
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg., 12 August 2022
Sec. Genitourinary Surgery and Interventions
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.990049
This article is part of the Research TopicTelemedicine and Telementoring in Urology PracticeView all 7 articles
 Xingjun Bao1,2,†
Xingjun Bao1,2,† Fengze Sun2,†
Fengze Sun2,† Huibao Yao2
Huibao Yao2 Di Wang2
Di Wang2 Hongquan Liu2
Hongquan Liu2 Gonglin Tang2
Gonglin Tang2 Xiaofeng Wang2
Xiaofeng Wang2 Zhongbao Zhou3
Zhongbao Zhou3 Jitao Wu2*
Jitao Wu2* Yuanshan Cui2*
Yuanshan Cui2*
Background: Most patients suffer from ureteral stent-related symptoms (USRS) caused by indwelling ureteral stents. Nevertheless, various medications to alleviate discomfort as well as novel stents are continually being developed, and in recent years, some researchers have believed that proper intravesical stent placement can relieve USRS.
Objective: To determine appropriate intravesical ureteral stent position may alleviate USRS.
Methods: Up to May 1, 2022, the PubMed, Embase, Scopus and Web of Science databases were thoroughly searched, and two independent reviewers included relevant studies that met the PICO (Patient, Intervention, Comparison, Outcome) criteria. Studies methodological quality were assessed by ROB2 and ROBINS-I. Ureteral stent symptom questionnaire (USSQ), international prostate symptom score (IPSS) and quality of life (QoL) was used to quantify the USRS. According to intravesical ureteral stent position, Group A was defined as the contralateral group, that is distal end of ureteral stent crossed the bladder midline, whereas Group B was classified as ipsilateral group, meaning stent end did not cross the midline.
Results: Six studies incorporating a total of 590 patients were eligible. In terms of USSQ score, the meta-analysis showed that contralateral group was associated with a significant increase in USSQ total (MD, 17.55; 95% CI, 12.04 to 23.07; P < 0.001), urinary symptoms (MD, 2.74; 95% CI, 0.48 to 5.01; P = 0.02), general health (MD, 4.04; 95% CI, 2.66 to 5.42; P < 0.001), work performance (MD, 1.36; 95% CI, 0.75 to 1.98; P < 0.001) and additional problems (MD, 0.89; 95% CI, 0.47 to 1.32; P < 0.001) scores while not associated with a significant increase in body pain (MD, 3.13; 95% CI, −0.19 to 6.44; P = 0.06) and sexual matters (MD, 1.01; 95% CI, −0.03 to 2.06; P = 0.06). As for IPSS, although no significant differences in IPSS total (MD, 2.65; 95% CI, −0.24 to 5.54; P = 0.07) or voiding symptoms (MD, −0.84; 95% CI, −3.16 to 1.48; P = 0.48) scores were found, ipsilateral group was associated with a significant decrease in storage symptoms (MD, 1.92; 95% CI, 0.91 to 2.93; P = 0.0002). Furthermore, ipsilateral group was linked to a significant decrease in QoL score (MD, 1.00; 95% CI, 0.18 to 1.82; P = 0.02).
Conclusion: This meta-analysis proven that correct intravesical stent position was critical, and patients with stents crossing the midline experienced more severe USRS than those who did not. Further high-quality randomized controlled trials are needed to corroborate our findings.
Double-J ureteral stent (DJUS), also known as double-pigtail stent, is now the most often utilized stent type in urology. With the advantages of its security and convenience, DJUS was extensively employed in the adjuvant treatment of urolithiasis, the release of upper urinary tract obstruction caused by various reasons and the expansion treatment of ureteral stenosis (1). The history of DJUS may be traced back to 1978, when Finney first revealed its benefits and application experience (2). Notwithstanding, because its material was not absorbable, an indwelling stent will ultimately induce urinary discomfort and even complications. It was no exaggeration to say that over 80% of patients with ureteral stents suffered one or more urinary tract symptoms, especially storage symptoms, urinary incontinence, dysuria and hematuria (3).
