
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 06 January 2023
Sec. Obstetrics and Gynecological Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.988392
Objective: The aim of this study was to determine whether the addition of esketamine to morphine would improve postoperative analgesia after cesarean section.
Methods: Parturients who planned for a cesarean delivery using combined spinal–epidural anesthesia with a request for postoperative anesthesia were randomly divided into four groups (A, B, C, and D). When the surgery was completed, the parturients in groups A, B, C, and D were administered 2 mg morphine, 0.25 mg/kg of esketamine, 0.25 mg/kg of esketamine plus 2 mg morphine hydrochloride, and 0.25 mg/kg of esketamine plus 1 mg morphine through the epidural catheters, respectively. The postoperative pain at rest, pain with movement, the number of rescue analgesics, and adverse effects were evaluated for 48 h after cesarean delivery.
Results: A total of 119 parturients were included in this study, including 30 cases in group A, 30 cases in group B, 30 cases in group C, and 29 cases in group D. All visual analog scale (VAS) scores at rest and with movement were much lower in group C as compared with those in group A and group B (P < 0.05). Moreover, VAS scores at rest were also lower in Group C than in group D for 24 h (P < 0.05). Corresponding to the low pain scores, parturients in group C also required less rescue analgesia as compared with the other three groups (P = 0.021 for C vs. A, P < 0.001 for C vs. B, and P < 0.001 for C vs. D). There were no statistically significant differences between the four study groups with regard to the incidence of adverse events (P > 0.05).
Conclusions: The addition of esketamine to morphine improved postoperative analgesia after cesarean section without increasing the incidence of adverse events.
Cesarean section is the most common surgery in the obstetrics department, accounting for 25%–45% of all births in China (1, 2). Post-cesarean pain control is still a challenge for the postoperative nursing of parturients. Pain after cesarean section can result in a delay in the recovery of parturients and their ability to return to daily functional activities (3), increasing the risk of thromboembolic events and postpartum depression (4, 5). Furthermore, post-cesarean pain can delay the breastfeeding of newborns, affecting the intimate communication between mothers and their infants (6).
Satisfactory post-cesarean pain management has been reported to be associated with a shorter hospital stay, improved mobility, and greater satisfaction in parturients (7). Several pain reduction protocols have been suggested for parturients after cesarean section. Among them, morphine and its derivatives are the most widely used (8). Morphine exerts a rapid analgesic effect and lasts for a long time. Its effect in relieving post-cesarean pain is well established (9, 10). However, the side effects may limit the use of morphine after cesarean section.
Many non-opioid analgesics have been used in combination with morphine to maintain analgesic efficacy, reduce postoperative use of opioids, and decrease opioid-related adverse effects (11). Esketamine, a nonselective N-methyl-D-aspartic acid (NMDA) receptor inhibitor, possesses non-opioid analgesic properties. Esketamine is commonly used for the treatment of resistant depression (6). In recent years, the analgesic effect of esketamine has drawn increasing attention. Lei et al. (12) found that esketamine effectively reduced acute and chronic pain after thoracoscopy and pulmonary surgery under general anesthesia. Nielsen et al. (13) reported that intraoperative esketamine reduced postoperative pain and opioid use after spine surgery. A study conducted by Wang et al. (6) confirmed that esketamine not only reduced pain but also decreased the incidence of postpartum depression in parturients who underwent cesarean section. However, no study has been performed to determine the effects of esketamine combined with morphine on pain control after cesarean section.
Thus, this single-center prospective study was conducted to determine whether the addition of esketamine to morphine would improve postoperative analgesia after cesarean section.
The inclusion criteria were as follows: (1) nulliparous parturients who were scheduled for elective cesarean delivery under spinal–epidural anesthesia; (2) parturients who had requested postoperative analgesia; (3) parturients aged between 20 and 35 years old; (4) parturients who had a full-term pregnancy; (5) parturients who were identified as having a singleton pregnancy; and (6) parturients who were categorized as having an American Society of Anaesthesiologists (ASA) physical status of 1 or 2. This study has been registered, ClinicalTrials.gov Identifier: NCT05582135.
