- 1Emergency and Traumatology Department, The First Affiliated Hospital of Hainan Medical University, Hainan Medical University, Haikou, China
- 2Key Laboratory of Emergency and Trauma Ministry of Education, Hainan Medical University, Haikou, China
- 3Academy of Pediatrics, Hainan Medical University, Haikou, China
- 4Institute of Wound Repair and Regeneration Medicine, Southern University of Science and Technology School of Medicine; Department of Wound Repair, Southern University of Science and Technology Hospital, Shenzhen, China
Background: Wound repair is a new field that has emerged in China in the last 5 years. Exposed tendon wounds are one of the most common problems faced in wound treatment today, as the poor blood supply leads to low survival rates of skin grafts. This paper explores the feasibility of applying the Masquelet technique to repair tendon-exposed wounds.
Method: We examined 12 patients with tendon-exposed wounds, 5 males and 7 females, from January 2021 to November 2021, including 2 patients with post-traumatic wounds, 8 diabetic patients with dorsal wounds, and 2 patients with various chronic infections. The Masquelet technique was employed to treat these wounds. The wound surface was sealed with antibiotic bone cement to form an induction membrane, the cement was removed after 3–4 weeks, and the wound was repaired with skin grafts to observe survival, appearance, texture, healing, and related functions.
Results: All wounds were covered with antibiotic bone cement, and after 3–4 weeks, an induction membrane was applied, and in 10 out of 12 patients, full-thickness skin grafts were applied, and the patients survived. However, in 2 patients, the skin became partially necrotic, but these patients recovered by changing medications.
Conclusion: The current study found that direct skin grafting may effectively treat exposed tendon wounds once the Masquelet approach generates the induction membrane. Further, this method is less difficult, less expensive, and easier to care for the procedure that deserves to be used more frequently.
Introduction
The number of acute wounds and chronic conditions based on chronic wounds (such as diabetic foot, pressure ulcers, and venous ulcers) is rising quickly due to rapid economic development and the population's accelerated ageing. Skin and soft tissue loss in the limbs are the most prevalent, typically resulting in tendon-exposed wounds (1). For tendon-exposed wounds, we often use flap prostheses or skin grafts after long-term drug replacement, but flap prostheses are difficult, and manipulation is challenging (2). Some wounds require secondary flap prostheses because the thick flap partially affects appearance and function. Poor vascular and skin conditions can lead to flap necrosis, making this treatment option rarely available. In addition, long-term drug treatment can lead to long-term exposure to the tendon and corresponding functional impairment due to tendon necrosis, finally seriously affecting the patient's quality of life (3).
In recent years, some scholars have applied antibiotic-containing gentamicin bone cement to treat various wounds with some success. However, there are no reports of repair of tendon-exposed wounds after covering them with antibiotic-containing gentamicin bone cement, followed by skin grafting directly to the guiding membrane (4). From January 2021 to November 2021, this study successfully repaired tendon-exposed wounds using this technique in 12 patients. These wounds underwent prompt and complete cleaning before being sealed with gentamicin bone cement, which was removed 3–4 weeks later. An induced membrane started to develop. Thick skin grafts were used to treat the wounds directly, and they were successfully repaired.
Methods
Patients
Twelve patients (5 males and 7 females) ages 47–89 years (mean age 68.75) were recruited for the current study. Among all selected patients, 2 showed post-traumatic wounds, 8 diabetic foot wounds, 2 with other chronic infected wounds, 10 with ground and foot wounds, and 2 with dorsal hand wounds. The wound defects range from 3 cm × 2 cm to 18 cm × 12 cm, and disease duration ranges from 1 to 4 months.
After anaesthesia, disinfection, and draping, infected necrotic tissue was completely removed, and the wounds were cleaned until fresh blood drained from their wounds. The wound was cleaned with 3% hydrogen peroxide, diluted iodine, and normal saline. The wound blood was stopped by electrocoagulation or fine wire ligation and was checked and re-cleaned if necessary.
Treatment method
Gentamicin Bone Cement (Depuy Synthes, USA) powder cement (40 g) was taken and thoroughly mixed. The polymethyl methacrylate reagent (Depuy Synthes, USA) was added and mixed to create a paste. Following the drawing step, it was thinly tiled to fit the size and shape of the wound before being covered and moulded. Before the gentamicin bone cement hardened, a 1.5 mm Kirschner wire was used to drill holes in its surface. In order to cover the cement surface, sutures were used to attach the gentamicin bone cement to the wound surface. Postoperative anti-infective care involving intravenous static antibiotics was necessary.