Causes and mechanisms of ureteral stent-related symptoms (USRS) remain unclear. The current studies supported the conclusion that various parameters, including stent design (4, 5), material (6), diameter (7), length and position (8, 9), may be related to the USRS. Moreover, some researchers thought that mechanical stimulation and retrograde pressure transmission from a stent were the core causes of USRS (10, 11). Several studies have focused on the relationship between stent position and USRS have appeared in recent years. Most researchers concurred that if the distal end of DJUS crossed the bladder midline, individuals would experience more severe USRS than those who did not (8, 12, 13). Furthermore, Lee and colleagues suspected that the intravesical appropriate stent placement was much more effective than drugs treatment for alleviating the USRS (14). In contrast, Abt et al. demonstrated stent position did not significantly influence the USRS (15). Some meta-analyses focusing on stent diameter and length have reported up to now, but there was still a void for stent position (16, 17).
Since its inception in 2003, the ureteral stent symptom questionnaire (USSQ) has been regarded as a sensitive and comprehensive tool for assessing USRS (18). Despite its lack of specificity, the International Prostate Symptom Score (IPSS) was commonly utilized in this evaluation (19). In our meta-analysis, we first used USSQ, IPSS and quality of life (QoL) scores to assess whether the distal end of DJUS crossing the bladder midline resulted in more severe USRS than those not crossing.
We performed this systematic review and meta-analysis in accordance with the latest Preferred Reporting Items for Systematic Reviews of Interventions (PRISMA 2020) statements. Supplementary file provided the completed PRISMA 2020 checklist.
In PubMed, Embase, Scopus and Web of Science databases, search terms (“ureteral stent” AND “midline”) OR (“stent position” AND “symptoms”) were retrieved, and all literatures acquired up to 1 May 2022 were systematically reviewed. Case reports, editorials, conference abstracts, and non-English literature were all barred from consideration. Relevant articles from the selected articles' reference lists were also searched and reviewed. Two authors included relevant studies based on the PICO (Patient, Intervention, Comparison, Outcome) criteria. Any disagreements were resolved by discussion with a third author. The PRISMA flowchart is shown in Figure 1.
The study selection followed the PICO model (Patients: individuals with ureteral stents; Intervention: the distal end of ureteral stent crossed the midline of bladder; Comparison: the distal end of ureteral stent did not cross the midline of bladder; Outcomes: USSQ, IPSS and QoL). Furthermore, all included patients completed questionnaires and performed a plain radiograph of the kidney-ureter-urinary bladder prior to the stent removal procedure. The bladder midline was defined as a vertical line through the midline gap of the pubic symphysis based on imaging. Group A was defined as the contralateral group, that is distal end of ureteral stent crossed the bladder midline, whereas Group B was classified as ipsilateral group, meaning stent end did not cross the midline.
We used the revised Cochrane risk of bias tool for randomized trials (ROB2) and the risk of bias tool for non-randomized studies of interventions (ROBINS-I) to assess the risk of bias in randomized controlled trials (RCTs) and non-RCTs, respectively (20, 21). Disagreements among reviewers were resolved by consensus.
The following data was gathered from the included studies: (1) First author's name and year of publication; (2) country of study; (3) sample size in each study group; (4) type, diameter, length and retention period of the stent; (5) primary outcomes, including USSQ, IPSS and QoL; and (6) age of the included population and indications of stent indwelling. Two authors worked independently to finish the procedure.
Outcomes analysis was performed with RevMan v.5.4.0 (Cochrane Collaboration, Oxford, UK). Using the Quantile Estimation (QE) method recently developed by McGrath et al. (22), the interquartile range was turned into mean and standard deviation (SD). The formula given by Zhang et al. was used to combine SD of different subgroups (23). The mean difference (MD) with 95% confidence interval (CI) was employed to describe continuous outcomes. The I-square (I2) and Q tests were used to assess heterogeneity among studies included. The random-effect model was utilized if the heterogeneity was considerable (P < 0.05 and I2 ≥ 50%). In contrast, fixed-effect model was selected for meta-analysis. For the overall effect, a P < 0.05 value was considered statistically significant.
A total of 224 articles were retrieved. Following a review of the title and abstract, 214 papers were excluded. After further examination of the full-text, 4 articles (8, 9, 12, 24)were excluded due to the absence of available data. The remaining 6 papers (13–15, 25–27)were eventually included in the meta-analysis. Three of the included studies were randomized controlled trials (RCTs) (13, 14, 25), and three were prospective observational studies (15, 26, 27). The characteristics of the included studies are summarized in Table 1.
