- 1Department of Orthopedics, Xuanwu Hospital Capital Medical University, Beijing, China
- 2National Clinical Research Center for Geriatric Diseases, Beijing, China
- 3Department of Pathology, West China Hospital, Sichuan University, Sichuan, China
Background: Enhanced recovery after surgery (ERAS) is an evidence-based multimodal perioperative management designed to reduce the length of stay (LOS) and complications. The purpose of the present study is to evaluate the recovery of physiological function, LOS, complications, pain score, and clinical efficacy in frail elderly patients undergoing multisegment fusion surgery after the implementation of the ERAS protocol.
Methods: Frail patients older than 75 years undergoing multilevel lumbar fusion surgery for degenerative discogenic conditions, lumbar spinal stenosis, and lumbar spondylolisthesis from January 2017 to December 2018 (non-ERAS frail group) and from January 2020 to December 2021 (ERAS frail group) were enrolled in the present study. Propensity score matching for age, sex, body mass index, and smoking status was performed to keep comparable characteristics between the two groups. Further recovery of physiological function, LOS, complications, pain score, and clinical efficacy were compared between the groups.
Results: There were 64 pairs of well-balanced patients, and the clinical baseline data were comparable between the two groups. There was significant improvement in terms of recovery of physiological function (10.65 ± 3.51 days vs. 8.31 ± 3.98 days, p = 0.011) and LOS (12.18 ± 4.69 days vs. 10.44 ± 4.60 days, p = 0.035), while no statistical discrepancy was observed with regard to complications between the groups, which indicated favorable outcomes after the implementation of the ERAS protocol. Further analysis indicated that more patients were meeting a minimally clinical important difference for the visual analog score for the legs and the Oswestry Disability Index in the ERAS frail group. With regard to postoperative pain, the score was higher in the ERAS frail group than in the non-ERAS frail group on postoperative day (POD) 1 (4.88 ± 1.90 in the ERAS frail group vs. 4.27 ± 1.42 in the non-ERAS frail group, p = 0.042), while there was no significant discrepancy on POD 2 (3.77 ± 0.88 in the ERAS frail group vs. 3.64 ± 1.07 in the non-ERAS frail group, p = 0.470) and POD 3 (3.83 ± 1.89 in the ERAS frail group vs. 3.47 ± 1.75 in the non-ERAS frail group, p = 0.266).
Conclusions: In this retrospective cohort study, we found a significant improvement in terms of LOS, recovery of physiological function, and clinical efficacy after the implementation of the ERAS protocol in elderly and frail patients undergoing multilevel lumbar fusion surgery, while there was no significant discrepancy with regard to complications, 90-day readmission, and postoperative pain.
Introduction
Enhanced recovery after surgery (ERAS) is an evidence-based, multidisciplinary perioperative approach adopted to decrease postoperative adverse events by mitigating stress response in patients following surgical intervention (1–4). First introduced for colon surgery, the ERAS protocol has been implemented successfully in various surgical specialties. Substantial attention has been paid to spine surgery, and several studies have found that patients undergoing lumbar fusion surgery (short-segment or multilevel) can benefit from the implementation of the ERAS protocol (5–10). Studies have demonstrated that ERAS for lumbar fusion could reduce hospitalization costs, postoperative pain, and complications, while facilitating the recovery of physiological function without adversely affecting readmission rates; this is the case irrespective of whether the protocol is implemented preoperatively, intraoperatively, or postoperatively (11, 12).
There is now an increased focus on desirable perioperative outcomes in vulnerable patients due to the increasing incidence of age-related disorders. Frailty is clinically defined as a syndrome characterized by decreased physiological reserve that can predispose patients undergoing surgery to suboptimal outcomes (13, 14). Moreover, previous studies have shown that frail patients are susceptible to an increased risk of complications, a longer length of stay (LOS), and more hospitalization expenditures arising from lumbar surgery (15, 16). Accurate risk stratification and predicting postoperative complications in time are imperative in older patients undergoing lumbar fusion surgery. The Fried frailty phenotype was described by Fried and colleagues (17), which is comprised of five variables, namely unintentional weight loss, self-reported exhaustion, low physical activity, slowness, and weakness. The score assigns one point for any of these factors and is calculated by adding each variable.
