- 1Department of Joint Surgery and Sports Medicine, Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Beijing University of Chinese Medicine, Beijing, China
Background: Cutibacterium acnes (C. acnes), a common pathogen, contributes significantly to infections in shoulder surgery. Prevention of shoulder infection is crucial to improve postoperative functional recovery and reduce costs. This study aimed to perform a systematic review and meta-analysis to assess the safety and efficacy of 5% benzoyl peroxide (BPO) application in the shoulder to decrease C. acnes.
Methods: Three electronic databases were searched as follows: PubMed, Embase, and the Cochrane Library databases. Data extraction for this study was performed by two independent reviewers, and only level I and level II studies were included. The outcome data sources of individual studies were pooled. The fixed-effect model was used to determine the meta-analysis.
Results: There were five level I studies and five level II studies. The results showed that the 5% BPO group had a lower risk of C. acnes positivity [OR, 0.21 (0.15, 0.30), I2 = 24, p < 0.00001]. The pooled analysis results showed that there was no significant difference in the ability of 5% BPO and 5% BPO + clindamycin to reduce C. acnes. However, the lower rate of adverse events was significantly in favour of the non-BPO group compared with the 5% BPO group.
Conclusion: BPO can decrease C. acnes in the shoulder to prevent infection. However, the combination of BPO and clindamycin does not enhance this effect further.
Level of evidence: II, Systematic review and meta-analysis.
1. Introduction
Infection following shoulder surgery remains a devastating complication undesired by both surgeons and patients (1, 2). Common risk factors include diabetes, male sex, age under 75 years, previous shoulder arthroplasty, and rotator cuff arthropathy (3, 4). The incidence of shoulder joint infection was approximately 0.9%–1.8%, 3%–4%, and 0.01%–0.3% for primary arthroplasty, revision arthroplasty, and shoulder arthroscopy, respectively (5–10). Prosthetic infections (PJIs) are often more challenging to manage than postarthroscopic infections. Once infection events occur, poor functional outcomes, disability, more extended hospital stays, and higher costs after surgery are inevitable (11–13). The diagnostic process of infection is complex and time-consuming, involving the integration of clinical symptoms, laboratory exams, radiological studies, and microbiological swabs (4).
Currently, the organism responsible for microorganisms in PJI is Staphylococcus aureus, Staphylococcus epidermidis, coagulase-negative Staphylococci, and Cutibacterium acnes (C. acnes) (4, 14). C. acnes is the most implicated pathogen in shoulder PJI (14–17). The discovery and treatment of C. acnes are complex compared to that of other organisms easily diagnosed in PJI and treated with two-stage revisions (14). In addition, these bacteria inhabit the pilosebaceous units of the normal skin, and this part is also a challenging area for shoulder preoperative skin sterilization (18–20). Therefore, reducing the number of C. acnes organisms before shoulder joint arthroplasty is essential to preventing PJI.
Benzoyl peroxide (BPO) consists of white crystal agglomerates that are soluble in chloroform or in other organic solvents such as organic peroxides (21). BPO was first identified in the 19th century and is now recommended by dermatologists to treat C. acne (22).
Previous studies on the inclusion of BPO were limited, and the evidence level was low, which lacked convincing evidence (23, 24). As several new studies (25–28) have been published, an updated systematic review and meta-analysis should be conducted. This study aimed to assess the safety and efficiency of BPO application in the shoulder to decrease C. acnes. The primary outcome was the rate of positivity for C. acnes, and the secondary outcome was complications. We hypothesized that BPO would significantly decrease C. acnes in the shoulder.
2. Methods
2.1. Identification and selection of trials
Two independent investigators performed the literature search based on the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, and the PRISMA checklist was used (29). The third investigator resolved any discrepancies. A comprehensive search was performed in three databases, Embase, PubMed, and the Cochrane Library, until the last check on July 1st, 2022 (PROSPERO: CRD42021261880). The following search terms were used {[(Benzoyl Peroxide) OR (Adapalene)] OR (Benzoyl Peroxide Drug Combination)} AND (Shoulder). The titles, abstracts, and full texts were screened, and the reference lists of all included studies were also checked to ensure that no studies were missed.
2.2. Inclusion criteria
We included studies that met the following criteria:
P: Participants ≥18 years;
I: Any treatment that contains 5% BPO;
C: No-treatment shoulder, or shoulder receiving other treatment without 5% BPO or with placebo, or shoulder before treatment;
O: Rate of positivity for C. acnes, adverse events (including any abnormal signs and symptoms);
S: Level I and II evidence studies.
2.3. Exclusion criteria
(1) Review studies;
(2) Animal studies;
(3) Cadaver studies;
(4) Biomechanical studies;
(5) Cohort studies;
(6) Case report or case series studies;
(7) Full text unavailable;
(8) Not published in English;
(9) Conference abstracts;
(10) Clinical registration records.
2.4. Data extraction
Two blinded investigators independently extracted the characteristics of the included studies. The third investigator resolved any disagreement. The following information was recorded: first author's name, year, journal, age, gender, LOE (level of evidence), and BPO intervention. Statistical analyses of this study were performed using RevMan 5.3 software (Cochrane Collaboration) for data management.
2.5. Risk of bias
We followed the Cochrane Handbook for Systematic Reviews of Interventions, and the Cochrane risk-of-bias tool was used for all included studies (30). This tool categorized bias into six domains, and each domain was assigned a level of risk of bias (low risk, unclear risk, and high risk). The Kappa score was used to calculate the degree of agreement between reviewers (31). A score of 0–0.20 represents poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, perfect agreement.