Figure 2 shows the detailed results of the risk of bias. Two RCTs (13, 25) explained their randomization protocol, and one study (13) performed intention-to-treat (ITT) analysis. According to RoB2, two (13, 25) of the three RCTs were classified as having a low risk of bias and one (14) as having some concerns due to an uncertain randomization sequence. Based on RoBIN-I, one non-RCT (27) had a critical risk of bias due to strong confounding variables and reporting bias. The remaining two non-RCTs (15, 26) were classified as having a serious risk of bias and moderate risk of bias, respectively.
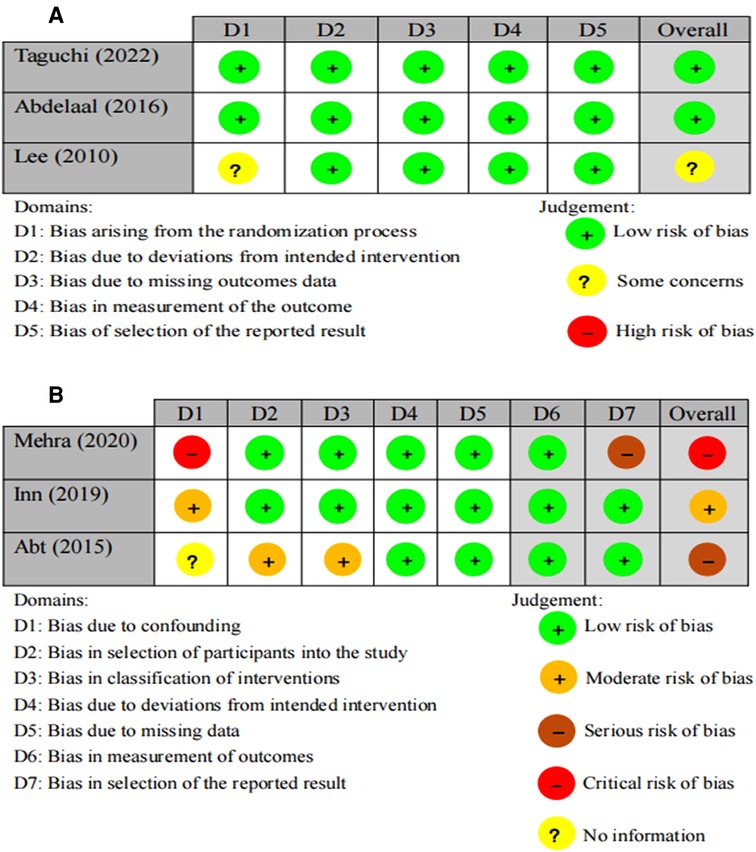
Figure 2. Risk of bias graph of the included studies. (A) Risk of bias rating of RCTs using ROB2. (B) Risk of bias rating of non-RCTs using ROBIN-I.
According to plain radiograph of the kidney-ureter-urinary bladder, patients with indwelling stents crossing the bladder midline were defined as group A, whereas patients with indwelling stents not crossing the midline were classified as group B.
Three studies (15, 25, 26), incorporating a total of 277 patients (113 in group A and 164 in group B), revealed the differences in USSQ total and additional problems score. There was no heterogeneity (P = 0.80, I2 = 0%) and low heterogeneity (P = 0.15, I2 = 48%) among studies, hence the fixed-effect model was used for both analyses. The MDs was 17.55 (95% CI, 12.04 to 23.07; P < 0.001) and 0.89 (95% CI, 0.47 to 1.32; P < 0.001), respectively, as seen in Figures. 3A,G. These results demonstrated patients in group A experienced more severe discomfort than group B.
Five studies (13, 15, 25–27), incorporating a total of 537 patients (283 in group A and 254 in group B), revealed the difference in urinary symptoms scores. Because of the considerable heterogeneity (P = 0.0006, I2 = 80%) among studies, the random-effect model was used. Compared with Group B, Group A was significantly associated with a higher score (MD, 2.74; 95% CI, 0.48 to 5.01; P = 0.02), as seen in Figure 3B. We came to the conclusion that patients with indwelling stents that did not cross the midline had better urinary symptoms.