The key exclusion criteria were as follows: (1) parturients with severe internal, surgical, or obstetric comorbidities (including spinal deformities, hypertension, placental abruption, cholestasis in pregnancy, asthma, heart disease, and abnormal coagulation parameters); (2) parturients with a known allergy to the drugs used in this study; (3) parturients with severe mental illness who could not comply with doctors’ instructions; and (4) parturients with chronic pain syndrome, which is defined as pain that persists for a period longer than 3 months (14).
To ensure allocation concealment, the allocation sequence was randomly generated by computer by someone independent of the trial. The parturients enrolled were randomly divided by a computer-generated simple randomization schedule into four groups (A, B, C, and D) in a 1:1:1:1 ratio. The analgesic drugs were prepared by the nurse according to the random allocation and numbered accordingly. Enrolled parturients were given the corresponding numbered analgesic drugs. Neither the trial participants nor researchers were aware of the treatment allocation after randomization.
During the preoperative visit, the parturients were taught how to use the visual analog scale (VAS) for assessing pain. After entering the operation room, the standard anesthesia monitors were used for the parturients, including electrocardiogram, noninvasive blood pressure (BP), heart rate (HR), and peripheral oxygen saturation (SPO2). Spinal anesthesia was performed in the left lateral position at the L3–4 level using the needle-through-needle technique. Then, 2 ml of bupivacaine (5 mg/ml) was injected into the subarachnoid space, and an epidural catheter was inserted cephalad 3 cm rapidly. The parturients were then placed in the supine position, and surgery was started when the T6 sensory block was achieved.
The analgesic drugs were given to the parturients immediately upon completion of surgery. The parturients in group A were administered 2 mg morphine hydrochloride through the epidural catheter. The parturients in group B were administered 0.25 mg/kg of esketamine. The parturients in group C were administered 0.25 mg/kg of esketamine in combination with 2 mg morphine hydrochloride. The parturients in group D were administered 0.25 mg/kg of esketamine in combination with 1 mg morphine hydrochloride. Sterile saline was added to all combinations of the analgesic drugs to make a total volume of 8 ml. If the parturients still complained of pain after analgesic drug administration, rescue analgesia was administered by intramuscular injection of 100 mg tramadol, which was repeated if necessary.
The pain scores at rest and on movement were evaluated at 2, 4, 8, 12, 24, and 48 h after cesarean delivery by the parturients themselves using a VAS based on a linear scale from 0 to 10, where 0 represented an absence of pain and 10 represented maximal pain. The number of rescue analgesics required within 48 h of surgery was also recorded and compared. For safety, the systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, and SpO2 were recorded at 2, 4, 8, 12, 24, and 48 h after drug administration, postoperatively. Adverse events that occurred after the administration of study drugs were also recorded.
Based on previous data (7), we assumed that the average VAS score at rest at 24 h after cesarean delivery in the combination groups (group C and D) would be 1 point lower than that in the morphine hydrochloride alone (group A) group and that the average VAS score at 24 h after cesarean delivery in the esketamine alone (group B) group would be similar to group A. To detect a 1-point difference in the 0-to-10 VAS score, assuming a 1.15 SD (7), a sample size of 96 women (24 in each group) was calculated to suffice, with a power of 95% and an alpha error of 0.05. Considering that the randomized subjects might be lost for follow-up for various reasons, approximately 120 subjects were suggested to be included in this study. The sample size was processed using PASS 15.0 software.