2.6. Data synthesis and analysis
This systematic review's results were prioritized using a fixed-effects model; dichotomous data were calculated as odds ratios (OR) with a 95% confidence interval (CI). If there was more than one non-BPO control group in a study, we only extracted data from one control group and preferentially selected the placebo group for analysis. The I2 statistic was used to quantify heterogeneity: 0%–40% low heterogeneity, 40%–60% moderate heterogeneity, and >60% high heterogeneity. All these values can be examined via forest plots.
2.6.1. Subgroup analysis
To better explain the effects of BPO, we classified the different uses of BPO as follows:
1. 5% BPO vs. non-BPO Non-BPO group including a control group, the condition before use employed as the comparison group, a chlorhexidine gluconate group, and a pHisoHex group (1% triclosan; sodium benzoate, 5 mg/ml; and benzyl alcohol, 5 mg/ml)
2. 5% BPO + clindamycin vs. non-BPO
3. 5% BPO + blue Light vs. non-BPO
4. 5% BPO + chlorhexidine/alcohol vs. 2% chlorhexidine/alcohol.
2.6.2. Publication bias
To examine the possibility of publication bias, a funnel plot was used.
3. Results
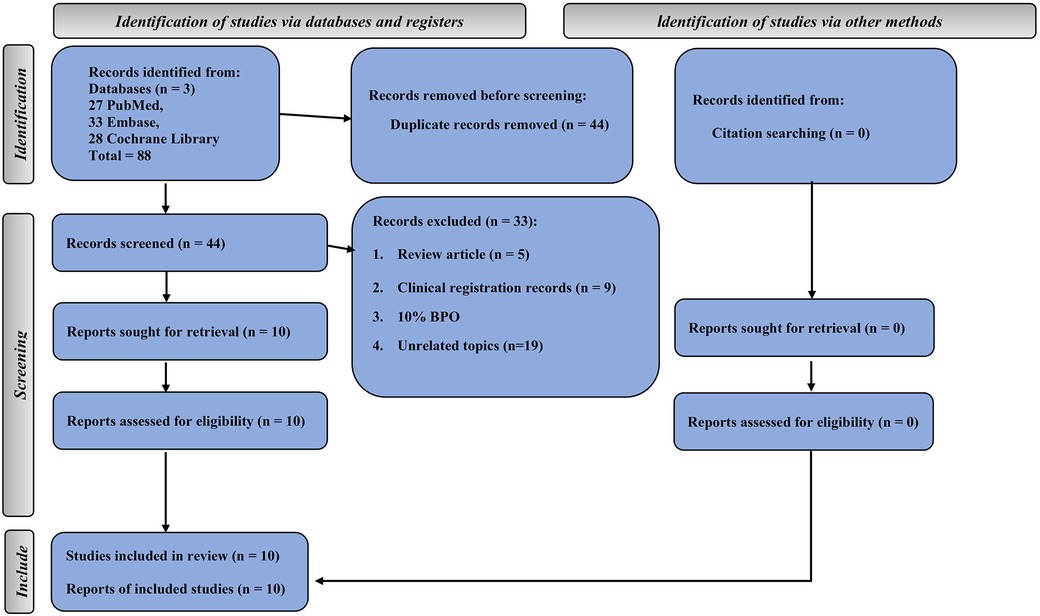
The comprehensive search yielded 88 studies from three databases (27 from PubMed, 33 from Embase, and 28 from the Cochrane Library). Half of these were duplicate studies. In addition, five studies were review articles, 9 studies were clinical registration records, 1 study used 10% BPO, and 19 studies had unrelated topics. Finally, 10 studies met our inclusion criteria (25–28, 32–37) (Figure 1).
3.1. Study characteristics
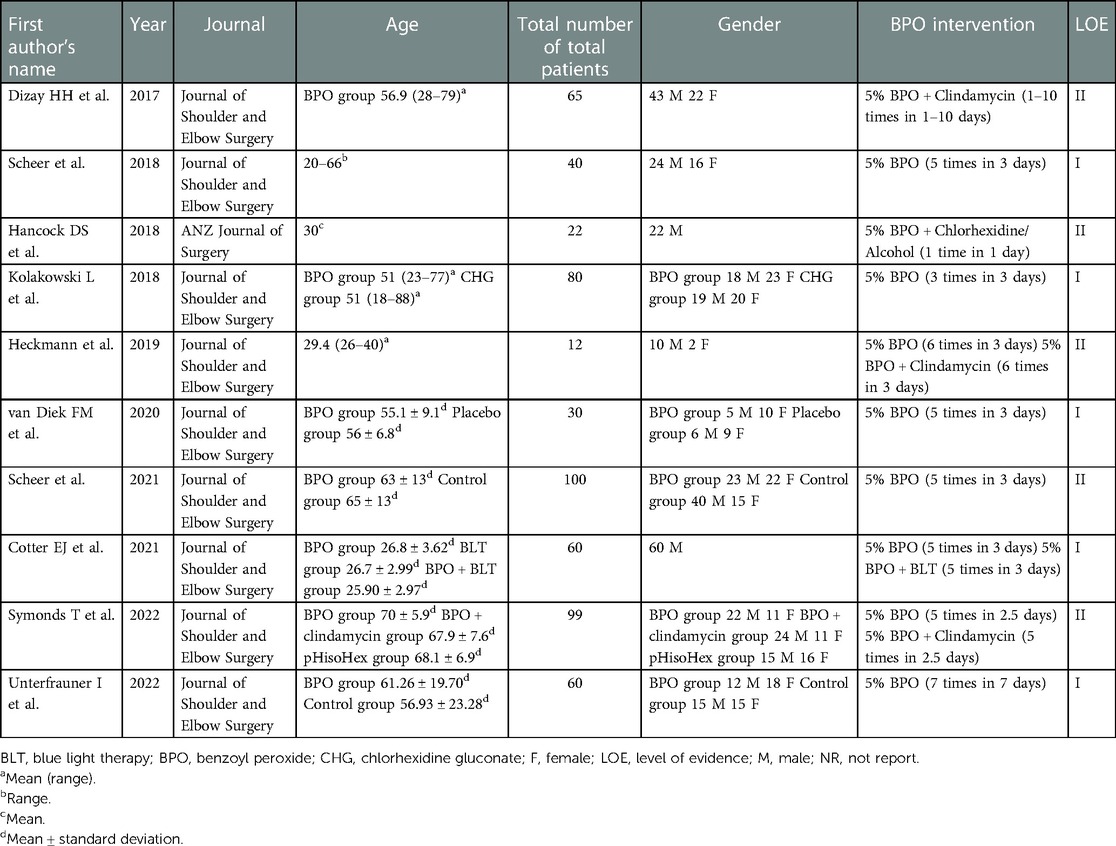
There were five level I studies (26, 27, 33, 35, 37) and five level II studies (25, 28, 32, 34, 36). A total of 9 studies were from the same journal. Two studies included only males (26, 32). Only one paper reported that BPO was used between 1 and 10 times (Table 1).