Previous studies have demonstrated the clinical efficacy of the ERAS protocol in lumbar fusion surgery; however, there has been a lack of sufficient data pool for evaluating ERAS in frail patients following lumbar fusion surgery, especially multisegment lumbar fusion surgery (8). Furthermore, with increasing age, elderly patients often suffer from comorbidities, making the vulnerable among them more prone to an increased risk of suboptimal outcomes (18). Against this background, this study aims to evaluate the return of physiological function, LOS, complication rates, pain scores, and clinical efficacy in frail elderly patients undergoing multisegment fusion surgery after the implementation of the ERAS protocol.
Methods
Population
This was a retrospective cohort study. This study was approved by the institutional review board in Xuanwu Hospital Capital Medical University (No. 2018086). Informed consent was waived due to the nature of the study design. Consecutive patients who underwent multilevel lumbar fusion surgery, defined as fusion segments greater than or equal to 3 before and after the implementing the ERAS protocol, were reviewed in this study. Inclusion criteria were (1) age >75 years; (2) undergoing multilevel lumbar fusion surgery for degenerative discogenic conditions, lumbar spinal stenosis, and lumbar spondylolisthesis; and (3) completed preoperative data. A multidisciplinary appraisal team was established in 2019 at our institution with the aim of minimizing selection bias, and the Fried phenotype score was evaluated by specially trained nurses. A patient was defined as frail if the score was >2. Exclusion criteria were (1) history of spinal surgery; (2) concomitant cervical surgery or thoracic spine surgery; and (3) lack of clinical data. The ERAS protocol was implemented in July 2019 to increase the reliability and comparability of the data, patients reviewed from January 2017 to December 2018 were classified as non-ERAS frail group, and those reviewed from January 2020 to December 2021 were classified as ERAS frail group. Propensity score matching for age, sex, body mass index (BMI), and smoking status was performed to maintain comparable clinical characteristics.
Enhanced recovery after surgery interventions
The ERAS protocol for multilevel lumbar fusion surgery was fully implemented in our department in July 2019 and a multidisciplinary assessment team was established. The ERAS program is a patient-specific perioperative management approach, and a tailor-made management regimen is adopted for patients by following ERAS principles. Our ERAS protocol consisted of preoperative, intraoperative, and postoperative interventions. The perioperative measures were (1) perioperative education and counseling: informing the patients about the risk of surgery and describing the ERAS pathway to ensure their understanding; (2) nutritional assessment: patients with malnutrition were provided with personalized and diet guidance and nutritional supplements from an expert nutritionist before surgery; (3) cessation of smoking and alcohol: 2 weeks before surgery; (4) no prolonged fasting: eating was permitted up to 6 h prior to surgery and carbohydrate-containing drinks were allowed up to 2 h before surgery; (5) multimodal analgesia: various analgesics were used according to pain stratifications; (6) antibiotic prophylaxis: within 1 h of the incision. Intraoperative interventions were (1) tranexamic acid: within half an hour of incision; (2) maintenance of normothermia: keeping temperature at 36–37°C; (3) local infiltration analgesia: 10 ml ropivacaine and 10 ml lidocaine; (4) standard anesthetic protocol: total intravenous anesthesia-based anesthetic technique with propofol, lidocaine, ketamine, ketorolac, antiemetics, and with up to 0.5% minimum alveolar concentration–inhaled anesthetics. Postoperative interventions were (1) early oral feeding: oral feeding after recovery from anesthesia; (2) early ambulation: patients with multilevel lumbar fusion surgery were suggested to ambulate out-of-bed with or without assistance within 48 h after surgery; (3) early removal of the bladder catheter: consider removing the catheter after 24 h; (4) multimodal analgesia: similar to the preoperative multimodal analgesia regimen with a patient-controlled analgesia pump.