The data in this study were analyzed using the IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY, United States) statistical package. Quantitative data were described as mean ± SD or median with 95% confidence interval. Categorical data were described as numbers and percentages. The Kolmogorov–Smirnov test was used to determine whether the quantitative data followed a normal distribution. The Chi-square test or Fisher's exact test was used for comparison of categorical data. One-way analysis of variance (ANOVA) was used for comparison of quantitative data. Repeated measures such as the VAS pain scores were assessed by the repeated measures ANOVA. The post hoc Bonferroni correction was done for multiple comparisons. A P-value <0.05 was considered as statistically significant.
From July 2020 to December 2021, 127 parturients were assessed for enrollment in this study. Eight were excluded before randomization based on the exclusion criteria (two parturients with a known allergy to esketamine, three with chronic pain syndrome, and three with severe internal comorbidities). A total of 119 parturients were finally included in this study, including 30 cases in group A, 30 cases in group B, 30 cases in group C, and 29 cases in group D (Figure 1). The baseline characteristics, including age, height, body weight, body mass index, duration of surgery, gestational age, and ASA physical status, were comparable among the four groups (Table 1).
Postoperative VAS scores for pain at rest are shown in Table 2. VAS scores were much lower for group C compared with group A or B for all the time points (P < 0.05). Moreover, group C had lower VAS scores compared to group D at all the time points except for at 24 h (P = 0.103) and 48 h (P = 0.098). There was no significant difference between groups A and D for the VAS scores at rest and at all the time points. However, group D had lower VAS scores when compared with group B at the time points of 2, 4, 8, and 12 h (P < 0.05).
Postoperative VAS scores for pain with movement are shown in Table 3. From the time point of 4 h, VAS scores were much lower for group C compared with groups A or B (P < 0.05). Furthermore, at the time points of 8, 12, and 24 h, group C had lower VAS values compared to group D (P < 0.05). There was no significant difference between groups A and D regarding the VAS scores with movement at all the time points. When compared with group B, group D only had significantly lower VAS scores at the time points of 4 and 8 h (P = 0.003 and P < 0.001, respectively).
The results for rescue analgesics are shown in Figure 2. Compared with groups A, B, and D, group C required less rescue analgesia within the first 48 h after surgery (P = 0.021 for C vs. A, P < 0.001 for C vs. B, and P < 0.001 for C vs. D, respectively).
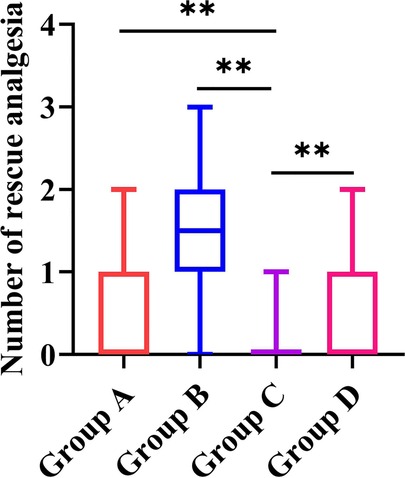
Figure 2. The results for rescue analgesia in the four study groups. Group A: administrated with 2 mg morphine hydrochloride; group B: administrated with 0.25 mg/kg of esketamine; group C: administrated with 0.25 mg/kg of esketamine in combination with 2 mg morphine hydrochloride; and group D: administrated with 0.25 mg/kg of esketamine in combination with 1 mg morphine hydrochloride. Data are presented as median (interquartile range). **P < 0.05.
There were no significant differences in the SBP, DBP, HR, and SpO2 among the four groups at the six time points after surgery (two-way ANOVA, overall P = 0.099, 0.354, 0.105, and 0.476, respectively, Figure 3). No serious complications occurred during the study period. Nausea and/or vomiting occurred in five patients in group A, three in group B, four in group C, and three in group D. Pruritus occurred in four patients in group A, two in group B, two in group C, and one in group D. Dizziness occurred in one patient in group A, one in group B, two in group C, and three in group D. There was no statistically significant difference between the four groups regarding the incidence of adverse events (Table 4).