3.2. Risk of bias
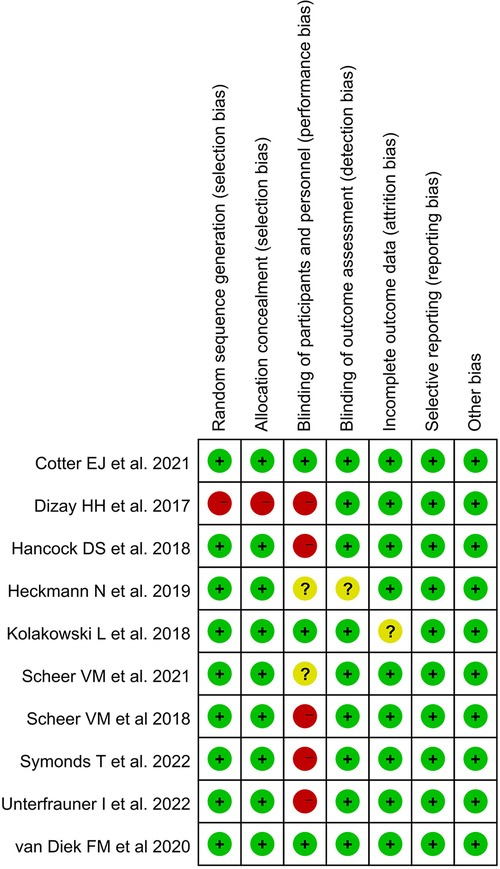
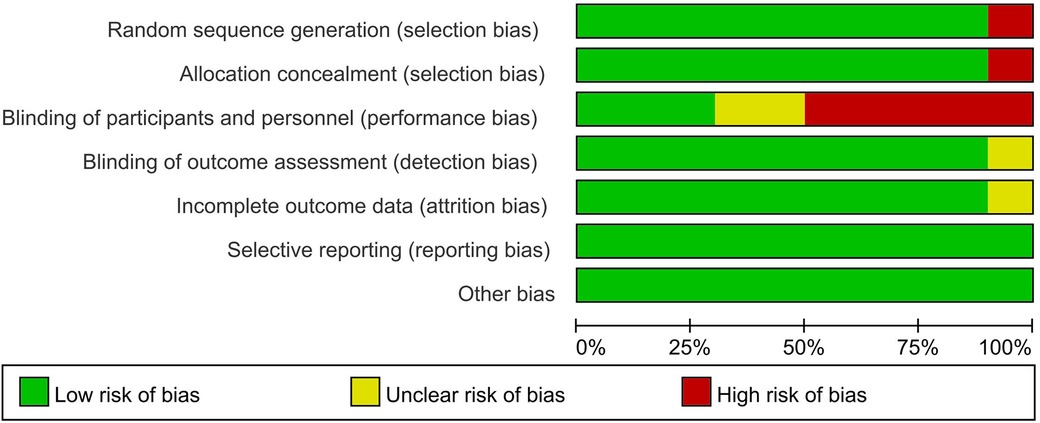
Only one study (34) was at high risk of selection bias because participants compared themselves before and after using BPO. Three studies (26, 33, 37) were at low risk of performance bias, and one study (36) was at unclear risk of detection bias. In addition, only one study had an unclear risk of attrition bias that may have obscured the analysis results (33). All studies were low risk in selective reporting and other biases. The Kappa score was 0.82 among reviewers (Figures 2, 3).
3.3. The rate of positivity for Cutibacterium acnes in different 5% BPO groups vs. non-BPO groups
A total of 9 articles were included in this analysis (25–28, 32, 34–37). In the 5% BPO vs. non-BPO subgroup, the results indicated that the rate of positivity for C. acnes was significantly lower in the 5% BPO group [OR, 0.16 (0.09, 0.27), I2 = 5, p < 0.00001].
In 5% BPO + clindamycin vs. non-BPO, the results indicated that the rate of positivity for C. acnes was significantly lower in the 5% BPO with clindamycin group [OR, 0.20 (0.11, 0.40), I2 = 0, p < 0.00001].
Collectively, the 5% BPO group had a lower risk of C. acnes positivity [OR, 0.21 (0.15, 0.30), I2 = 24, p < 0.00001]. The test for subgroup differences was 58.8% (Figure 4).