Collected variables
Patient-specific and procedure-specific variables were reviewed from the medical records. The patient-specific perioperative variables included age, sex, BMI, smoking status, visual analog score (VAS) for the back and legs, Oswestry Disability Index (ODI) score before and after surgery, American Society of Anesthesiologists (ASA) classification, and Charlson comorbidity index (CCI) (19). The procedure-specific variables included operation time, intraoperative blood loss, intraoperative blood transfusion, LOS, fusion segments, 90-day readmission, and postoperative complications (i.e., deep vein thrombosis, pneumonia, surgical site infection, bacteremia, uroschesis, urinary tract infection, myocardial ischemia, neurological deficit, hematoma, delirium, spinal fluid leakage, and nausea and vomiting). We recorded the time to first ambulation, time to first bowel movement, and time to void, and the return of physiological function was defined as the sum of these parameters. Clinical efficacy was compared between the two groups according to the minimal clinically important difference (MCID) with a cutoff of 12.8 points for the ODI, 1.2 points for back pain, and 1.6 points for leg pain (20).
Surgical technique
A standard midline approach was performed to expose the posterior elements. The nerve roots were decompressed by hemilaminectomy or laminectomy according to the preoperative lumbar symptoms, radicular symptoms, and MRI. Spinal instrumentation was performed using a pedicle screw-rod construct, followed by a decompression of responsible segments with transforaminal lumbar interbody fusion. All surgeries were performed by the same team.
Statistical methods
Continuous variables were summarized as mean ± standard deviation when data were normally distributed, while categorical variables were expressed as frequencies and percentages. The continuous variables were analyzed using independent two-sample t-tests and categorical variables were compared using a chi-square test or the Fisher’s exact test. All statistical analyses were performed using SPSS software version 25.0 (SPSS, Inc., Armonk, NY, USA). P-values < 0.05 were considered statistically significant.
Results
Demographics
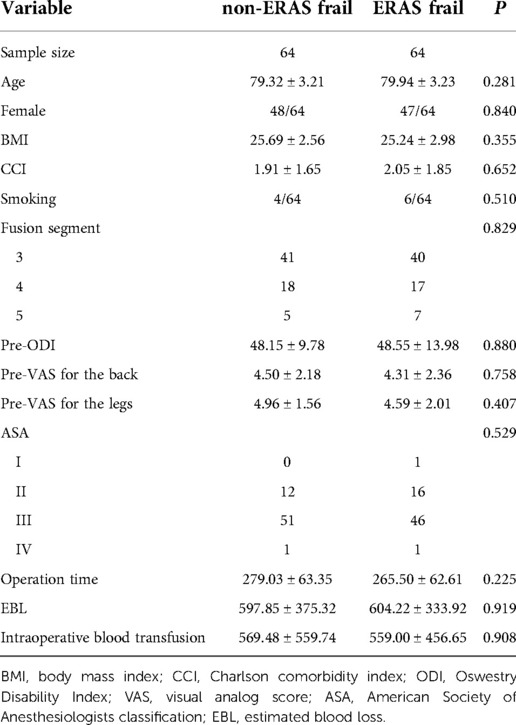
The detailed demographic patient data are presented in Table 1. After propensity score matching for age, sex, BMI, and smoking status, there were 64 pairs of well-balanced patients. The mean age was 79.94 ± 3.23 years and BMI was 25.24 ± 2.98 kg/m2 with 73.44% of women in the ERAS frail group. Analogously, the mean age was 79.32 ± 3.21 years and BMI was 25.69 ± 2.56 kg/m2 with 75.00% of women in the non-ERAS frail group. Patient-specific and procedure-specific baseline characteristics were comparable in both cohorts. CCI, ASA, pre-ODI, and pre-VAS for the back and legs were similar. In addition, there were no significant differences in terms of fusion segment, operation time, estimated blood loss, or intraoperative blood transfusions.