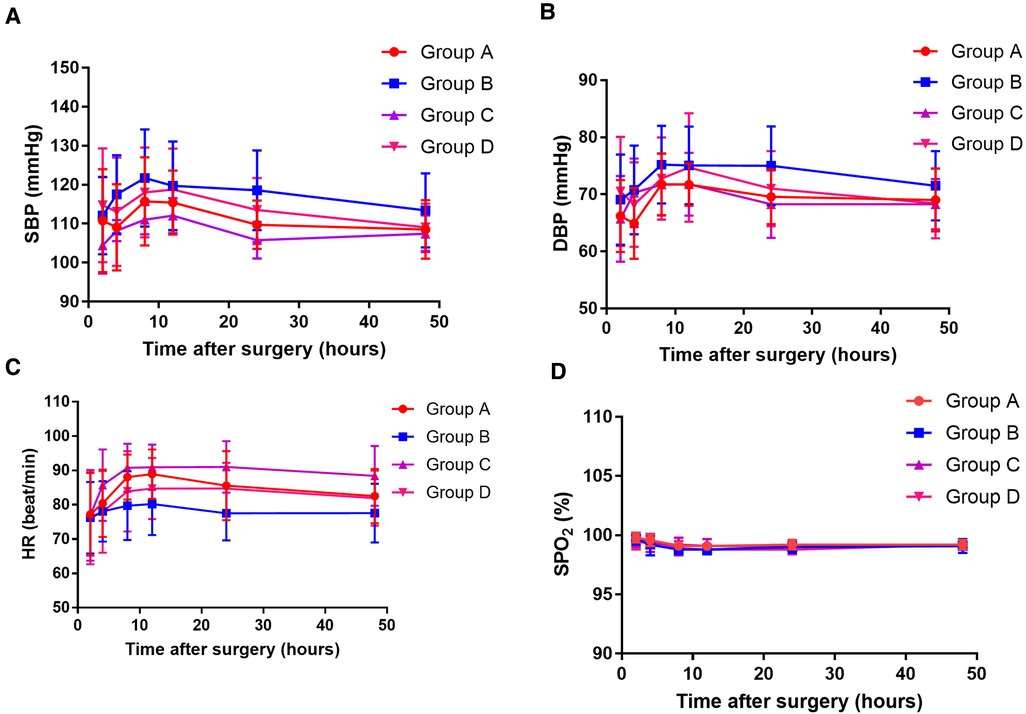
Figure 3. The systolic blood pressure (SBP) value (A), diastolic blood pressure (DBP) value (B), heart rate (HR) value (C), and oxygen saturation (SPO2) value (D) at 10, 20, 30, 40, and 50 h after surgery among the four groups. Group A: administrated with 2 mg morphine hydrochloride; group B: administrated with 0.25 mg/kg of esketamine; group C: administrated with 0.25 mg/kg of esketamine in combination with 2 mg morphine hydrochloride; and group D: administrated with 0.25 mg/kg of esketamine in combination with 1 mg morphine hydrochloride.
In the present study, we found that the analgesic regime of epidural esketamine and morphine loaded immediately after cesarean delivery could significantly relieve postoperative pain and reduce the requirement of rescue analgesia compared with morphine or esketamine alone. Furthermore, this combination was not associated with an increased incidence of adverse events.
As an NMDA-receptor antagonist, esketamine has been used effectively to treat depressive disorders (6). Furthermore, esketamine has been reported to be useful for preventing hyperalgesia and controlling pain after surgery (13, 15). Suppa et al. (16) reported that esketamine had an anti-hyperalgesic action that could effectively control postoperative pain in parturients who underwent cesarean delivery. Han et al. (17) found that low-dose esketamine used as an adjuvant in patient-controlled intravenous analgesia significantly reduced the incidence of postpartum depression and relieved pain after cesarean delivery. Similarly, Wang et al. (6) also confirmed that low-dose esketamine could reduce both post-cesarean pain and the incidence of postpartum depression in parturients.