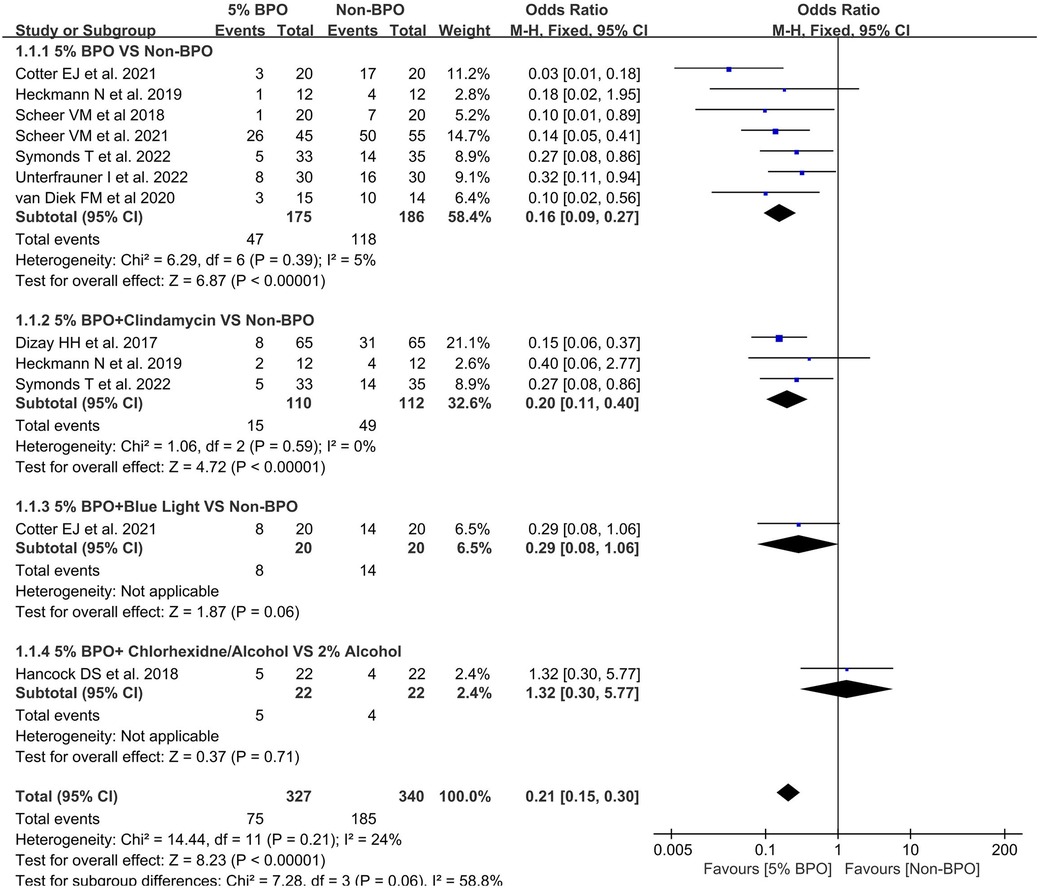
Figure 4. Forest plot showing the rate of positivity for Cutibacterium acnes in different 5% benzoyl peroxide groups vs. non- benzoyl peroxide groups.
3.4. The rate of positivity for Cutibacterium acnes with 5% BPO vs. 5% BPO with clindamycin
A total of 9 articles were included in this analysis (25, 26, 32–34, 36, 37). The pooled result found no significant difference between the BPO group and the BPO with clindamycin group [OR, 1.00 (0.32, 3.13), I2 = 0, p = 1.00] (Figure 5).
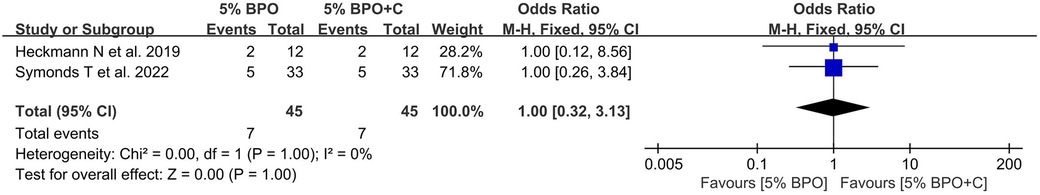
Figure 5. Forest plot showing the rate of positivity for Cutibacterium acnes with 5% benzoyl peroxide vs. 5% benzoyl peroxide with clindamycin.
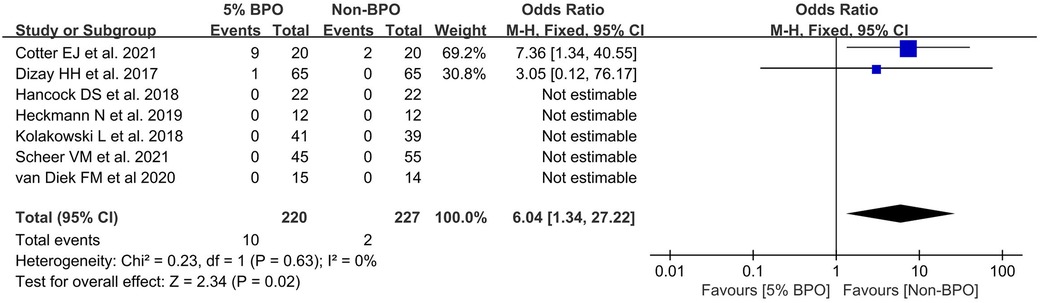
3.5. Adverse events
A total of 7 articles reported adverse events (25, 26, 32–34, 36, 37). The results indicated that the rate of lower adverse events was significantly higher in the non-5% BPO group [OR, 6.04 (1.34, 27.22), I2 = 0, p = 0.02] (Figure 6).