Four studies (15, 25–27) disclosed changes in the score of the other four USSQ subgroups. The random-effect model was only employed to body pain score owing to the significant heterogeneity among studies (P = 0.008, I2 = 74%), yet no significant difference (MD, 3.13; 95% CI, −0.19 to 6.44; P = 0.06) was found between the Group A (186 patients) and Group B (184 patients), as seen in Figure 3C. Furthermore, there were significant differences in general health (MD, 4.04; 95% CI, 2.66 to 5.42; I2 = 0%; P < 0.001) and work performance (MD, 1.36; 95% CI, 0.75 to 1.98; I2 = 0%; P < 0.001) scores while no significant difference in sexual matters (MD, 1.01; 95% CI, −0.03 to 2.06; I2 = 48%; P = 0.06) score. No heterogeneity was found among studies and the fixed-effect models were selected in the three subgroups, which included a total of 434 (224 in group A and 210 in group B), 420 (212 in group A and 208 in group B) and 398 patients (195 in group A and 203 in group B) separately, as seen in Figures 3D–F. All in all, except for body pain and sexual matters, patients with indwelling stents not crossing the midline reported greater satisfaction in general health and work performance than patients with indwelling stents crossing the midline.
Two studies (13, 14), incorporating a total of 121 patients (65 in group A and 56 in group B), revealed the changes in IPSS total and it subgroups score. No heterogeneity (I2 = 0%) was found among studies, and the fixed-effect models were applied to meta-analysis. IPSS total and voiding symptoms scores by a mean of 2.65 (95% CI, −0.24 to 5.54; P = 0.07) and −0.84 (95% CI, −3.16 to 1.48; P = 0.48) respectively were no significant differences, as seen in Figure 4A,B. Intriguingly, the MD of storage symptoms subscore was 1.92 (95% CI, 0.91 to 2.93; P = 0.0002), as shown in Figure 4C. Although IPSS total score and subscore of group A were higher than group B, we only had evidence to conclude that individuals in group A experienced more severe storage symptoms.
Two studies (13, 14), incorporating a total of 121 patients (65 in group A and 56 in group B). Revealed the difference in QoL score. There was no heterogeneity (P = 0.74, I2 = 0%) among studies, so the fixed-effect model was used for the meta-analysis. The results of integrative data analysis revealed that patients in Group B were associated with a significant decrease in QoL score (MD, 1.00; 95% CI, 0.18 to 1.82; P = 0.02; Figure 4D). Therefore, patients in group B had a greater quality of life than group A.
Despite the wide range of indications for DJUS, the ensuing USRS were indeed vexing (1). Hao et al. showed that approximately 19.6% of individuals with ureteral stents experienced one or more discomforts, whereas Joshi and colleagues reported that up to 80% of patients with ureteral stents suffered a variety of urinary symptoms, with storage symptoms, incontinence, dysuria and hematuria being the most bothersome (3, 28). However, the pathogenesis of USRS has not been fully elucidated to date. It has been suggested that stent-related flank pain was due to the backflow of urine from the stent into the renal collecting system during urination. In addition, stent-related irrigative symptoms may be attributed to irritation of the mucosa of the bladder associated with stent migration due to active during the day (1, 10). In a word, the mechanisms of the USRS were still poorly studied, and treatment options for USRS were limited.
Although pharmacologic interventions were the mainstay of treatment for USRS, it adverse effects caused some patients to fail to take their prescription (29). At present, experts studies have revealed that stent material, shape, diameter, length, and position all had the potential to influence the USRS (13). RANE et al. (24) proposed in 2001 that stent position was linked with the USRS. This study included 60 patients showed that the incidence of urinary urgency and asymptomatic cases was as high as 72% and 33.3% respectively in the contralateral group compared to 33.3% and 66.6%, respectively, in the ipsilateral group, and the differences were statistically significant. Furthermore, AL-KANDARI et al. research (8), which included 120 individuals, reported that 53 individuals (88%) in the contralateral group had moderate to severe dysuria compared to 11 individuals (18%) in the ipsilateral group (P < 0.001). It was noteworthy that correct intravesical stent placement has been repeatedly proven to improve the USRS (13, 26, 27). Thus we included 6 studies with 590 individuals to explore the impact of stent position on the USRS using a meta-analysis of USSQ, IPSS, and QoL score. This is, to the best of our knowledge, the first literature review and meta-analysis evaluating the effect of stent position on the USRS. The analyses demonstrated that contralateral group had higher USSQ, storage symptoms, and QoL scores than ipsilateral group. This also served as a reminder to urologists to carefully inspect indwelling stents to ensure that they were in the proper location.