Perioperative outcomes
Perioperative characteristics are given in Table 2. There was no significant difference with regard to complication rates, 90-day readmission, post-ODI, post-VAS for the back, and post-VAS for the legs for both cohorts. However, there was a significant reduction in the LOS in the ERAS frail group (12.18 ± 4.69 days vs. 10.44 ± 4.60 days, p = 0.035).
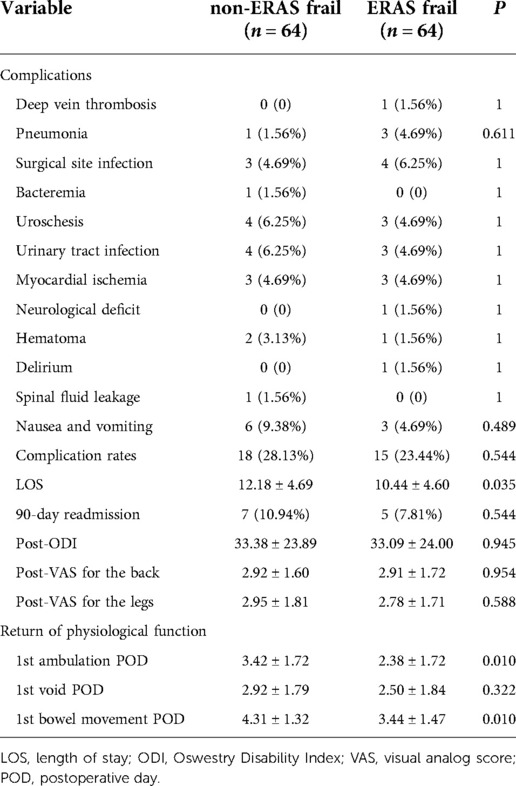
Table 2. Perioperative outcomes between the enhanced recovery after surgery frail group and the non-enhanced recovery after surgery frail group.
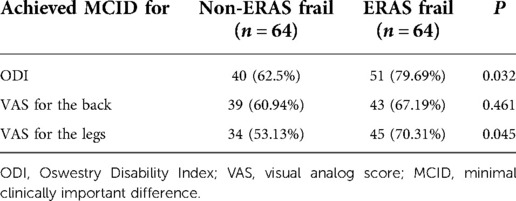
Table 3. Clinical efficacy described by recovery for the Oswestry Disability Index and visual analog score according to the minimal clinically important difference between groups.
Recovery of physiological function
Significant improvements were seen on the first day of ambulation (3.42 ± 1.72 days vs. 2.38 ± 1.72 days, p = 0.010) and the first day of bowel movement (4.31 ± 1.32 days vs. 3.44 ± 1.47 days, p = 0.010) in the ERAS frail group. On average, the first day of bladder voiding occurred 0.42 days earlier (2.92 ± 1.79 days vs. 2.50 ± 1.84 days, p = 0.322) in the ERAS frail group, although no significant difference was observed. There was significant improvement in terms of recovery of physiological function in the ERAS frail group (10.65 ± 3.51 days vs. 8.31 ± 3.98 days, p = 0.011). The detailed characteristics are displayed in Figure 1.
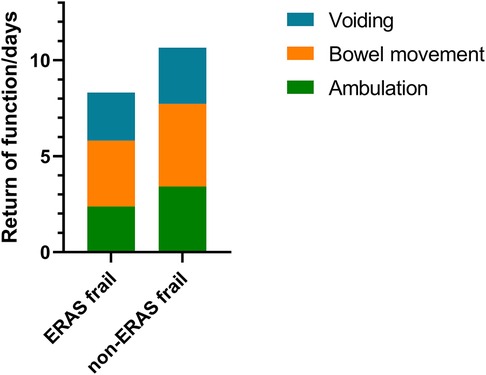
Figure 1. Stacked bar graph denoting recovery of physiological function for the ERAS frail group and the non-ERAS frail group. ERAS, enhanced recovery after surgery.