Because of its ability to prevent hyperalgesia and central sensitization without respiratory depression, ketamine has been suggested as an adjuvant drug in opioid analgesia (18). Esketamine has a higher affinity for NMDA receptors than ketamine (6). It is also reported to have a shorter sedation time and fewer side effects than ketamine (19). Thus, esketamine has been used as an adjuvant drug in multimodal analgesia. The administration of esketamine in combination with opioids in the nonpregnant population has been described previously. Brinck et al. (20) found that patient-controlled opioid analgesia containing esketamine could decrease cumulative opioid consumption at 24 h after major lumbar spinal fusion surgery without additional adverse effects. Snijdelaar et al. (21) reported that pain scores and cumulative morphine consumption were significantly lower in patients who received esketamine/morphine compared to those who received morphine alone after radical prostatectomy. Lyu et al. (22) demonstrated that esketamine combined with sufentanil could provide satisfactory analgesia in patients who had undergone laparoscopic radical resection.
In the present study, esketamine in combination with morphine was demonstrated to be effective for parturients who underwent cesarean delivery. We found that epidural 0.25 mg/kg esketamine and 2 mg morphine immediately after delivery could significantly relieve postoperative pain at rest and with movement. Epidural anesthesia is one of the treatment protocols that have been suggested for women who undergo cesarean delivery (23). Previous studies reported that epidural morphine significantly prolongs analgesia after cesarean delivery (24). Here, we found that parturients who received epidural 0.25 mg/kg esketamine combined with 2 mg morphine had much lower pain scores for 48 h after cesarean delivery (including pain at rest and pain with movement) than those who received 2 mg morphine or 0.25 mg/kg esketamine alone. At most of the time points in the study period, parturients who received epidural 0.25 mg/kg esketamine combined with 2 mg morphine also had lower pain scores than those who received epidural 0.25 mg/kg esketamine and 1 mg morphine. Corresponding to the results of VAS scores, parturients in the group who received 0.25 mg/kg esketamine combined with 2 mg morphine required less rescue analgesia within the first 48 h postoperatively as compared with the other three groups. These results indicate that the addition of esketamine to morphine improves postoperative analgesia after cesarean section.
There was no statistically significant difference between the four study groups with regard to the incidence of adverse events. Additionally, there were no significant differences in the SBP, DBP, HR, and SpO2 among the four groups at all the six time points after surgery. Thus, the addition of esketamine to morphine does not increase the risk of adverse events.
Furthermore, in this study, it was also found that parturients who received epidural 0.25 mg/kg esketamine combined with 1 mg morphine had similar pain scores compared with those who received 2 mg morphine. Thus, the addition of esketamine to morphine may reduce the dose of morphine required and yet achieve the same effect. This result should be confirmed in further studies.
The present study has several limitations. First, this study was carried out in a single center with a small sample size. A multicenter study with a larger sample size is needed to confirm the conclusions of this study. Second, the assessment of post-cesarean pain was based on the VAS, which is completely subjective. More objective methods should be considered in further studies. Finally, previous studies have shown that esketamine reduces the incidence of postpartum depression in parturients (6). Due to the short study period and small sample size, we did not observe the effect of esketamine in reducing postpartum depression. This effect should be investigated in further studies.
This study showed that epidural esketamine and morphine immediately after cesarean delivery significantly relieves postoperative pain and reduces the requirement of rescue analgesia, compared with using morphine or esketamine alone. Thus, the addition of esketamine to morphine improves postoperative analgesia after cesarean section without increasing the risk of adverse events.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by ethics committee of Xuanhan County People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Conception, design of the work, supervision, and drafting the manuscript: JT and ZZ. Data collection, analysis, and interpretation of the data: QR, FZ, YW, FH, CY, and XT. Statistical analysis and critical revision of the manuscript: JT, ZZ, QR and FZ. All authors contributed to the article and approved the submitted version.
