- 1Department of Pathology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, China
- 3Department of Pathology, Longquanyi District of Chengdu Maternity and Child Health Care Hospital, Chengdu, China
- 4Department of Robotic Minimally Invasive Surgery Center, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
Background: Uterine leiomyomas are the most common gynecological tumors in women of child-bearing age and premenopausal women, while benign metastasizing leiomyomas of the heart are rare.
Case presentation: We report a rare case of metastasizing leiomyoma in the heart of a 54-year-old woman 10 years after a uterine leiomyoma was discovered during hysterectomy. Echocardiography, cardiac plain scan and enhanced MRI at presentation showed a soft tissue signal mass in the right ventricle. A large cardiac mass attached to the chordae of the tricuspid valve and later shown to be histopathologically consistent with uterine leiomyoma was successfully resected through a right atriotomy.
Conclusions: Our case report highlights a rare type of tumor of the heart and suggests that metastasizing leiomyoma should be considered in the differential diagnosis of right-sided cardiac tumors. The complete surgical resection of the tumor was considered to be the best treatment.
Introduction
Primary cardiac tumors are rare, and most cardiac tumors are secondary to metastatic disease (1). Overall, smooth muscle tumors of the heart are extremely rare. Most of the smooth muscle tumors are occurring in reproductive women who have a history of uterine leiomyoma resection or hysterectomy (2). The presenting symptoms of cardiac tumor depend on the size, location of the mass, and eventual obstruction to the inflow or outflow tracts. Most cardiac tumors are asymptomatic, and diagnosis is made on the basis of heart murmur, arrythmias. Echocardiography, cardiac magnetic resonance imaging (MRI), and computed tomography (CT) usually are useful for establishing the diagnosis, although pathological diagnosis remains the gold standard for diagnostic confirmation (3). Here, we report a rare case of metastasis of uterine leiomyoma to the heart. The aim of this report is to increase awareness of this metastatic tumor. This study was reported in agreement with principles of the CARE guidelines (4).
Case presentation
A 54-year-old woman presented to the hospital due to with a heart murmur detected at a routine examination. On physical examination, the patient's general condition and vital signs were quite unremarkable; only the 2nd to 3rd costal segment of the left margin of the sternum appeared abnormal, and 3/6 systolic blow-like murmurs could be heard; pericardial frictional sounds were not heard, and there was no edema in either lower limb. Echocardiography indicated right ventricular space occupation. Cardiac plain scan and enhanced MRI showed soft tissue signal mass shadows in the outflow tract of the right ventricle (Figure 1). CT angiography showed no macrovascular lesions. What's more is that patient had undergone hysterectomy in a local hospital 10 years previously due to excessive vaginal bleeding from uterine leiomyoma. The family histories were noncontributory to the diagnosis.
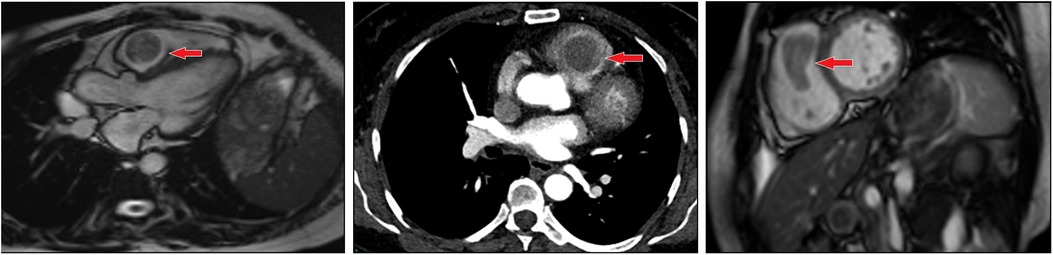
Figure 1. Imaging of metastasizing leiomyoma. Enhanced MRI revealed soft tissue signal mass shadows in the outflow tract of the right ventricle.