One debridement was performed 5–7 days after admission and covered with gentamicin bone cement after 1–2 thorough debridements. Every 2 days after Stage I surgery, the wound dressing was changed. The wound exudate and redness and swelling around the wound were observed. Two weeks later, the gentamicin bone cement was removed. The physician monitored infection control and new granulation growth, collected wound secretions, and performed bacterial culture and drug sensitivity testing. Depending on the wound's size and the granulation's growth, the decision was made to use relaxing sutures, subsequent drug replacement, or skin grafting. If was no improvement in wound infection or only a small amount of fresh granulation tissue has begun to grow, another debridement and antibiotic gentamicin bone cement can be used. Suppose there was no bacterial infection and a large amount of granulation tissue begins to grow in the wound culture. In that case, the physician should observe a smooth guiding membrane formed on the tendon and wound, perform a full-layer skin graft, and secure the plaster externally using “packing” combined with a compression bandage to prevent skin loosening and necrosis due to activity. The compression bandages were removed 7–10 days after surgery. If some skin grafts were not viable, the dressing was changed every 2 days, and recombinant bovine basic fibroblast growth factor (Essex Bio-Technology, Hong Kong) gel was applied externally until the wound healed.
The Masquelet technique must be used to treat these wounds. This method involves sealing the wound with gentamicin-containing bone cement to produce an induction membrane, removing the cement after 3–4 weeks, covering the wound with a direct skin graft, and monitoring the grafted skin's survival, shape, feel, and wound healing.
Hematoxylin-eosin staining
Human wound tissues covered with gentamicin bone cement were collected during surgery, fixed with 4% formaldehyde, embedded in paraffin, and then cut into 8 μm-thick serial sections. The sixth layer of each section was selected for hematoxylin-eosin (HE) staining, and the images were observed and recorded under a light microscope with a 40× objective.
Results
After complete debridement, antibiotic gentamicin bone cement was applied to all wounds, and in 3–4 weeks, an induction membrane developed. The two patients with partial necrosis of the grafted skin recovered with the replacement of dressings, and all 10 of the 12 patients who underwent all-layer skin grafting survived.
Typical case 1
A 47-year-old female patient was admitted to our hospital with “left leg pain from a car accident for the past 5 days”. She had a normal medical history, and the left sole was identified upon admission. The patient was treated with wound sealing with debridement + gentamicin bone cement coverslip + VSD for skin liquefaction necrosis after admission while receiving a nerve block (Figure 1A). The injury was on the left dorsal foot. In the surgery, liquefied necrotic tissue was excised, a culture specimen was obtained, and an irregular wound measuring approximately 8.0 cm × 10.0 cm was formed, exposing two to five extensor toe tendons (Figure 1B). The wound surface was covered and shaped. The gentamicin bone cement surface was perforated with a 1.5 mm Kirschner wire before the cement solidified, and sutures were used to secure the gentamicin bone cement to the wound surface (Figure 1C). On postoperative day 21, the gentamicin bone cement with antibiotics was harvested. It was evident that an inductive membrane had formed on the surface of the tendon (Figure 1D). Therefore, a full-layer skin graft was performed, and the entire wound was wrapped with packing and compression bandages (Figure 1E). The skin graft survived 14 days after skin grafting (Figure 1F). At the 3 month postoperative follow-up, the affected area had recovered well in appearance and function (Figures 1G,H).

Figure 1. (A) Pre-operative. (B) Exposed tendons. (C) Gentamicin bone cement covers the wound. (D) Induction of membrane formation. (E) After implant surgery. (F) Fourteen days after implant surgery, the dressing was removed. (G,H) Three months after implant surgery.