This article was supported by Xuanhan County Education and Science and Technology Bureau Project (2020SF01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Su Y, Heitner J, Yuan C, Si Y, Wang D, Zhou Z, et al. Effect of a text messaging-based educational intervention on cesarean section rates among pregnant women in China: quasirandomized controlled trial. JMIR Mhealth Uhealth. (2020) 8(11):e19953. doi: 10.2196/19953
2. Huang L, Zhang J, Sun H, Dong H, Li R, Cai C, et al. Association of gestational weight gain with cesarean section: a prospective birth cohort study in southwest China. BMC Pregnancy Childbirth. (2021) 21(1):57. doi: 10.1186/s12884-020-03527-1
3. Kintu A, Abdulla S, Lubikire A, Nabukenya MT, Igaga E, Bulamba F, et al. Postoperative pain after cesarean section: assessment and management in a tertiary hospital in a low-income country. BMC Health Serv Res. (2019) 19(1):68. doi: 10.1186/s12913-019-3911-x
4. Cao X, Zhang X. Comparison of different sufentanil-tramadol combinations for pain relief within the first 24 h after cesarean section: a retrospective study. J Pain Res. (2018) 11:2445–51. doi: 10.2147/JPR.S177500
5. Shen D, Hasegawa-Moriyama M, Ishida K, Fuseya S, Tanaka S, Kawamata M. Acute postoperative pain is correlated with the early onset of postpartum depression after cesarean section: a retrospective cohort study. J Anesth. (2020) 34(4):607–12. doi: 10.1007/s00540-020-02789-5
6. Wang Y, Zhang Q, Dai X, Xiao G, Luo H. Effect of low-dose esketamine on pain control and postpartum depression after cesarean section: a retrospective cohort study. Ann Palliat Med. (2022) 11(1):45–57. doi: 10.21037/apm-21-3343
7. Dafna L, Herman HG, Ben-Zvi M, Bustan M, Sasson L, Bar J, et al. Comparison of 3 protocols for analgesia control after cesarean delivery: a randomized controlled trial. Am J Obstet Gynecol MFM. (2019) 1(2):112–8. doi: 10.1016/j.ajogmf.2019.04.002
8. Sharpe EE, Molitor RJ, Arendt KW, Torbenson VE, Olsen DA, Johnson RL, et al. Intrathecal morphine versus intrathecal hydromorphone for analgesia after cesarean delivery: a randomized clinical trial. Anesthesiology. (2020) 132(6):1382–91. doi: 10.1097/ALN.0000000000003283
9. Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. (1999) 90(2):437–44. doi: 10.1097/00000542-199902000-00018
10. Gehling M, Tryba M. Risks and side-effects of intrathecal morphine combined with spinal anaesthesia: a meta-analysis. Anaesthesia. (2009) 64(6):643–51. doi: 10.1111/j.1365-2044.2008.05817.x
11. Nie Y, Tu W, Shen X, Yu W, Yu Y, Song X, et al. Dexmedetomidine added to sufentanil patient-controlled intravenous analgesia relieves the postoperative pain after cesarean delivery: a prospective randomized controlled multicenter study. Sci Rep. (2018) 8(1):9952. doi: 10.1038/s41598-018-27619-3
12. Lei Y, Liu H, Xia F, Gan S, Wang Y, Huo W, et al. Effects of esketamine on acute and chronic pain after thoracoscopy pulmonary surgery under general anesthesia: a multicenter-prospective, randomized, double-blind, and controlled trial. Front Med (Lausanne). (2021) 8:693594. doi: 10.3389/fmed.2021.693594
13. Nielsen RV, Fomsgaard JS, Nikolajsen L, Dahl JB, Mathiesen O. Intraoperative S-ketamine for the reduction of opioid consumption and pain one year after spine surgery: a randomized clinical trial of opioid-dependent patients. Eur J Pain. (2019) 23(3):455–60. doi: 10.1002/ejp.1317