Postoperative pain
The mean pain scores on postoperative days (PODs) 1–3 between the cohorts are illustrated in Figure 2. A significant difference was observed in the pain score on POD 1 (4.88 ± 1.90 in the ERAS frail group vs. 4.27 ± 1.42 in the non-ERAS frail group, p = 0.042), while there was no significant difference on POD 2 (3.77 ± 0.88 in the ERAS frail group vs. 3.64 ± 1.07 in the non-ERAS frail group, p = 0.470) and POD 3 (3.83 ± 1.89 in the ERAS frail group vs. 3.47 ± 1.75 in the non-ERAS frail group, p = 0.266).
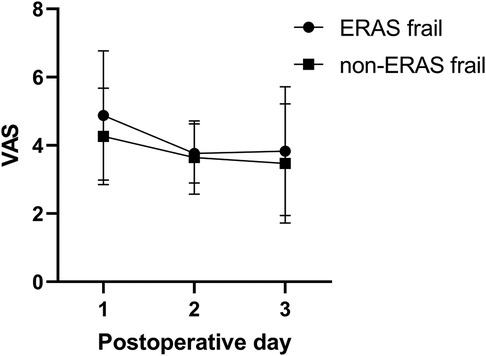
Figure 2. Pain scores on POD 1–3 between the ERAS frail group and the non-ERAS group. POD, postoperative day; ERAS, enhanced recovery after surgery.
Clinical efficacy
There were 51 (79.69%) patients in the ERAS group and 40 (62.50%) patients in the non-ERAS frail group meeting an MCID for the ODI, respectively (p = 0.032). In addition, there was substantial improvement in the VAS for the legs in the ERAS frail group compared with that in the non-ERAS frail group (70.31% vs. 53.13%, p = 0.045). More patients were meeting an MCID for the VAS for the back in the ERAS frail group, without a significant discrepancy (67.19% vs. 60.94%, p = 0.461).
Discussion
ERAS is an evidence-based multidisciplinary perioperative pathway designed to achieve early convalescence, a reduction of LOS, and postoperative complications (5, 21, 22). Conspicuous perioperative outcomes in previous studies resulted in ERAS gaining in popularity in spine surgery. Although ERAS studies have increased exponentially, there is a dearth of studies investigating the implementation of the ERAS protocol in frail older patients (>75 years) (8). The present study indicated that the implementation of the protocol amplified the recovery of physiological function, improvement of clinical efficacy, and reduction of LOS.
Frailty is clinically defined as a syndrome characterized by a decreased physiological reserve, predisposing patients to undergo surgery to avoid suboptimal outcomes. Further, multilevel lumbar fusion surgery exhibits higher complication rates and a longer LOS than their short-level counterparts (18). Therefore, if there are no external meticulous interventions, frail elderly patients would incur an increased risk of suboptimal postoperative outcomes. In a recently published retrospective study of frail patients following 1- or 2-level transforaminal lumbar interbody fusion, Porche et al. (8) indicated that ERAS significantly improved the LOS compared with their non-frail counterparts. In the present study, we found a significant reduction in the LOS in the ERAS frail group, though there was no significant difference in complications. Based on clinical experience, postoperative wound pain is correlated with patient satisfaction (23). Hence, a multimodal analgesia regimen as a part of an ERAS protocol should help maintain pain in the tolerable range. In this study, there was a significant difference in the pain score on POD 1, while there was no significant discrepancy on POD 2 and POD 3. The pain score appeared to have decreased from POD 1 to POD 3, especially in the non-ERAS frail group. In our previously published study (24), we stated that the patient-controlled multimodal analgesia pump is usually removed on POD 3 in our department, and this practice might account for the pain score being a little higher on POD 3 than on POD 2 in the ERAS frail group.
Early ambulation is the backbone of the ERAS protocol, and ERAS is designed to reduce adverse events based on a theoretical rationale for diminishing surgical-related stress response and insulin resistance (25). Hence, early recovery of physiological function occurs after implementing the ERAS protocol theoretically. Consistent with Proche et al., the total days for recovery of physiological function were significantly lower in the ERAS frail group (pre-ERAS: 6.7 days, post-ERAS: 3.4 days, p < 0.001). In this study, the first day of ambulation occurred on average 1.04 days earlier, the first day of bowel movement occurred on average 0.87 days earlier, and the first day of bladder voiding occurred 0.42 days earlier in the ERAS frail group than in the non-ERAS frail group, respectively. Furthermore, the number of days to the recovery of physiological function was significantly less, with an average of 2.34 days earlier in the ERAS frail group.