3.6. Publication bias
A funnel plot of the positive C. acnes rate in different 5% BPO groups vs. non-BPO was performed to ensure that this study had publication bias. This funnel plot summarized a mild degree of asymmetry, indicating a publication bias in the data (Figure 7).
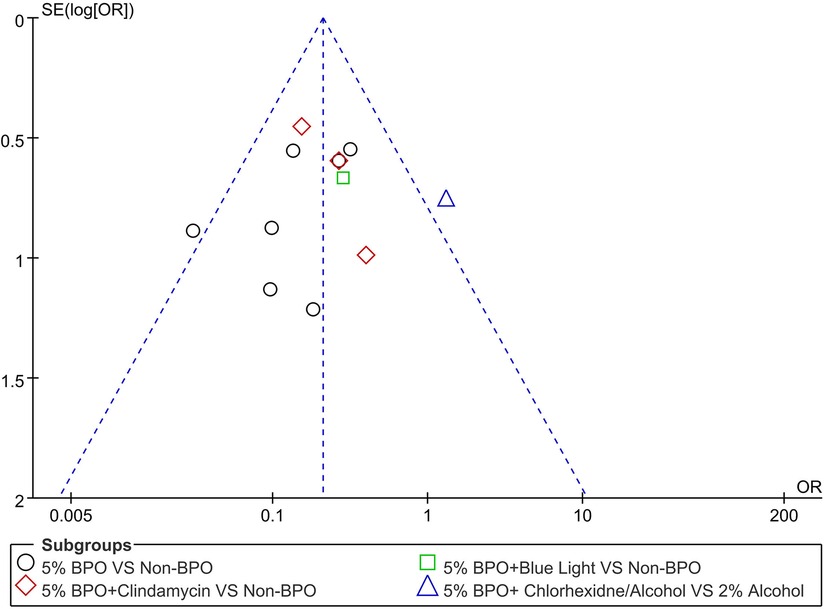
Figure 7. A funnel plot showing the positive C. acnes rate in different 5% benzoyl peroxide groups vs. non- benzoyl peroxide.
4. Discussion
In this meta-analysis, we found that BPO significantly reduced the amount of C. acnes in the skin, which is consistent with previous studies (23, 24). Theoretically, BPO and clindamycin (antibacterial) are synergistic in their activity (38–40). To better explore the method of BPO, we conducted a comparative analysis of BPO alone or combined with clindamycin, but the results showed no significant difference. This pooled result was similar to those of a double-blind clinical trial that found no significant difference in efficacy between the combination therapy of niosomal BPO 1% and clindamycin 1% compared with niosomal clindamycin in acne vulgaris (41). Therefore, the combined use of clindamycin is unnecessary based on the current results.
As a drug to inhibit the proliferation of C. acnes, BPO is often used in the surgical area before surgery. In the literature we included, the use of BPO is not uniform. However, it is worth noting that BPO is used more than 3 times in most studies. This is because a single use of BPO is not effective in preventing infection (32, 34). Conversely, the longer the application time is and the more applications there are, the stronger the effect of BPO. Dizay et al. found that when BPO was applied only once, the gel is two-thirds effective in eliminating surface colonization. When used more than once, the efficiency will reach approximately 80%. Therefore, BPO should be used several times to achieve good results.
In addition to the number of BPO applications, gender also affected the outcome. C. acnes prefers to live within pilosebaceous glands (42). In the literature we included, two of the patients were male, and in the other several pieces of literature, the proportion of males was much higher than that of females. Chuang et al. found that despite standard skin preparation and prophylactic antibiotics, men still have higher colonization rates than women (43). In the use of BPO to prevent infection, if the ratio of men to women included in the two groups is inconsistent, the authenticity of the results may be masked. At the same time, it is also worth considering whether excluding all male patients would underestimate the effect of BPO.
At present, the indications for the application of BPO are mostly concentrated in dermatology, and the indications for the use of BPO to prevent infection in the shoulder joint have not yet formed a unified understanding due to the lack of literature. However, there has been much literature on risk factors for shoulder infections, including age, sex, hair, history of surgery, cortisone injections prior to surgery, and diabetes (44–47). Therefore, BPO can be used to reduce the likelihood of shoulder infection in patients with multiple risk factors.
The adverse events of topical BPO include mild dryness, concentration-dependent irritant dermatitis with erythema, angioedema, scaling and itching (48–50). In this study, we also found a similar situation. Direct contact with the epidermal active ingredient is the basis for BPO side effects (50). Once symptoms are detected, benzoyl peroxide use should be discontinued (51).
BPO is an over-the-counter, FDA-approved prescription medication that has long been used in dermatology (39). It breaks down into benzoic acid and hydrogen peroxide in the pilosebaceous duct, releasing free radicals against C. acnes (52). Compared with other antibiotics for surgical infection prevention, there was no resistance to benzoyl peroxide (53). BPO is usually formulated at concentrations of 2.5%, 5%, and 10%. However, the optimal use of BPO for shoulder prophylaxis has not been determined. In a network meta-analysis study, the combinations of BPO with adapalene or clindamycin (54% vs. 35% or 49% vs. 35%) were a more effective treatment for acne than BPO alone (54). Whether there is a similar efficiency in preventing shoulder infection remains to be further verified.
In addition, when using BPO to prevent shoulder infection, it is also necessary to pay attention to patients’ skin allergies to BPO. A patient who had undergone total knee arthroplasty developed a systemic reaction and intractable pain. A patient undergoing total knee replacement developed systemic reactions and intractable pain due to hypersensitivity to BPO (55). Bircher A et al. reported five patients who had relevant sensitization to BPO with complications from a knee or a shoulder joint implant (56). Typical symptoms include pain, swelling, and inflammation of the skin.