The patient underwent right ventricular mass resection under general anesthesia with low-temperature extracorporeal circulation. The patient recovered well after the surgery. The surgical findings showed that there was a 3 cm × 3 cm oblong tomato-like mass with a smooth surface and tough texture occupying the right ventricle. The tumor pedicle was connected to the chordae of the tricuspid valve and protruding into the right ventricular outflow tract, but other portions of the tumor had no adhesion to the heart. The specimens were laid out along their longitudinal axes and fixed in 10% neutral formalin for 12 h. Ten representative tissue samples were taken from different areas, dehydrated, embedded in paraffin, and sequentially sectioned into 3-μm-thick sections. The sections were incubated with primary antibodies against desmin, ER, PR, myogenin, MyoD1, caldesmon, pan-CK, Ki-67, SMA, CD117, FH, RB-1, MDM2, and CD34. PBS replaced the primary antibody as a negative control. DAB color development and hematoxylin counterstaining were performed. The primary antibodies and the kit were purchased from Beijing Zhongshan Jinqiao Company.
On histopathologic examination, the tumor appeared as a grayish-white tubercle, with no exact capsule; it was solid and tough and measured 3 cm × 3 cm × 1.5 cm in size (Figure 2). Under the microscope, the spindle cells within the tumor showed clear boundaries, mild cell morphology, fascicular arrangement, and no mitotic figures (Figure 3). Immunohistochemical staining for desmin, caldesmon, ER, PR and SMA was positive, and the Ki-67 index was approximately 2% (Figure 4). These morphologic features and the immunohistochemical staining pattern confirmed a diagnosis of benign metastasizing leiomyoma to the heart.

Figure 2. Macropathology of metastasizing leiomyoma. The leiomyoma appeared as a solid, tough, grayish-white tubercle with no exact capsule.
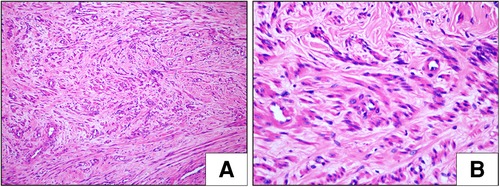
Figure 3. Histological examination (HE) of metastasizing leiomyoma. (A,B) The spindle cell tumor showed clear boundaries, mild cell morphology, fascicular arrangement, and no mitotic figures (H&E staining, A: ×100, B: ×400).

Figure 4. Immunohistochemical (IHC) features of metastasizing leiomyoma. (Envision × 400) The spindle cells expressed desmin (A), caldesmon (B), PR (C), ER (D) and SMA (E). The calculated Ki67 labeling index was less than approximately 2% (F).
Discussion
Case review
Benign metastasizing leiomyoma is very rare. Benign metastasizing leiomyoma involving the heart was first reported by Timmis in 1980, and at least 8 other cases were subsequently reported (Table 1).
Clinical manifestations, diagnosis, treatment and prognosis
Metastasizing leiomyomas of the heart are most common in middle-aged women. Most patients with metastasizing leiomyoma have a history of hysterectomy/myomectomy combined with an existing hysteromyoma. In the case described here, the patient had a history of hysteromyoma resection, and the time from the first hysteromyoma resection to the discovery of cardiac leiomyoma was 10 years. The clinical symptoms of metastasizing leiomyoma are nonspecific; the most common symptoms are dyspnea, syncope, edema of the lower extremities, and palpitations. The patient described here had no clinical symptoms, and the leiomyoma was found by chance during physical examination. TEE, CT and MRI can effectively assist preoperative diagnosis and guide surgical treatment. Although clinical manifestations and imaging can provide early and accurate assessment of the disease, the final diagnosis depends on postoperative pathological examination. Patients in whom there was complete tumor resection had a good prognosis, and no postoperative recurrence or death was reported. Postoperative recurrence is common in patients with incomplete tumor resection. In cases in which the tumor cannot be completely removed, some researchers have suggested the use of antiestrogen drugs (including tamoxifen and letrozole) after surgery. Awonuga (13) and Rivera (14) found that the tumor subsided after the use of antiestrogen drugs after surgery. In our case, the patient underwent right ventricular mass resection by surgery, and have not received any medication. Postoperative cardiac MRI indicated that the mass was completely removed, and there was no recurrence after 12 months follow-up.