Typical case 2
A 68-year-old woman was admitted to our hospital with a skin ulcer and pus on the left foot that had persisted for over a month and worsened over a week in the affected area. The patient had a history of diabetes for over 3 years. She used insulin for blood glucose control and did not regularly measure blood glucose levels. On admission, there was a 3 cm × 4 cm skin defect on the anterior edge of the right instep with erythema and swelling of the surrounding tissue. Numerous purulent secretions with exposed tendons were noted (Figure 2A). After appropriate testing, the patient underwent surgery for wound closure with debridement of the left dorsum + gentamicin bone cement covering + vacuum aspiration under a nail block of the left dorsum 7 days after admission. After initial thorough debridement, a 5 cm × 6 cm wound was formed, the tendon was exposed, a specimen was taken and cultured (Figure 2B), and then gentamicin bone cement was sutured and fixed to the wound (Figure 2C). On postoperative day 30, the gentamicin bone cement with antibiotics was removed. It was evident that a layer of induction membrane had formed on the surface of the tendon (Figure 2D). During the pre-implant surgery, a substantial amount of fresh granulation tissue was found encircling the exposed tendon and indicating the attachment of a new induction membrane (Figures 2E,F). A full-layer skin graft was placed. The skin graft began to survive after 7 days (Figure 2G). Finally, the wound healed well after 1 month of follow-up (Figure 2H).
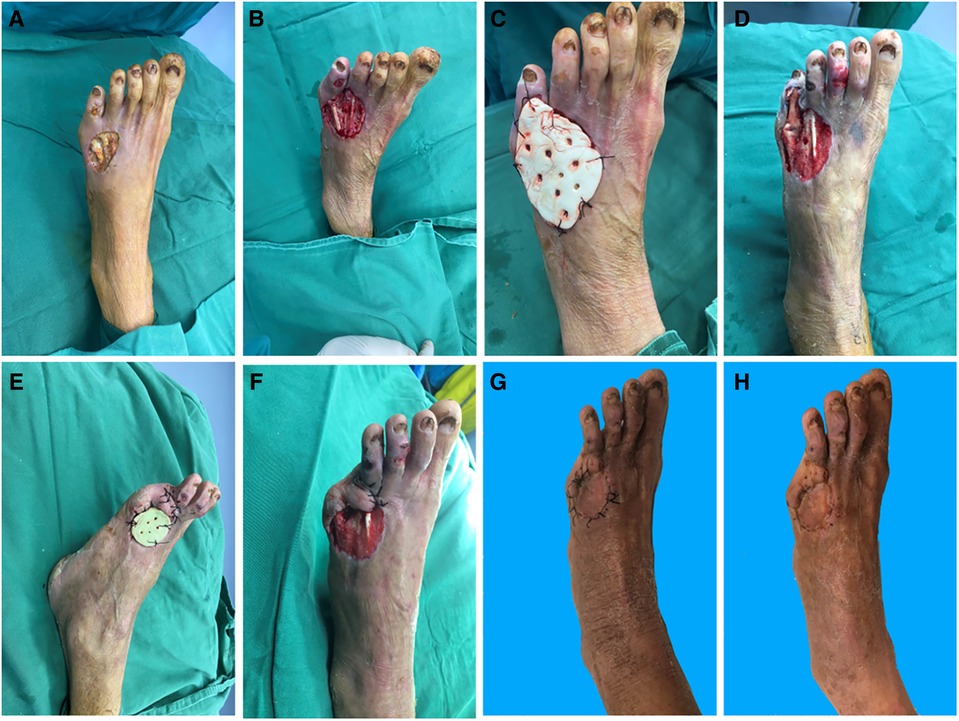
Figure 2. (A) Pre-operative. (B) Exposed tendons. (C) Gentamicin bone cement covers the wound. (D) Induction of membrane formation. (E,F) Before implantation. (G) Fourteen days after implant surgery. (H) One month after implant surgery.
Discussion
Tendons are a special type of tissue in which the tendon receives little blood supply, except at the bony ends, but the tendon depends on the surrounding interstitial fluid for its survival (5). When the tendon is exposed, the tendon membrane can become deficient due to trauma or infection. The wound is prone to bacterial contamination, so the tendon must be repaired and covered. Otherwise, the wound can easily lead to serious complications such as tendon wound infection and ischemic tendon necrosis (6). Some patients have complex conditions in clinical practice, such as unstable general conditions due to multiple underlying medical complications, combined wound infections that prevent thorough debridement, too large wound defects, and limited flap zone conditions. Stage-I repair of the exposed tendon cannot be performed, so only the wound can be temporarily placed and await secondary repair (7). The conventional approach to treating putting-aside requires debridement followed by a dressing change with a sterile dressing, which takes time and saves just a small amount of the tendon. Vacuum drainage has been increasingly popular among medical practitioners in recent years. Although tendon yellowing, degeneration, and necrosis are seen after generating VSD, it is generally utilised for various acute and chronic wounds (8).