From the payer's perspective, BPO is a cost-effective strategy for preventing infection. A randomized controlled study reported that the application of BPO three times cost less than $10 per patient (33). In contrast, the minimum institutional cost of treating arthroscopic rotator cuff repair infection was $24,991.31 (57). The average total cost of a shoulder prosthesis infection was $46,745 (58). In addition, a recent study estimated that joint infections around hip and knee implants will cost $1.85 billion a year in hospital costs by 2030 due to increased surgery volumes (59). Two break-even analysis studies suggested that using BPO for infection prevention in shoulder surgery is a highly economically justified practice (57, 58).
5. Limitations
This study has several limitations. Most importantly, the nine studies obtained from the same journal may have caused bias in the results. Second, some studies involved participants without shoulder surgery, which could have influenced the results. Third, more studies are needed to analyse the effect of BPO on the specific colony number of C. acnes. At the same time, more literature is needed to compare the effect of BPO at different concentrations (2.5%, 5%, 10%) on the prevention of surgical infection of the shoulder joint.
6. Conclusion
BPO can decrease C. acnes in the shoulder to prevent infection. However, the combination of BPO and clindamycin does not enhance this effect further.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
DF: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing; JiM, XL, SZ: conceptualization, data curation, investigation, validation; JS, BJ, YL, LZ: data curation, investigation, validation. All authors contributed to the article and approved the submitted version..
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Marcheggiani Muccioli GM, Huri G, Grassi A, Roberti di Sarsina T, Carbone G, Guerra E, et al. Surgical treatment of infected shoulder arthroplasty. A systematic review. Int Orthop. (2017) 41(4):823–30. doi: 10.1007/s00264-017-3399-0
2. Vopat BG, Lee BJ, DeStefano S, Waryasz GR, Kane PM, Gallacher SE, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. (2016) 32(3):428–34. doi: 10.1016/j.arthro.2015.08.021
3. Kunutsor SK, Barrett MC, Whitehouse MR, Craig RS, Lenguerrand E, Beswick AD, et al. Incidence, temporal trends and potential risk factors for prosthetic joint infection after primary total shoulder and elbow replacement: systematic review and meta-analysis. J Infect. (2020) 80(4):426–36. doi: 10.1016/j.jinf.2020.01.008
4. Franceschini V, Chillemi C. Periprosthetic shoulder infection. Open Orthop J. (2013) 7:243–9. doi: 10.2174/1874325001307010243
5. Moor BK, Léger B, Steffen V, Troillet N, Emonet S, Gallusser N. Subcutaneous tissue disinfection significantly reduces Cutibacterium acnes burden in primary open shoulder surgery. J Shoulder Elbow Surg. (2021) 30(7):1537–43. doi: 10.1016/j.jse.2020.11.018
6. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. (2006) 88(10):2279–92. doi: 10.2106/JBJS.F.00125
7. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. (2012) 21(11):1534–41. doi: 10.1016/j.jse.2012.01.006
8. Zavala JA, Clark JC, Kissenberth MJ, Tolan SJ, Hawkins RJ. Management of deep infection after reverse total shoulder arthroplasty: a case series. J Shoulder Elbow Surg. (2012) 21(10):1310–5. doi: 10.1016/j.jse.2011.08.047
9. Herrera MF, Bauer G, Reynolds F, Wilk RM, Bigliani LU, Levine WN. Infection after mini-open rotator cuff repair. J Shoulder Elbow Surg. (2002) 11(6):605–8. doi: 10.1067/mse.2002.127302
10. Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. (2014) 42(2):437–41. doi: 10.1177/0363546513510686
11. Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. (2002) 23(4):183–9. doi: 10.1086/502033
12. Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. (2014) 35(6):605–27. doi: 10.1086/676022
13. Patel VV, Ernst SMC, Rangarajan R, Blout CK, Lee BK, Itamura JM. Validation of new shoulder periprosthetic joint infection criteria. J Shoulder Elbow Surg. (2021) 30(7S):S71–6. doi: 10.1016/j.jse.2021.04.009
14. Paxton ES, Green A, Krueger VS. Periprosthetic infections of the shoulder: diagnosis and management. J Am Acad Orthop Surg. (2019) 27(21):e935–44. doi: 10.5435/JAAOS-D-18-00232
15. Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg. (2009) 18(6):897–902. doi: 10.1016/j.jse.2009.01.023
16. Pottinger P, Butler-Wu S, Neradilek MB, Merritt A, Bertelsen A, Jette JL, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. (2012) 94(22):2075–83. doi: 10.2106/JBJS.K.00861
17. Richards J, Inacio MC, Beckett M, Navarro RA, Singh A, Dillon MT, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. (2014) 472(9):2809–15. doi: 10.1007/s11999-014-3696-5