Clinical efficacy is evaluated according to patient-reported health-related quality-of-life questionnaires including the ODI and VAS in spinal surgery studies. However, even subtle changes can yield statistically significant differences in sample sizes and measurement accuracy, and these are sufficient. Therefore, the MCID suggests a threshold to assess clinical efficacy, which makes intergroup analysis intuitive and explicit (20). In a retrospective study, Ayling et al. (26) indicated that there was no significant difference in the ODI and numeric rating scale between patients undergoing 1- to 2-level open transforaminal lumbar interbody fusion or minimally invasive transforaminal lumbar interbody fusion. Further analysis suggested that a higher baseline leg pain score predicted achieving the MCID in both cohorts. Jacob et al. (23) conducted a retrospective study suggesting that meeting an MCID for the back and leg pain was associated with patient satisfaction in lumbar decompression patients. Our study showed a significant improvement in the ODI and VAS after the performance of the procedures both in the ERAS frail group and in the non-ERAS frail group. In addition, despite finding the analogous preoperative and postoperative ODI, VAS for the back, and VAS for the legs, we found a significantly increased number of patients who met an MCID for the ODI and VAS for the legs after the implementation of the ERAS protocol. More patients in the ERAS frail group met an MCID in the VAS for the back; however, there was no significant difference because the postoperative patient-reported outcomes included in this study were evaluated before discharge, which provides favorable evidence for immediate recovery for daily activities after implementing the ERAS protocol in clinical practice.
This study was not without limitations. First, the study suffered from inherent limitations associated with retrospective analysis. Second, we did not perform multivariate analysis for patients who did not meet an MCID on the grounds of insufficient statistical power due to the small sample size. Finally, long-term patient-reported outcomes were not included in this study, as this study primarily focused on ERAS exposure with frailty as the variable, whereas ERAS is a multimodal management approach focusing on perioperative outcomes. Further multicenter studies with large cohorts are required to confirm our findings.
Conclusion
In this retrospective cohort study, we found a significant improvement in terms of the LOS, recovery of physiological function, and clinical efficacy after the implementation of the ERAS protocol in elderly and frail patients undergoing multilevel lumbar fusion surgery, while there was no significant discrepancy with regard to complications, 90-day readmission rates, and postoperative pain.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board in Xuanwu Hospital Capital Medical University (No.2018086). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
PC and SW were responsible for conceptualization, preparation of the methodology, data curation, and writing of the original draft; PW and LY were responsible for supervision and reviewing of statistical analysis; CK and SL were responsible for project administration, supervision, and evaluation of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Beijing Municipal Health Commission (Jing 2019-2), Beijing Hospitals Authority’s Ascent Plan (DFL20190802), and Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX202116). The funders played no role in the design of this study, the collection, analysis, and interpretation of data, or the preparation of the manuscript.