Pathological etiology
The behavior of cardiac leiomyoma is benign. Microscopically, the tumor in our patient was composed of fasciculate spindle smooth muscle cells with scattered stroma in small blood vessels. No obvious bleeding or necrosis was observed, and nuclear atypia, pleomorphism and mitoses were also rare. The rare types of pathological variation previously reported include angiomyoma (15), adipose leiomyoma (16), lymphangiomyoma (17) and borderline leiomyoma (18). In our case, immunohistochemistry showed positive results for smooth muscle markers such as SMA and desmin, and the lack of expression of vimentin ruled out a myofibroblastic nature of the tumor. Intrauterine tumors have histopathological and immunohistochemical characteristics that are the same as those of cardiac leiomyomas originating in the uterus. Some researchers have conducted molecular genetic analysis of cardiac leiomyoma; the results of those studies showed that almost all of the analyzed samples had abnormal genotypes such as 45, XX, Der (14) T (12;14) (Q15; Q24) (19), However, as cardiac leiomyomas are clinically rare, their etiology remains to be further studied.
Histological origin
At present, there are two views on the histological occurrence of leiomyomas. One is that the tumor originates from uterine fibroids and extends into veins; this is the case for the metastasizing leiomyomas reported in most studies (20–22). The second is that primary cardiac leiomyomas may originate from the smooth muscle tissue of the vein wall. In this case, the tumor extends upward along the venous system and may exceed the inferior vena cava and enter the right atrium, right ventricle and even the pulmonary artery (15). In our case, medical history, cardiac imaging findings and the immunohistochemical staining pattern confirmed a diagnosis of benign metastasizing leiomyoma originated from uterine fibroids.
Differential diagnosis
The differential diagnosis of cardiac leiomyoma mainly includes cardiac myxoma, cardiac leiomyosarcoma, and other metastasizing cardiac tumors. Cardiac myxoma mostly occurs in the left cardiac system, with an incidence of 87.3% to 85.5%; in this respect, it differs from cardiac leiomyoma, which only affects the right cardiac system (23–25). Most atrial myxomas are pedicled to the atrial septum or wall and may be adherent to the wall. Histological morphology, preoperative imaging examination and history of uterine leiomyoma are helpful for differential diagnosis. Leiomyosarcomas in the heart are malignant tumors with similar clinical and imaging manifestations as leiomyomas of the heart, and the two need to be differentiated through postoperative pathology and immunohistochemical diagnosis. Moreover, leiomyosarcomas have the behavioral characteristics of malignant tumors and often metastasize to other organs or sites such as the lung, lymph nodes, bone, skeletal muscle, subcutaneous tissue and retroperitoneal space but do not extend and grow in the venous system (26). Other metastasizing cardiac neoplasms often invade the pericardium or myocardial tissue, and their primary foci can be determined by systemic radionuclide scanning.
Conclusion
Although metastasizing leiomyoma is rare, this tumor should be considered in the differential diagnosis of cardiac tumors, especially in patients with a history of uterine fibroids. Future research is needed to better understand the pathogenesis and treatment of such tumors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LXL and LJ contributed equally to this work; LXL and LJ wrote the manuscript; ZH and HSY was responsible for literature search and proofreading; RSQ collected the clinicopathologic data. All authors contributed to the article and approved the submitted version.