18. Falconer TM, Baba M, Kruse LM, Dorrestijn O, Donaldson MJ, Smith MM, et al. Contamination of the surgical field with Propionibacterium acnes in primary shoulder arthroplasty. J Bone Joint Surg Am. (2016) 98(20):1722–8. doi: 10.2106/JBJS.15.01133
19. MacNiven I, Hsu JE, Neradilek MB, Matsen FA 3rd. Preoperative skin-surface cultures can help to predict the presence of Propionibacterium in shoulder arthroplasty wounds. JBJS Open Access. (2018) 3(1):e0052. doi: 10.2106/JBJS.OA.17.00052
20. Rao AJ, Chalmers PN, Cvetanovich GL, O'Brien MC, Newgren JM, Cole BJ, et al. Preoperative doxycycline does not reduce Propionibacterium acnes in shoulder arthroplasty. J Bone Joint Surg Am. (2018) 100(11):958–64. doi: 10.2106/JBJS.17.00584
22. Merker PC. Benzoyl peroxide: a history of early research and researchers. Int J Dermatol. (2002) 41(3):185–8. doi: 10.1046/j.1365-4362.2002.01371.x
23. Meyer LE, Lazarides AL, Hendren S, Lassiter T, Klifto C, Anakwenze O. The efficacy of peroxide solutions in decreasing Cutibacterium acnes burden around the shoulder. J Am Acad Orthop Surg. (2022) 30(1):e91–8. doi: 10.5435/JAAOS-D-21-00457
24. Nhan DT, Woodhead BM, Gilotra MN, Matsen FA 3rd, Hsu JE. Efficacy of home prophylactic benzoyl peroxide and chlorhexidine in shoulder surgery: a systematic review and meta-analysis. JBJS Rev. (2020) 8(8):e2000023. doi: 10.2106/JBJS.RVW.20.00023
25. Scheer VM, Jungeström MB, Serrander L, Kalén A, Scheer JH. Benzoyl peroxide treatment decreases Cutibacterium acnes in shoulder surgery, from skin incision until wound closure. J Shoulder Elbow Surg. (2021) 30(6):1316–23. doi: 10.1016/j.jse.2020.12.019
26. Cotter EJ, Cotter LM, Franczek EB, Godfrey JJ, Hetzel SJ, Safdar N, et al. Efficacy of combinational therapy using blue light and benzoyl peroxide in reducing Cutibacterium acnes bioburden at the deltopectoral interval: a randomized controlled trial. J Shoulder Elbow Surg. (2021) 30(12):2671–81. doi: 10.1016/j.jse.2021.08.008
27. Unterfrauner I, Wieser K, Catanzaro S, Uçkay I, Bouaicha S. Acne cream reduces the deep Cutibacterium acnes tissue load before elective open shoulder surgery: a randomized controlled pilot trial. J Shoulder Elbow Surg. (2022) 31(5):897–905. doi: 10.1016/j.jse.2022.01.115
28. Symonds T, Grant A, Doma K, Hinton D, Wilkinson M, Morse L. The efficacy of topical preparations in reducing the incidence of Cutibacterium acnes at the start and conclusion of total shoulder arthroplasty: a randomized controlled trial. J Shoulder Elbow Surg. (2022) 31(6):1115–21. doi: 10.1016/j.jse.2022.01.133
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
30. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
31. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. (2012) 22(3):276–82. doi: 10.11613/BM.2012.031
32. Hancock DS, Rupasinghe SL, Elkinson I, Bloomfield MG, Larsen PD. Benzoyl peroxide+chlorhexidine versus chlorhexidine alone skin preparation to reduce Propionibacterium acnes: a randomized controlled trial. ANZ J Surg. (2018) 88(11):1182–6. doi: 10.1111/ans.14848
33. Kolakowski L, Lai JK, Duvall GT, Jauregui JJ, Dubina AG, Jones DL, et al. Neer award 2018: benzoyl peroxide effectively decreases preoperative Cutibacterium acnes shoulder burden: a prospective randomized controlled trial. J Shoulder Elbow Surg. (2018) 27(9):1539–44. doi: 10.1016/j.jse.2018.06.012
34. Dizay HH, Lau DG, Nottage WM. Benzoyl peroxide and clindamycin topical skin preparation decreases Propionibacterium acnes colonization in shoulder arthroscopy. J Shoulder Elbow Surg. (2017) 26(7):1190–5. doi: 10.1016/j.jse.2017.03.003
35. Scheer VM, Bergman Jungeström M, Lerm M, Serrander L, Kalén A. Topical benzoyl peroxide application on the shoulder reduces Propionibacterium acnes: a randomized study. J Shoulder Elbow Surg. (2018) 27(6):957–61. doi: 10.1016/j.jse.2018.02.038
36. Heckmann N, Heidari KS, Jalali O, Weber AE, She R, Omid R, et al. Cutibacterium acnes persists despite topical clindamycin and benzoyl peroxide. J Shoulder Elbow Surg. (2019) 28(12):2279–83. doi: 10.1016/j.jse.2019.06.016
37. van Diek FM, Pruijn N, Spijkers KM, Mulder B, Kosse NM, Dorrestijn O. The presence of Cutibacterium acnes on the skin of the shoulder after the use of benzoyl peroxide: a placebo-controlled, double-blinded, randomized trial. J Shoulder Elbow Surg. (2020) 29(4):768–74. doi: 10.1016/j.jse.2019.11.027
38. Leyden JJ, Wortzman M, Baldwin EK. Antibiotic-resistant Propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis. (2008) 82(6):417–21.19181031
39. Foster AL, Cutbush K, Ezure Y, Schuetz MA, Crawford R, Paterson DL. Cutibacterium acnes in shoulder surgery: a scoping review of strategies for prevention, diagnosis, and treatment. J Shoulder Elbow Surg. (2021) 30(6):1410–22. doi: 10.1016/j.jse.2020.11.011
40. Yang Z, Zhang Y, Lazic Mosler E, Hu J, Li H, Zhang Y, et al. Topical benzoyl peroxide for acne. Cochrane Database Syst Rev. (2020) 3(3):CD011154. doi: 10.1002/14651858.CD011154.pub2