The impact of the wound microenvironment on wound repair is progressively becoming understood because of developments in histology and cell function (9). Masquelet technique, also called induced membrane technique, is an emerging field based on human foreign body reaction and bone regeneration capabilities. It was first reported by French scholar Masquelet in 1986 as a treatment for large bone defects (10). The thickness of the induction membrane and synovium created by Masquelet is almost the same, ranging from 0.5 to 2.0 mm. There are small blood vessels on the induction membrane (Figure 3), and immunohistochemical analysis showed that the induction membrane capsule is composed of type I collagen, its inner layer is composed of synovial epithelial cells, and its outer layer is rich in fibroblasts and myofibroblasts. At the same time, the inductive membrane capsule is not only densely populated with small blood vessels in the long axis direction. However, it can also secrete VEGF, TGF-β1, and BMP-2 to contribute to local vessel formation (11–13). To date, the Masquelet technique has been used in, but not limited to, the reconstruction of large bone defects (14), the treatment of infected femoral defects after trauma (15), the reconstruction of finger bones (16), chronic osteomyelitis (17), the reconstruction of the chest wall (18), and in the treatment of wounds. Gentamicin bone cement is applied to the wound to form an induction membrane. An inflammatory reaction sets the stage for wound healing, followed by granulation and tissue growth. Angiogenesis is crucial for tissue repair at this point. As a result, the author hypothesises that gentamicin bone cement also treats wounds infected with soft tissue (19, 20).
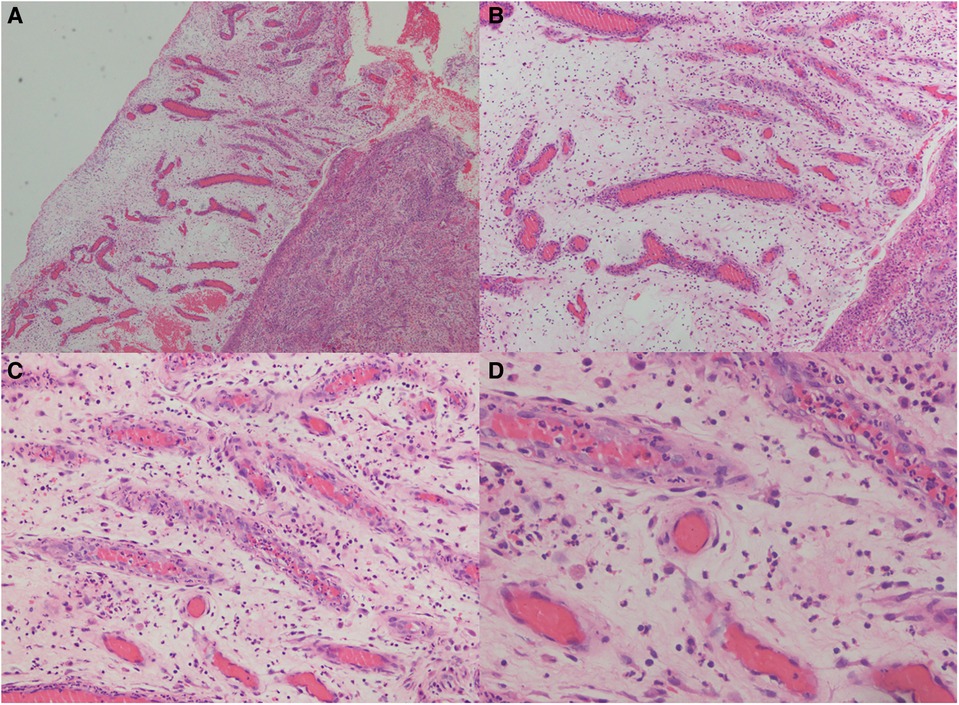
Figure 3. Hematoxylin and eosin (HE) staining of the induced membrane tissue at different magnifications. The developed membranes were widely dispersed and had considerable neovascularization, which increased the likelihood that the flap transplant would survive (magnification from A–D are: 40x, 100x, 200x, 400x).