Acknowledgments
We thank the staff at the Department of Orthopedics, Xuanwu Hospital Capital Medical University, and all the patients who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Debono B, Corniola MV, Pietton R, Sabatier P, Hamel O, Tessitore E. Benefits of enhanced recovery after surgery for fusion in degenerative spine surgery: impact on outcome, length of stay, and patient satisfaction. Neurosurg Focus. (2019) 4:E6. doi: 10.3171/2019.1.FOCUS18669
2. Elsarrag M, Soldozy S, Patel P, Norat P, Sokolowski JD, Park MS, et al. Enhanced recovery after spine surgery: a systematic review. Neurosurg Focus. (2019) 4:E3. doi: 10.3171/2019.1.FOCUS18700
3. Smith J, Probst S, Calandra C, Davis R, Sugimoto K, Nie L, et al. Enhanced recovery after surgery (ERAS) program for lumbar spine fusion. Perioper Med (Lond). (2019) 8:4. doi: 10.1186/s13741-019-0114-2
4. Soffin EM, Vaishnav AS, Wetmore DS, Barber L, Hill P, Gang CH, et al. Design and implementation of an enhanced recovery after surgery (ERAS) program for minimally invasive lumbar decompression spine surgery: initial experience. Spine. (2019) 9:E561–70. doi: 10.1097/brs.0000000000002905
5. Brusko GD, Kolcun JPG, Heger JA, Levi AD, Manzano GR, Madhavan K, et al. Reductions in length of stay, narcotics use, and pain following implementation of an enhanced recovery after surgery program for 1- to 3-level lumbar fusion surgery. Neurosurg Focus. (2019) 4:E4. doi: 10.3171/2019.1.FOCUS18692
6. Wang P, Wang Q, Kong C, Teng Z, Li Z, Zhang S, et al. Enhanced recovery after surgery (ERAS) program for elderly patients with short-level lumbar fusion. J Orthop Surg Res. (2020) 1:299. doi: 10.1186/s13018-020-01814-3
7. Chen J, Li D, Wang R, Wang S, Shang Z, Wang M, et al. Benefits of the enhanced recovery after surgery program (ERAS) in short-segment posterior lumbar interbody fusion surgery. World Neurosurg. (2021) 159:e303–10. doi: 10.1016/j.wneu.2021.12.046
8. Porche K, Yan S, Mohamed B, Garvan C, Samra R, Melnick K, et al. Enhanced recovery after surgery (ERAS) improves return of physiological function in frail patients undergoing 1-2 level TLIFs: an observational retrospective cohort study. Spine J. (2022) 22(9):1513–22. doi: 10.1016/j.spinee.2022.04.007
9. Dagal A, Bellabarba C, Bransford R, Zhang F, Chesnut RM, O’Keefe GE, et al. Enhanced perioperative care for major spine surgery. Spine (Phila Pa 1976). (2019) 13:959–66. doi: 10.1097/BRS.0000000000002968
10. Angus M, Jackson K, Smurthwaite G, Carrasco R, Mohammad S, Verma R, et al. The implementation of enhanced recovery after surgery (ERAS) in complex spinal surgery. J Spine Surg. (2019) 1:116–23. doi: 10.21037/jss.2019.01.07
11. d’Astorg H, Fière V, Dupasquier M, Vieira TD, Szadkowski M. Enhanced recovery after surgery (ERAS) protocol reduces LOS without additional adverse events in spine surgery. Orthop Traumatol Surg Res. (2020) 6:1167–73. doi: 10.1016/j.otsr.2020.01.017
12. Sun Z, Qi Y. Application of enhanced recovery after surgery care protocol in the perioperative care of patients undergoing lumbar fusion and internal fixation. J Orthop Surg Res. (2022) 1:240. doi: 10.1186/s13018-022-03099-0
13. Shahrestani S, Ton A, Chen XT, Ballatori AM, Wang JC, Buser Z. The influence of frailty on postoperative complications in geriatric patients receiving single-level lumbar fusion surgery. Eur Spine J. (2021) 12:3755–62. doi: 10.1007/s00586-021-06960-8
14. Agarwal N, Goldschmidt E, Taylor T, Roy S, Dunn SC, Bilderback A, et al. Impact of frailty on outcomes following spine surgery: a prospective cohort analysis of 668 patients. Neurosurgery. (2021) 3:552–7. doi: 10.1093/neuros/nyaa468
15. Sun W, Lu S, Kong C, Li Z, Wang P, Zhang S. Frailty and post-operative outcomes in the older patients undergoing elective posterior thoracolumbar fusion surgery. Clin Interv Aging. (2020) 15:1141–50. doi: 10.2147/CIA.S245419
16. Beauchamp-Chalifour P, Flexman AM, Street JT, Fisher CG, Ailon T, Dvorak MF, et al. The impact of frailty on patient-reported outcomes after elective thoracolumbar degenerative spine surgery. J Neurosurg. (2021):1–9. doi: 10.3171/2021.2.Spine201879
17. Fried L, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol. (2001) 3:M146–56. doi: 10.1093/gerona/56.3.m146
18. Li G, Patil CG, Lad SP, Ho C, Tian W, Boakye M. Effects of age and comorbidities on complication rates and adverse outcomes after lumbar laminectomy in elderly patients. Spine. (2007) 33:1250–55.