Besides, a retrospective study (12) found that the contralateral group had worse overactive bladder symptom score (OABSS) total score and sub scores than the ipsilateral group, and multivariate analysis revealed that stent position was an independent predictor of the USRS. Remarkably, Lee et al. (14) demonstrated that correct stent position was more significant than medication treatment for relieving the USRS in a prospective randomized study. This begs the question, what exactly causes patients with stent crossing the midline of the bladder to have more severe USRS?
Distal end of ureteral stent crossing the midline was associated with more severe USRS, possibly as a result of direct physical contact with the intravesical stent with the contralateral bladder wall (30). Our study demonstrated the contralateral group had more severe storage symptoms, which were directly related to irritation of the bladder trigone. It was not difficult to imagine that intravesical stent crossing the midline would increase the risk of irritation to the bladder trigone, especially when the patient was active (11). Interestingly, there was no significant link between ureteral stent length and intravesical stent position. This might be due to the fact that intravesical stent location varies with time and patient position, and a study has indicated that shifts from ipsilateral through midline to contralateral were more prevalent, which could also explain why around 80% of patients suffered USRS (31). A study (17) found that there was no significant correlation between stents with small diameter and stent migration, therefore whether using stents with smaller diameters and without crossing the bladder midline may effectively relieve USRS has to be examined further.
In recent years, drug-eluting expandable metal stents and biodegradable stents have emerged owing to the prevalence of USRS, stent encrustation, stent migration and stent-related urinary tract infection (32, 33). To promote stent development and avoid ureteral stent migration to the contralateral side to trigger severe USRS, can we focus stent innovation on limiting stent migration? All in all, stent related technology is constantly improving, and we will be able to totally eradicate stent related symptoms.
Our analysis had apparently limits. We were unable to incorporate more high-quality RCTs to support our findings due to a paucity of previous research. The studies included in the meta-analysis may have biases. The patient characteristics, stent parameters, stent duration, and questionnaire scoring time was not consistent. These variables may have an impact on the primary outcome of our study. But, to our knowledge, this was the first systematic review and meta-analysis assessing the effect of stent position on the USRS.
In conclusion, our meta-analysis revealed that patients with stents crossing the midline suffered more severe discomforts in subgroups such as urinary symptoms, general health, work performance, additional problems, storage symptoms, and QoL. When indwelling a ureteral stent, urologists must take the time to ensure that the stent is properly positioned. However, better quality randomized controlled trials are urgently required to validate our outcomes.
The original contributions presented in the study are included in the article/Suplementary Material, further inquiries can be directed to the corresponding author/s.
Guarantor of integrity of the entire study: XB, FS, JW; Conceptualization: JW, YC; Literature search: XB, FS, HL; Data collection: XB, DW, HY, GT; Statistical analysis: XB, ZZ, XW; Manuscript preparation: XB, FS; Manuscript editing and revising: XB, FS. All authors contributed to the article and approved the submitted version.
This work was supported by Joint fund of Shandong Natural Science Foundation, under Code ZR2021LSW019, grants from the National Nature Science Foundation of China (Nos. 81870525; 81572835) and Taishan Scholars Program of Shandong Province (No. tsqn201909199).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.990049/full#supplementary-material.