Funding
The Universal Application Project of Sichuan Provincial Health and Family Planning Commission of China (serial number 16PJ490). The funder is Xing-Lan Li, who wrote the manuscript. Doctoral Science Foundation of Sichuan Provincial People's Hospital (serial number 2015BS08). The funder is Xing-Lan Li, who wrote the manuscript. Key research and development projects of Sichuan Science and Technology Department (serial number 2022YFS0135). The funder is Shang-Qing Ren, who collected the clinicopathologic data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Galvin SD, Wademan B, Chu J, Bunton RW. Benign metastasizing leiomyoma: a rare metastatic lesion in the right ventricle. Ann Thorac Surg. (2010) 89(1):279–81. doi: 10.1016/j.athoracsur.2009.06.050
2. Kayser K, Zink S, Schneider T, Dienemann H, Andre S, Kaltner H, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Archiv. (2000) 437(3):284–92. doi: 10.1007/s004280000207
3. Cho JM, Danielson GK, Puga FJ, Dearani JA, McGregor CG, Tazelaar HD, et al. Surgical resection of ventricular cardiac fibromas: early and late results. Ann Thorac Surg. (2003) 76(6):1929–34. doi: 10.1016/S0003-4975(03)01196-2
4. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE Guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
5. Timmis AD, Smallpeice C, Davies AC, Macarthur AM, Gishen P, Jackson G. Intracardiac spread of intravenous leiomyomatosis with successful surgical excision. N Engl J Med. (1980) 303(18):1043–4. doi: 10.1056/NEJM198010303031806
6. Takemura G, Takatsu Y, Kaitani K, Ono M, Ando F, Tanada S, et al. Metastasizing uterine leiomyoma. A case with cardiac and pulmonary metastasis. Pathol Res Pract. (1996) 192(6):622–33. doi: 10.1016/S0344-0338(96)80116-6
7. Cai A, Li L, Tan H, Mo Y, Mo Y, Zhou Y. Benign metastasizing leiomyoma. Case report and review of the literature. Herz. (2014) 39(7):867–70. doi: 10.1007/s00059-013-3904-1
8. Consamus EN, Reardon MJ, Ayala AG, Schwartz MR, Ro JY. Metastasizing leiomyoma to heart. Methodist Debakey Cardiovasc J. (2014) 10(4):251–4. doi: 10.14797/mdcj-10-4-251
9. Williams M, Salerno T, Panos AL. Right ventricular and epicardial tumors from benign metastasizing uterine leiomyoma. J Thorac Cardiovasc Surg. (2016) 151(2):e21–4. doi: 10.1016/j.jtcvs.2015.09.059
10. Meddeb M, Chow RD, Whipps R, Haque R. The heart as a site of metastasis of benign metastasizing leiomyoma: case report and review of the literature. Case Rep Cardiol. (2018) 2018:7231326. doi: 10.1155/2018/7231326
11. Gad MM, Găman MA, Bazarbashi N, Friedman KA, Gupta A. Suspicious right heart mass: a rare case of benign metastasizing leiomyoma of the tricuspid valve. JACC Case Rep. (2020) 2(1):51–4. doi: 10.1016/j.jaccas.2019.12.004
12. Karnib M, Rhea I, Elliott R, Chakravarty S, Al-Kindi SG. Benign metastasizing leiomyoma in the heart of a 45-year-old woman. Tex Heart Inst J. (2021) 48(1):e197066. doi: 10.14503/THIJ-19-7066
13. Awonuga AO, Shavell VI, Imudia AN, Rotas M, Diamond MP, Puscheck EE. Pathogenesis of benign metastasizing leiomyoma: a review. Obstet Gynecol Surv. (2010) 65(3):189–95. doi: 10.1097/OGX.0b013e3181d60f93
14. Rivera JA, Christopoulos S, Small D, Trifiro M. Hormonal manipulation of benign metastasizing leiomyomas: report of two cases and review of the literature. J Clin Endocrinol Metab. (2004) 89(7):3183–8. doi: 10.1210/jc.2003-032021