19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying pronnostic comorbidity in long itudinal studies: development and validation. Pergamon J. (1987) 40:373–83.
20. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. (2008) 6:968–74. doi: 10.1016/j.spinee.2007.11.006
21. Porche K, Samra R, Melnick K, Brennan M, Vaziri S, Seubert C, et al. Enhanced recovery after surgery (ERAS) for open transforaminal lumbar interbody fusion: a retrospective propensity-matched cohort study. Spine J. (2021) 22(3):399–410. doi: 10.1016/j.spinee.2021.10.007
22. Adeyemo EA, Aoun SG, Barrie U, Nguyen ML, Badejo O, Pernik MN, et al. Enhanced recovery after surgery reduces postoperative opioid use and 90-day readmission rates after open thoracolumbar fusion for adult degenerative deformity. Neurosurgery. (2021) 2:295–300. doi: 10.1093/neuros/nyaa399
23. Jacob KC, Patel MR, Collins AP, Park GJ, Vanjani NN, Prabhu MC, et al. Meeting patient expectations and achieving a minimal clinically important difference for back disability, back pain, and leg pain may provide predictive utility for achieving patient satisfaction among lumbar decompression patients. World Neurosurg. (2022) 162:e328–35. doi: 10.1016/j.wneu.2022.03.002
24. Cui P, Wang P, Kong C, Li XY, Wang SK, Wang JL, et al. Patients older than 75 years undergoing polysegmental lumbar fusion surgery can also benefit from enhanced recovery after surgery program. Clin Interv Aging. (2022) 17:245–52. doi: 10.2147/CIA.S353511
25. Ripolles-Melchor J, Abad-Motos A, Diez-Remesal Y, Aseguinolaza-Pagola M, Padin-Barreiro L, Sanchez-Martin R, et al. Association between use of enhanced recovery after surgery protocol and postoperative complications in total hip and knee arthroplasty in the postoperative outcomes within enhanced recovery after surgery protocol in elective total hip and knee arthroplasty study (POWER2). JAMA Surg. (2020) 4:e196024. doi: 10.1001/jamasurg.2019.6024
26. Ayling OG, Rampersaud YR, Dandurand C, Yuan PHS, Ailon T, Dea N, et al. Surgical outcomes of patients who fail to reach minimal clinically important differences: comparison of minimally invasive versus open transforaminal lumbar interbody fusion. J Neurosurg. (2022):1–8. doi: 10.3171/2022.2.Spine211210
Keywords: enhanced recovery after surgery, frail, multilevel, lumbar fusion surgery, propensity score matching
Citation: Cui P, Wang S, Wang P, Yang L, Kong C and Lu S (2022) Comparison of perioperative outcomes in frail patients following multilevel lumbar fusion surgery with and without the implementation of the enhanced recovery after surgery protocol. Front. Surg. 9:997657. doi: 10.3389/fsurg.2022.997657
Received: 19 July 2022; Accepted: 5 October 2022;
Published: 2 November 2022.
Edited by:
Vadim Byvaltsev, Irkutsk State Medical University, RussiaReviewed by:
Dalong Yang, Third Hospital of Hebei Medical University, ChinaXinyu Liu, Shandong University, China
© 2022 Cui, Wang, Wang, Yang, Kong and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Kong a29uZzk4ODUwMEAxNjMuY29t Shibao Lu c3BpbmVsdUB4d2hvc3Aub3Jn
†These authors contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Peng Cui
Peng Cui Shuaikang Wang
Shuaikang Wang Peng Wang1,2
Peng Wang1,2 Chao Kong
Chao Kong