rotator cuff repair in a rabbit model of chronic rotator cuff tear. Am J Sports Med. (2020) 48(3):706–14. doi: 10.1177/0363546519896350
60. Chen H, Hoemann CD, Sun J, Chevrier A, McKee MD, Shive MS, et al. Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res. (2011) 29(8):1178–84. doi: 10.1002/jor.21386
61. Hayashi S, Nakasa T, Ishikawa M, Nakamae A, Miyaki S, Adachi N. Histological evaluation of early-phase changes in the osteochondral unit after microfracture in a full-thickness cartilage defect rat model. Am J Sports Med. (2018) 46(12):3032–9. doi: 10.1177/0363546518787287
62. Eldracher M, Orth P, Cucchiarini M, Pape D, Madry H. Small subchondral drill holes improve marrow stimulation of articular cartilage defects. Am J Sports Med. (2014) 42(11):2741–50. doi: 10.1177/0363546514547029
Keywords: rotator cuff retear, bone marrow stimulation, microfracture, arthroscopic rotator cuff repair, glenohumeral osteoarthritis, meta-analysis
Citation: Zhang L, Zhu Y, Xu T and Fu W (2023) Bone marrow stimulation in arthroscopic rotator cuff repair is a cost-effective and straightforward technique to reduce retear rates: A systematic review and meta-analysis. Front. Surg. 10:1047483. doi: 10.3389/fsurg.2023.1047483
Received: 18 September 2022; Accepted: 23 January 2023;
Published: 21 February 2023.
Edited by:
Jan Zabrzynski, Poznan University of Medical Sciences, PolandReviewed by:
Jakub Pękala, Jagiellonian University Medical College, Poland© 2023 Zhang, Zhu, Xu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fu Weili Zm94d2luMjAwOEAxNjMuY29t
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.