By collecting clinical data, the author found that Masquelet technology can temporarily cover a wound. Covering with gentamicin bone cement creates a separate space, isolates it from the external environment, avoids exogenous infection, and seals the wound to promote the metabolism of wound tissue fluids (21). Keeping the wound moist is beneficial for epidermal cell regeneration, activation of growth factors, and promotion of vasculature and granulation. It is especially important for the nutritional supply to the tendon, i.e., creating a physiological survival environment for the tendon. In other words, it is similar to creating a condition after flap repair (22). A typical colour characterises the covered tendon, and an inductive membrane gradually develops. This offers favourable circumstances for subsequent tendon-exposed wound healing. In addition, antibiotic gentamicin bone cement works well as a temporary filler for the space left behind after the removal of necrotic tissue, lowering the bacterial load of the wound, preventing further tendon injury, delaying tendon and wound healing, lowering the need for intravenous antibiotics, and lowering toxic effects on the body's liver and kidney function (11, 23). Stage-1 Thorough debridement is the cornerstone of successful follow-up surgery. Local infection should be prevented and treated with gentamicin bone cement. However, thorough debridement is a prerequisite for successful follow-up surgery, and ultimately care must be taken to protect the induced membrane during surgery (24). Stage-2 When removing gentamicin bone cement during surgery, the gentamicin bone cement must be gently removed, and the induced cement integrity must be maintained; 1–2 weeks is a period of thorough debridement, infection control, and systemic remediation. The vascular activity of the induced membrane peaks at 2–4 weeks. Based on the clinical data collected, the author has determined that approximately 3 weeks postoperatively is the optimal time for induced epithelial grafting, with better results.
Debridement of the wound and antibiotics to prevent infection and lessen pressure on the foot are the current gold standard of treatment for diabetic foot ulcers. Neuropathic, ischemic, and neuro-ischemic diabetic foot ulcers can be distinguished at this stage, with neuro-ischemic being the most prevalent. The ulcerative lesions are made worse by the absence of neurotrophic support and the accompanying vascular disease, resulting in amputation and a bad prognosis for the patient. Current treatment options include interventional therapy, antibiotic therapy, hyperbaric oxygen therapy, electrical stimulation therapy, multiple dressings for wounds, vacuum sealing drainage (VSD), biological therapy, surgical debridement, skin grafting, flap grafting, joint fusion, etc. The main reason for the differences in the treatment of tendon outgrowth due to diabetic foot ulcers is the differences in the bacterial species of the wounds, especially when it comes to fungal infections similar to MASA and other drug-resistant bacteria. On the other hand, age and diabetic neuropathy cause the elderly to be more prone to gangrene and even amputation. The length of hospitalization for tendon exenteration compared to ordinary diabetic foot ulcer wounds and the economic pressure leading to treatment interruptions in the patient's treatment process are also important factors in the difference in treatment outcome.
The first patient in this study has experienced trauma, while the second patient has a diabetic foot ulcer. Poor blood perfusion decreased blood flow to the limb tissues, the patient's obesity or poor health status, persistent, localised pressure on the torso, and a considerable decline in skin elasticity and thickness distinguish diabetic foot wound patients from those with traumatic ulcers. As a result, tendon outgrowth brought on by trauma heals more quickly than tendon outgrowth brought on by a diabetic foot.
Limitations
This study discovered that the Masquelet technique and skin grafting procedures do not have complete efficacy metrics for tendon debridement wounds. However, we noticed an increase in wound granulation and a percentage reduction in the size of the wound. This study did not employ comparison studies or other research techniques due to the brief observation duration. More samples are required to establish the efficacy of the Masquelet technique and skin grafting for the treatment of exposed tendon wounds due to the small sample size of this study.