1. Miyaoka R, Monga M. Ureteral stent discomfort: etiology and management. Indian J Urol. (2009) 25(4):455–60. doi: 10.4103/0970-1591.57910
2. Finney RP. Experience with new double J ureteral catheter stent. J Urol. (1978) 120(6):678–81. doi: 10.1016/S0022-5347(17)57326-7
3. Joshi HB, Okeke A, Newns N, Keeley FX Jr, Timoney AG. Characterization of urinary symptoms in patients with ureteral stents. Urology. (2002) 59(4):511–6. doi: 10.1016/S0090-4295(01)01644-2
4. Lingeman JE, Preminger GM, Goldfischer ER, Krambeck AE, Comfort Study T. Assessing the impact of ureteral stent design on patient comfort. J Urol. (2009) 181(6):2581–7. doi: 10.1016/j.juro.2009.02.019
5. Taguchi M, Inoue T, Muguruma K, Murota T, Kinoshita H, Matsuda T. Impact of loop-tail ureteral stents on ureteral stent-related symptoms immediately after ureteroscopic lithotripsy: comparison with pigtail ureteral stents. Investig Clin Urol. (2017) 58(6):440–6. doi: 10.4111/icu.2017.58.6.440
6. Bosio A, Alessandria E, Agosti S, Vitiello F, Vercelli E, Bisconti A, et al. Pigtail suture stents significantly reduce stent-related symptoms compared to conventional Double J stents: a prospective randomized trial. Eur Urol Open Sci. (2021) 29:1–9. doi: 10.1016/j.euros.2021.03.011
7. Taguchi M, Yoshida K, Sugi M, Kinoshita H, Matsuda T. Effect of ureteral stent diameter on ureteral stent-related symptoms. Low Urin Tract Symptoms. (2019) 11(4):195–9. doi: 10.1111/luts.12259
8. Al-Kandari AM, Al-Shaiji TF, Shaaban H, Ibrahim HM, Elshebiny YH, Shokeir AA. Effects of proximal and distal ends of double-J ureteral stent position on postprocedural symptoms and quality of life: a randomized clinical trial. J Endourol. (2007) 21(7):698–702. doi: 10.1089/end.2007.9949
9. Ho CH, Tai HC, Chang HC, Hu FC, Chen SC, Lee YJ, et al. Predictive factors for ureteral double-J-stent-related symptoms: a prospective, multivariate analysis. J Formos Med Assoc. (2010) 109(11):848–56. doi: 10.1016/S0929-6646(10)60130-1
10. Sameh WM, Eid AA. Pressure transmission through ureteric stents: a novel in vivo human study. Urology. (2012) 79(4):766–70. doi: 10.1016/j.urology.2011.10.056
11. Fischer KM, Louie M, Mucksavage P. Ureteral stent discomfort and its management. Curr Urol Rep. (2018) 19(8):64. doi: 10.1007/s11934-018-0818-8
12. Taguchi M, Yoshida K, Sugi M, Matsuda T, Kinoshita H. A ureteral stent crossing the bladder midline leads to worse urinary symptoms. Cent European J Urol. (2017) 70(4):412–7. doi: 10.5173/ceju.2017.1533
13. Taguchi M, Yasuda K, Kinoshita H. Prospective randomized controlled trial comparing a ureteral stent crossing versus not crossing the bladder midline. World J Urol. (2022) 40(6):1–7. doi: 10.1007/s00345-022-03978-5
14. Lee SJ, Yoo C, Oh CY, Lee YS, Cho ST, Lee SH, et al. Stent position is more important than α-blockers or anticholinergics for stent-related lower urinary tract symptoms after ureteroscopic ureterolithotomy: a prospective randomized study. Korean J Urol. (2010) 51(9):636–41. doi: 10.4111/kju.2010.51.9.636
15. Abt D, Mordasini L, Warzinek E, Schmid HP, Haile SR, Engeler DS, et al. Is intravesical stent position a predictor of associated morbidity? Korean J Urol. (2015) 56(5):370–8. doi: 10.4111/kju.2015.56.5.370
16. Wu G, Sun F, Sun K, Zhang D, Yao H, Wu J, et al. Impact of differential ureteral stent diameters on clinical outcomes after ureteroscopy intracorporeal lithotripsy: a systematic review and meta-analysis. Int J Urol. (2021) 28(10):992–9. doi: 10.1111/iju.14631
17. Diatmika A, Djojodimedjo T, Kloping YP, Hidayatullah F, Soebadi MA. Comparison of ureteral stent diameters on ureteral stent-related symptoms: a systematic review and meta-analysis. Turk J Urol. (2022) 48(1):30–40. doi: 10.5152/tud.2022.21255
18. Joshi HB, Newns N, Stainthorpe A, MacDonagh RP, Keeley FX Jr, Timoney AG. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. (2003) 169(3):1060–4. doi: 10.1097/01.ju.0000049198.53424.1d
19. Pecoraro A, Peretti D, Tian Z, Aimar R, Niculescu G, Alleva G, et al. Treatment of ureteral stent-related symptoms. Urol Int. (2021):1–16. doi: 10.1159/000518387. [Epub ahead of print]34818261
20. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
22. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020):962280219889080. doi: 10.1177/0962280219889080. [Epub ahead of print]32292115
23. Bing ZHANG, Jie KANG, Xm CHEN. Methods to combine standard deviations of diff erent subgroups in meta-analysis. Chinese Journal of evidence based medicine. (2016) 16(7):851–4. doi: 10.7507/1672-2531.20160130.