14. Ostovar-Kermani T, Arnaud D, Almaguer A, Garcia I, Gonzalez S, Mendez Martinez YH, et al. Painful sleep: insomnia in patients with chronic pain syndrome and its consequences. Folia Med (Plovdiv). (2020) 62(4):645–54. doi: 10.3897/folmed.62.e50705
15. Liu P, Li P, Li Q, Yan H, Shi X, Liu C, et al. Effect of pretreatment of S-ketamine on postoperative depression for breast cancer patients. J Invest Surg. (2021) 34(8):883–8. doi: 10.1080/08941939.2019.1710626
16. Suppa E, Valente A, Catarci S, Zanfini BA, Draisci G. A study of low-dose S-ketamine infusion as “preventive” pain treatment for cesarean section with spinal anesthesia: benefits and side effects. Minerva Anestesiol. (2012) 78(7):774–81.22374377
17. Han Y, Li P, Miao M, Tao Y, Kang X, Zhang J. S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: a randomized controlled trial. BMC Anesthesiol. (2022) 22(1):49. doi: 10.1186/s12871-022-01588-7
18. Imani F, Varrassi G. Ketamine as adjuvant for acute pain management. Anesth Pain Med. (2019) 9(6):e100178. doi: 10.5812/aapm.100178
19. Wang H, Duan CY, Huang WQ, Zhao P, Zhou LZ, Liu YH, et al. Perioperative intravenous S(+)-ketamine for acute postoperative pain in adults: study protocol for a multicentre, randomised, open-label, positive-controlled, pragmatic clinical trial (SAFE-SK-A trial). BMJ Open. (2021) 11(12):e054681. doi: 10.1136/bmjopen-2021-054681
20. Brinck ECV, Virtanen T, Mäkelä S, Soini V, Hynninen VV, Mulo J, et al. S-ketamine in patient-controlled analgesia reduces opioid consumption in a dose-dependent manner after major lumbar fusion surgery: a randomized, double-blind, placebo-controlled clinical trial. PLoS One. (2021) 16(6):e0252626. doi: 10.1371/journal.pone.0252626
21. Snijdelaar DG, Cornelisse HB, Schmid RL, Katz J. A randomised, controlled study of peri-operative low dose s(+)-ketamine in combination with postoperative patient-controlled s(+)-ketamine and morphine after radical prostatectomy. Anaesthesia. (2004) 59(3):222–8. doi: 10.1111/j.1365-2044.2003.03620.x
22. Lyu SG, Lu XH, Sun XT, Li CJ, Miao C. Effects of S(+)-ketamine combined with sufentanil for patient-controlled intravenous analgesia on the early recovery in elderly patients undergoing laparoscopic radical resection of rectal cancer. Zhonghua Yi Xue Za Zhi. (2021) 101(39):3238–43. doi: 10.3760/cma.j.cn112137-20210504-01053
23. Bonnet MP, Mignon A, Mazoit JX, Ozier Y, Marret E. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain. (2010) 14(9):894.e1–9. doi: 10.1016/j.ejpain.2010.03.003
Keywords: esketamine, morphine, cesarean section, analgesia, pain
Citation: Tang J, Zheng Z, Ran Q, Zhao F, Wang Y, Hu F, Yang C and Tan X (2023) Epidural esketamine and morphine for postoperative analgesia after caesarean delivery: A pilot study. Front. Surg. 9:988392. doi: 10.3389/fsurg.2022.988392
Received: 7 July 2022; Accepted: 26 October 2022;
Published: 6 January 2023.
Edited by:
Tai-Ho Hung, Taipei Chang Gung Memorial Hospital, Taiwan© 2023 Tang, Zheng, Ran, Zhao, Wang, Hu, Yang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qijun Ran cmFucWlqdW5fcnFqQDE2My5jb20= Feng Zhao emhhb2Zfa29rb3BAMjFjbi5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.