Conclusion
In summary, Masquelet technology can, in essence, create a transparent guiding membrane over the wound, create a favourable angiogenic environment, cover exposed tendon wounds, protect tendons, prevent infection, lessen the overuse of antibiotics, decrease dead space, and encourage granulation and tissue formation. On the other hand, when used with skin grafting, it may successfully cover acute, chronic, and exposed tendons, producing better and faster results, making it worthwhile to promote and use.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JY, JY and YZ wrote the paper. QW and LC collected and edited the pathology imaging. LZ, SC, RW and HZ collected clinical case information and imaging data. HC, YH and SC proofread and reviewed the article content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Hainan Province Clinical Medical Center, Hainan Provincial Natural Science Found ation of China (822MS174, 822QN463), Hainan Province Science and Technology Special Fund (ZDYF2021SHFZ238, ZDKJ2021038), National Natural Science Foundation of China (81860347), Hainan Provincial Health and Family Planning Commission Project (21A200231, 21A200062); Youth cultivation fund of the First Affiliated Hospital of Hainan Medical University (HYFYPY2005); Hainan Medical College 2022 Student Innovation and Entrepreneurship Training Program (X202211810008).
Acknowledgments
We would like to thank the patients for participating in this case study, and all patients who participated in the study agreed to authorize the disclosure of personal information and surgical images. We thank Academician Workstation and the Department of Wound repair, The First Affiliated Hospital of Hainan Medical University for their support and assistance with this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
VSD, vacuum sealing drainage; VEGF, vascular endothelial growth factor; TGF-β1, transforming growth factor-β; BMP-2, bone morphogenetic protein-2
References
1. Jiang Y, Huang S, Fu X, Liu H, Ran X, Lu S, et al. Epidemiology of chronic cutaneous wounds in China. Wound Repair Regen. (2011) 19(2):181–8. doi: 10.1111/j.1524-475X.2010.00666.x
2. Zhang S, Cao D, Xie J, Li H, Chen Z, Bao Q. Platelet-rich fibrin as an alternative adjunct to tendon-exposed wound healing: a randomized controlled clinical trial. Burns. (2019) 45(5):1152–7. doi: 10.1016/j.burns.2019.01.007
3. Li MX, Ma J, Zheng ZJ, Niu LB, Yang L. Clinical effect of bi-layered artificial dermis and autologous skin graft in repairing bone and/or tendon exposed wounds. Zhonghua Shao Shang Za Zhi. (2020) 36(3):179–86. doi: 10.3760/cma.j.cn501120-20191119-00437
4. Liu C, You JX. Effect of induced membrane formation followed by polymethylmethacrylate implantation on diabetic foot ulcer healing when revascularization is not feasible. J Diabetes Res. (2019) 2019:2429136. doi: 10.1155/2019/2429136
5. Tsai SL, Nödl MT. Bringing tendon biology to heel: leveraging mechanisms of tendon development, healing, and regeneration to advance therapeutic strategies. Dev Dyn. (2021) 250(3):393–413. doi: 10.1002/dvdy.269
6. di Summa PG, Sapino G, Cherubino M. Reconstruction of complex soft tissue defects including tendons with anterolateral thigh flap extended to fascia lata: long term recovery and functional outcomes. Microsurgery. (2019) 39(5):405–15. doi: 10.1002/micr.30431
7. Nichols AEC, Oh I, Loiselle AE. Effects of type II diabetes mellitus on tendon homeostasis and healing. J Orthop Res. (2020) 38(1):13–22. doi: 10.1002/jor.24388
8. Liu X, Liang J, Zao J, Quan L, Jia X, Li M, et al. Vacuum sealing drainage treatment combined with antibiotic-impregnated bone cement for treatment of soft tissue defects and infection. Med Sci Monit. (2016) 22:1959–65. doi: 10.12659/MSM.896108
9. Chen Y, Liang Y, Liu J, Yang J, Jia N, Zhu C, et al. Optimizing microenvironment by integrating negative pressure and exogenous electric fields via a flexible porous conductive dressing to accelerate wound healing. Biomater Sci. (2021) 9(1):238–51. doi: 10.1039/D0BM01172J
10. Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. (2000) 45(3):346–53. PMID: 10929461
11. Wang W, Zuo R, Long H, Wang Y, Zhang Y, Sun C, et al. Advances in the Masquelet technique: myeloid-derived suppressor cells promote angiogenesis in PMMA-induced membranes. Acta Biomater. (2020) 108:223–36. doi: 10.1016/j.actbio.2020.03.010
12. Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. (2004) 22(1):73–9. doi: 10.1016/S0736-0266(03)00165-7
13. Alford AI, Nicolaou D, Hake M, McBride-Gagyi S. Masquelet's induced membrane technique: review of current concepts and future directions. J Orthop Res. (2021) 39(4):707–18. doi: 10.1002/jor.24978
14. Hatashita S, Kawakami R, Ejiri S, Sasaki N, Toshiki N, Ito M, et al. ‘Acute Masquelet technique’ for reconstructing bone defects of an open lower limb fracture. Eur J Trauma Emerg Surg. (2021) 47(4):1153–62. doi: 10.1007/s00068-019-01291-2
15. Pesciallo CA, Garabano G, Dainotto T, Ernst G. Masquelet technique in post-traumatic infected femoral and tibial segmental bone defects. Union and reoperation rates with high proportions (up to 64%) of allograft in the second stage. Injury. (2021) 52(11):3471–7. doi: 10.1016/j.injury.2021.08.031
16. Pruzansky ME, Lee Y, Pruzansky J. Masquelet technique for phalangeal reconstruction and osteomyelitis. Tech Hand Up Extrem Surg. (2020) 25(1):52–5. doi: 10.1097/BTH.0000000000000299
17. Hogan A, Heppert VG, Suda AJ. Osteomyelitis. Arch Orthop Trauma Surg. (2013) 133(9):1183–96. doi: 10.1007/s00402-013-1785-7
18. Taylor BC, Zink T, McGowan S. Masquelet technique for chest wall reconstruction: a case report. JBJS Case Connect. (2020) 10(1):e0011. doi: 10.2106/JBJS.CC.19.00011
19. Chakroborty D, Goswami S, Basu S, Sarkar C. Catecholamines in the regulation of angiogenesis in cutaneous wound healing. FASEB J. (2020) 34(11):14093–102. doi: 10.1096/fj.202001701R
20. Zhang D, Ouyang Q, Hu Z, Lu S, Quan W, Li P, et al. Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int J Biol Macromol. (2021) 173:591–606. doi: 10.1016/j.ijbiomac.2021.01.157
21. Oh Y, Yoshii T, Okawa A. Ankle arthrodesis using a modified Masquelet induced membrane technique for open ankle fracture with a substantial osteochondral defect: a case report of novel surgical technique. Injury. (2019) 50(11):2128–35. doi: 10.1016/j.injury.2019.09.020
22. Toth Z, Roi M, Evans E, Watson JT, Nicolaou D, McBride-Gagyi S. Masquelet technique: effects of spacer material and micro-topography on factor expression and bone regeneration. Ann Biomed Eng. (2019) 47(1):174–89. doi: 10.1007/s10439-018-02137-5
23. Ma YF, Jiang N, Zhang X, Qin CH, Wang L, Hu YJ, et al. Calcium sulfate induced versus PMMA-induced membrane in a critical-sized femoral defect in a rat model. Sci Rep. (2018) 8(1):637. doi: 10.1038/s41598-017-17430-x
Keywords: exposed tendon, wounds, skin grafting, Masquelet, gentamicin bone cement
Citation: Yao J, Zeng Y, Yang J, Wu Q, Chen L, Zheng L, Wang R, Zhu H, Cui H, Huang Y and Cheng S (2022) Repairing tendon-exposed wounds by combing the Masquelet technique with dermoplasty. Front. Surg. 9:995316. doi: 10.3389/fsurg.2022.995316
Received: 15 July 2022; Accepted: 10 October 2022;
Published: 2 November 2022.
Edited by:
Haihong Li, Southern University of Science and Technology Hospital, ChinaReviewed by:
Shang Ping, Wenzhou Medical University, ChinaShuming Ye, Second Hospital of Anhui Medical University, China
© 2022 Yao, Zeng, Yang, Wu, Chen, Zheng, Wang, Zhu, Cui, Huang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuesheng Huang eXNodWFuZzE5NThAMTYzLmNvbQ== Hongwang Cui Y3FjaHcyMDEzQHNpbmEuY29t Shaowen Cheng Y2hlbmdzaGFvd2VuMUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
 Jiangling Yao1,2,†
Jiangling Yao1,2,† Jian Yang
Jian Yang Linyang Zheng
Linyang Zheng Yuesheng Huang
Yuesheng Huang Shaowen Cheng
Shaowen Cheng