24. Rane A, Saleemi A, Cahill D, Sriprasad S, Shrotri N, Tiptaft R. Have stent-related symptoms anything to do with placement technique? J Endourol. (2001) 15(7):741–5. doi: 10.1089/08927790152596352
25. Abdelaal AM, Al-Adl AM, Abdelbaki SA, Al Azab MM, Al Gamal KA. Efficacy and safety of tamsulosin oral-controlled absorption system, solifenacin, and combined therapy for the management of ureteric stent-related symptoms. Arab J Urol. (2016) 14(2):115–22. doi: 10.1016/j.aju.2016.01.004
26. Inn FX, Ahmed N, Hou LG, Abidin ZAZ, Yi LL, Zainuddin ZM. Intravesical stent position as a predictor of quality of life in patients with indwelling ureteral stent. Int Urol Nephrol. (2019) 51(11):1949–53. doi: 10.1007/s11255-019-02262-7
27. Mehra K, Manikandan R, Dorairajan LN, Sreenivasan Kodakkattil S, Kalra S. Effect of ureteral stent length and position of stent coil in bladder on stent-related symptoms and quality of life of patients. Cureus. (2020) 12(11):e11669. doi: 10.7759/cureus.11669
28. Hao P, Li W, Song C, Yan J, Song B, Li L. Clinical evaluation of double-pigtail stent in patients with upper urinary tract diseases: report of 2685 cases. J Endourol. (2008) 22(1):65–70. doi: 10.1089/end.2007.0114
29. Park J, Yoo C, Han DH, Shin DW. A critical assessment of the effects of tamsulosin and solifenacin as monotherapies and as a combination therapy for the treatment of ureteral stent-related symptoms: a 2×2 factorial randomized trial. World J Urol. (2015) 33(11):1833–40. doi: 10.1007/s00345-015-1544-1
30. Giannarini G, Keeley FX Jr, Valent F, Manassero F, Mogorovich A, Autorino R, et al. Predictors of morbidity in patients with indwelling ureteric stents: results of a prospective study using the validated Ureteric Stent Symptoms Questionnaire. BJU Int. (2011) 107(4):648–54. doi: 10.1111/j.1464-410X.2010.09482.x
31. Tekin AC, Pratsinis M, Zumstein V, Güsewell S, Schmid HP, Abt D, et al. Intravesical ureteral stent position is highly variable over time and with patient position: an analysis of 1466 radiographic images. Minerva Urol Nephrol. (2021) 73(3):409–11. doi: 10.23736/S2724-6051.20.04224-1
32. Sampogna G, Grasso A, Montanari E. Expandable metallic ureteral stent: indications and results. Minerva Urol Nefrol. (2018) 70(3):275–85. doi: 10.23736/S0393-2249.18.03035-7
Keywords: meta-analysis, ureteral stent, bladder midline, USRS, USSQ, IPSS
Citation: Bao X, Sun F, Yao H, Wang D, Liu H, Tang G, Wang X, Zhou Z, Wu J and Cui Y (2022) Distal end of Double-J ureteral stent position on ureteral stent-related symptoms: A systematic review and meta-analysis. Front. Surg. 9:990049. doi: 10.3389/fsurg.2022.990049
Received: 9 July 2022; Accepted: 1 August 2022;
Published: 12 August 2022.
Edited by:
Nicola Pavan, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyReviewed by:
Mahdi Hadilou, Tabriz University of Medical Sciences, Iran© 2022 Bao, Sun, Yao, Wang, Liu, Tang, Wang, Zhou, Wu and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanshan Cui OTc4OTQ2NzAwQHFxLmNvbQ== Jitao Wu d2p0dXJvbG9neUAxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
Abbreviations DJUS, double-J ureteral stent; USRS, ureteral stent-related symptoms; USSQ, ureteral stent symptom questionnaire; IPSS, international prostate symptom score; QoL, quality of life; MD, mean difference; CI, confidence interval; RCTs, randomized controlled trials.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.