41. Mohammadi S, Pardakhty A, Khalili M, Fathi R, Rezaeizadeh M, Farajzadeh S, et al. Niosomal benzoyl peroxide and clindamycin lotion versus niosomal clindamycin lotion in treatment of acne vulgaris: a randomized clinical trial. Adv Pharm Bull. (2019) 9(4):578–83. doi: 10.15171/apb.2019.066
42. Lange-Asschenfeldt B, Marenbach D, Lang C, Patzelt A, Ulrich M, Maltusch A, et al. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol Physiol. (2011) 24(6):305–11. doi: 10.1159/000328728
43. Chuang MJ, Jancosko JJ, Mendoza V, Nottage WM. The incidence of Propionibacterium acnes in shoulder arthroscopy. Arthroscopy. (2015) 31(9):1702–7. doi: 10.1016/j.arthro.2015.01.029
44. Jensen ML, Jensen SL, Bolder M, Hanisch KWJ, Sørensen AKB, Olsen BS, et al. Previous rotator cuff repair increases the risk of revision surgery for periprosthetic joint infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg. (2023) 32(1):111–20. doi: 10.1016/j.jse.2022.07.001
45. Morris BJ, O'Connor DP, Torres D, Elkousy HA, Gartsman GM, Edwards TB. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg. (2015) 24(2):161–6. doi: 10.1016/j.jse.2014.05.020
46. Eck CF, Neumann JA, Limpisvasti O, Adams CR. Lack of level I evidence on How to prevent infection after elective shoulder surgery. Knee Surg Sports Traumatol Arthrosc. (2018) 26(8):2465–80. doi: 10.1007/s00167-018-4832-7
47. Seok HG, Park JJ, Park SG. Risk factors for periprosthetic joint infection after shoulder arthroplasty: systematic review and meta-analysis. J Clin Med. (2022) 11(14):4245. doi: 10.3390/jcm11144245
48. Foti C, Romita P, Borghi A, Angelini G, Bonamonte D, Corazza M. Contact dermatitis to topical acne drugs: a review of the literature. Dermatol Ther. (2015) 28(5):323–9. doi: 10.1111/dth.12282
49. Kawashima M, Sato S, Furukawa F, Matsunaga K, Akamatsu H, Igarashi A, et al. Twelve-week, multicenter, placebo-controlled, randomized, double-blind, parallel-group, comparative phase II/III study of benzoyl peroxide gel in patients with acne vulgaris: a secondary publication. J Dermatol. (2017) 44(7):774–82. doi: 10.1111/1346-8138.13798
50. Brammann C, Müller-Goymann CC. An update on formulation strategies of benzoyl peroxide in efficient acne therapy with special focus on minimizing undesired effects. Int J Pharm. (2020) 578:119074. doi: 10.1016/j.ijpharm.2020.119074
51. Matin T, Goodman MB. Benzoyl peroxide. In: Statpearls. Treasure island (FL): StatPearls Publishing (2020).
52. Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. (2009) 10(15):2555–62. doi: 10.1517/14656560903277228
53. Kircik LH. The role of benzoyl peroxide in the new treatment paradigm for acne. J Drugs Dermatol. (2013) 12(6):s73–6.23839205
54. Stuart B, Maund E, Wilcox C, Sridharan K, Sivaramakrishnan G, Regas C, et al. Topical preparations for the treatment of mild-to-moderate acne vulgaris: systematic review and network meta-analysis. Br J Dermatol. (2021) 185(3):512–25. doi: 10.1111/bjd.20080
55. Edwards SA, Gardiner J. Hypersensitivity to benzoyl peroxide in a cemented total knee arthroplasty: cement allergy. J Arthroplasty. (2007) 22(8):1226–8. doi: 10.1016/j.arth.2006.10.019
56. Bircher A, Friederich NF, Seelig W, Scherer K. Allergic complications from orthopaedic joint implants: the role of delayed hypersensitivity to benzoyl peroxide in bone cement. Contact Dermatitis. (2012) 66(1):20–6. doi: 10.1111/j.1600-0536.2011.01996.x
57. Lane PW, Griswold BG, Paré DW, Bushnell BD, Parada SA. Benzoyl peroxide is cost-effective for preventing infection by Cutibacterium acnes in arthroscopic rotator cuff repair. Arthrosc Sports Med Rehabil. (2021) 3(4):e1119–23. doi: 10.1016/j.asmr.2021.03.021
58. Hatch MD, Daniels SD, Glerum KM, Higgins LD. The cost effectiveness of vancomycin for preventing infections after shoulder arthroplasty: a break-even analysis. J Shoulder Elbow Surg. (2017) 26(3):472–7. doi: 10.1016/j.jse.2016.07.071
Keywords: P. acnes, C. acnes, benzoyl peroxide, clindamycin, shoulder, infection, surgery, chlorhexidine
Citation: Fan D, Ma J, Liu X, Zhang S, Sun J, Li Y, Jiang B and Zhang L (2023) The safety and efficiency of benzoyl peroxide for reducing Cutibacterium acnes in the shoulder: An updated systematic review and meta-analysis. Front. Surg. 10:1015490. doi: 10.3389/fsurg.2023.1015490
Received: 9 August 2022; Accepted: 20 February 2023;
Published: 10 March 2023.
Edited by:
John Jairo Aguilera-Correa, Health Research Institute Foundation Jimenez Diaz (IIS-FJD), SpainReviewed by:
Yongqiong Deng, The Affiliated Hospital of Southwest Medical University, ChinaVendela Scheer, Linköping University Hospital, Sweden
© 2023 Fan, Ma, Liu, Zhang, Sun, Li, Jiang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang YXJ0aHJvYXJ0aXN0QDE2My5jb20=
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 DingYuan Fan
DingYuan Fan Jia Ma1
Jia Ma1