15. Tamburino C, Russo G, Incognito C, Battaglia G, Monaca V, Lomeo A, et al. Intracardiac extension of a calcified ovarian hemangioma–a case report. Angiology. (1992) 43(3Pt1):249–52. doi: 10.1177/000331979204300310
16. Vural C, Özen Ö, Demirhan B. Intravenous lipoleiomyomatosis of uterus with cardiac extension: a case report. Pathol Res Pract. (2011) 207(2):131–4. doi: 10.1016/j.prp.2010.10.004
17. Stancanelli B, Seminara G, Vita A, Pantò A, Romano M. Extension of a pelvic tumor into the right atrium. N Engl J Med. (1995) 333(15):1013–4. doi: 10.1056/NEJM199510123331519
18. Lam PM, Lo KW, Yu MM, Lau TK, Cheung TH. Intravenous leiomyomatosis with atypical histologic features: a case report. Int J Gynecol Cancer. (2003) 13(1):83–7. doi: 10.1046/j.1525-1438.2003.13008.x
19. Quade BJ, Dal Cin P, Neskey DM, Weremowicz S, Morton CC. Intravenous leiomyomatosis: molecular and cytogenetic analysis of a case. Mod Pathol. (2002) 15(3):351–6. doi: 10.1038/modpathol.3880529
20. Gonzalez-Lavin L, Lee RH, Falk L, Gradman MD, McFadden PM, Basso LV, et al. Tricuspid valve obstruction due to intravenous leiomyomatosis. Am Heart J. (1984) 108(6):1544–6. doi: 10.1016/0002-8703(84)90705-1
21. Akatsuka N, Tokunaga K, Isshiki T, Asano K, Funaki H, Mizuno M, et al. Intravenous leiomyomatosis of the uterus with continuous extension into the pulmonary artery. Jpn Heart J. (1984) 25(4):651–9. doi: 10.1536/ihj.25.651
22. Ohmori T, Uraga N, Tabei R, Abe M, Sumimoto T, Hamada M, et al. Intravenous leiomyomatosis: a case report emphasizing the vascular component. Histopathology. (1988) 13(4):470–2. doi: 10.1111/j.1365-2559.1988.tb02066.x
23. Steger CM, Hager T, Ruttmann E. Primary cardiac tumours: a single-center 41-year experience. ISRN Cardiol. (2012) 2012:906109. doi: 10.5402/2012/906109
24. Pacini D, Careddu L, Pantaleo A, Berretta P, Leone O, Marinelli G, et al. Primary benign cardiac tumours: long-term results. Eur J Cardio-Thorac Surg. (2012) 41(4):812–9. doi: 10.1093/ejcts/ezr067
25. Wang JG, Li YJ, Liu H, Li NN, Zhao J, Xing XM. Clinicopathologic analysis of cardiac myxomas: seven years’ experience with 61 patients. J Thorac Dis. (2012) 4(3):272–83. doi: 10.3978/j.issn.2072-1439.2012.05.07
26. Canzonieri V, D’Amore ES, Bartoloni G, Piazza M, Blandamura S, Carbone A. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Archiv. (1994) 425(5):541–5. doi: 10.1007/BF00197559
Keywords: metastasizing leiomyoma, heart, case report, surgery, outcome
Citation: Li J, Zhu H, Hu S, Ren S and Li X (2022) Case report: Cardiac metastatic leiomyoma in an Asian female. Front. Surg. 9:991558. doi: 10.3389/fsurg.2022.991558
Received: 11 July 2022; Accepted: 8 August 2022;
Published: 23 August 2022.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Mihnea-Alexandru Găman, Carol Davila University of Medicine and Pharmacy, RomaniaGaetano Thiene, University of Padua, Italy
© 2022 Li, Zhu, Hu, Ren and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing-Lan Li bHhsMTk4NjA0MjFAMTI2LmNvbQ==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Abbreviations ER, estrogen receptor; PR, progesterone receptor; SMA, smooth muscle actin; FH, fumarate hydratase; TEE, transesophageal echocardiography; CT, Computed Tomography; MRI, Magnetic Resonance Imaging.
 Juan Li1,2
Juan Li1,2 Shang-Qing Ren
Shang-Qing Ren