- Department of Vascular Surgery, Wagga Wagga Base Hospital, Wagga Wagga, NSW, Australia
Background: Nutcracker syndrome (NS) is an uncommon condition resulting from the compression of the left renal vein (LRV) between the aorta and superior mesenteric artery (SMA), resulting in symptoms such as flank pain and hematuria.
Case presentation: We present the case of a 30-year-old woman complaining of abdominal pain who was found to have nutcracker syndrome and treated with endovascular stenting of the left renal vein.
Discussion: We review the literature related to endovascular treatment of NS with focus on the distribution of the sizes of stents and rates of stent migration.
Conclusion: NC is a rare condition requiring a high index of suspicion for diagnosis. Endovascular treatment is a reasonable option, but its limitations must be considered.
Introduction
Nutcracker syndrome (NS) is a rare disorder caused by extrinsic compression of the left renal vein (LRV) between the aorta and superior mesenteric artery (SMA), resulting in impaired blood outflow and congestion, causing dilation of the LRV distal to the compression (1, 2). Symptoms include gross or microscopic hematuria, flank, abdominal pain or pelvic pain, gonadal vein syndrome, varicocele, and proteinuria as well as nonspecific gastrointestinal derangements such as nausea and loss of appetite (3). It is important to distinguish NS from nutcracker phenomenon as the terms are often used interchangeably. Nutcracker phenomenon is the anatomical or radiological finding of LRV compression, while NS refers to patients who present with clinical symptoms (4).
The exact prevalence of NS is unclear because of the variability in symptoms and the absence of agreed diagnostic criteria (5, 6). Treatment options range from observation to nephrectomy depending on the degree of disease and clinician preferences. Observation is usually recommended for those who have mild hematuria or pain, and intervention should be used for patients with intractable pain, severe hematuria, renal insufficiency, and failure to respond to conservative management (1). Procedures include LRV transposition, SMA transposition, renal autotransplant, and endovascular renal vein stenting (4, 5).
Case description
A-30-year-old woman presented to her general practitioner with episodic epigastric pain radiating to the left flank over a period of 6 months. She had a history of migraines but was otherwise well with no previous abdominal operations.
The pain was somewhat positional, improving when the left side was dependent. It was exacerbated by food—solids more than fluids—and she had noted some recent weight loss. Colonoscopy was normal; gastroscopy revealed mild gastritis, but initiation of a proton pump inhibitor (omeprazole 20 mg daily) made no improvement in symptoms. There may have also been a component of superior mesenteric artery syndrome present alongside findings consistent with nutcracker syndrome; however, this could not be substantiated as there was no imaging to support this claim. Urinalysis revealed no significant findings and no hematuria.
A CT venogram demonstrated compression of the LRV between the superior mesenteric artery and the aorta with a prominent left ovarian vein (LOV) as seen in Figure 1. Her pain worsened and became constant despite negative findings on repeat gastroscopy, and the decision was made to proceed with angiography and angioplasty with a view for intervention on the LRV.
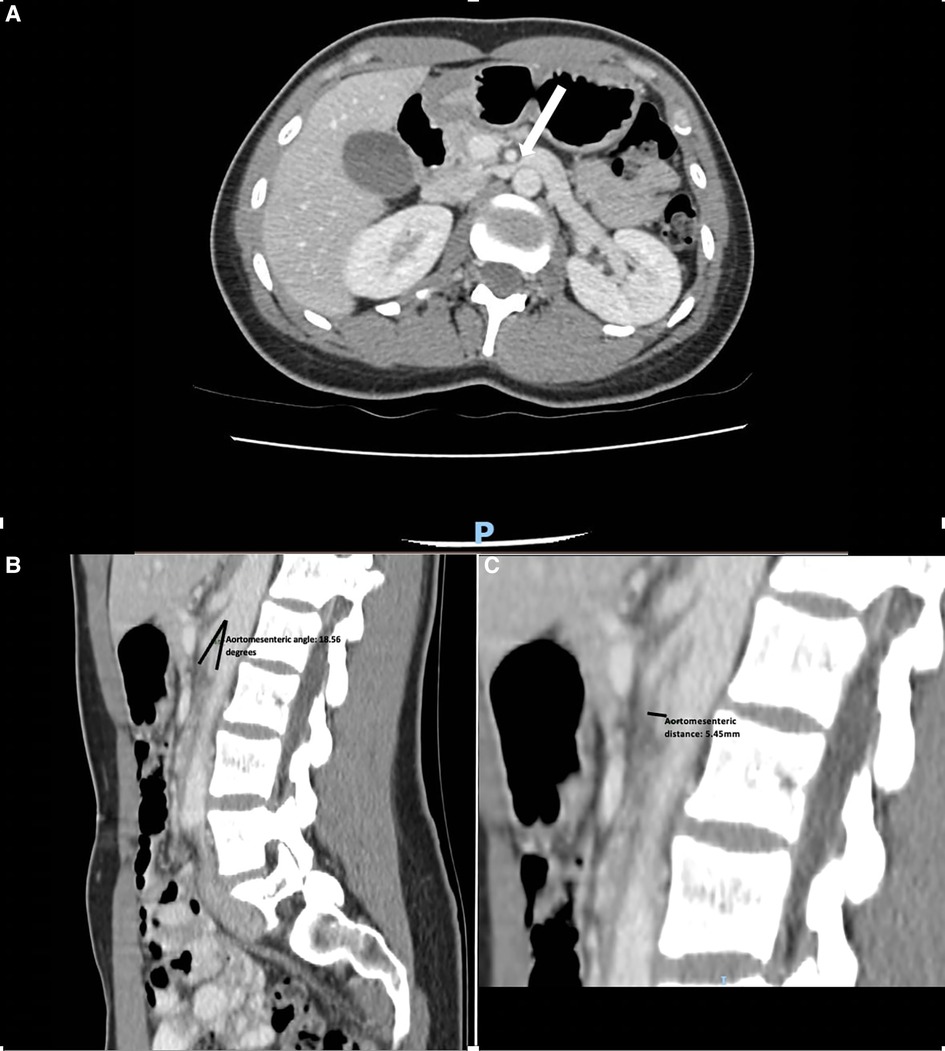
Figure 1. (A) CT scan demonstrating dilated left renal vein compressed between the superior mesenteric artery and aorta with an arrow pointing to the area of compression. (B) A sagittal CT scan demonstrating an aortomesenteric angle of 18.56°. (C) A sagittal CT scan demonstrating an aortomesenteric distance of 5.45 mm.
Access was gained via a 7F sheath placed in the right common femoral vein under local anesthesia. The LRV was cannulated, and angiograms were performed, demonstrating the venous dilatation and reflux into an engorged left ovarian vein (Figure 2). A 10 mm × 40 mm Amada 35 balloon (Abbot Laboratories, Chicago, IL, United States) was inflated across the point of maximal stenosis. Remarkably, the patient had instant and profound relief of symptoms, describing herself as pain-free for the first time in many months; however, once the balloon was deflated, the pain recurred over the course of 30–60 s, only to be relieved again by a second balloon inflation.
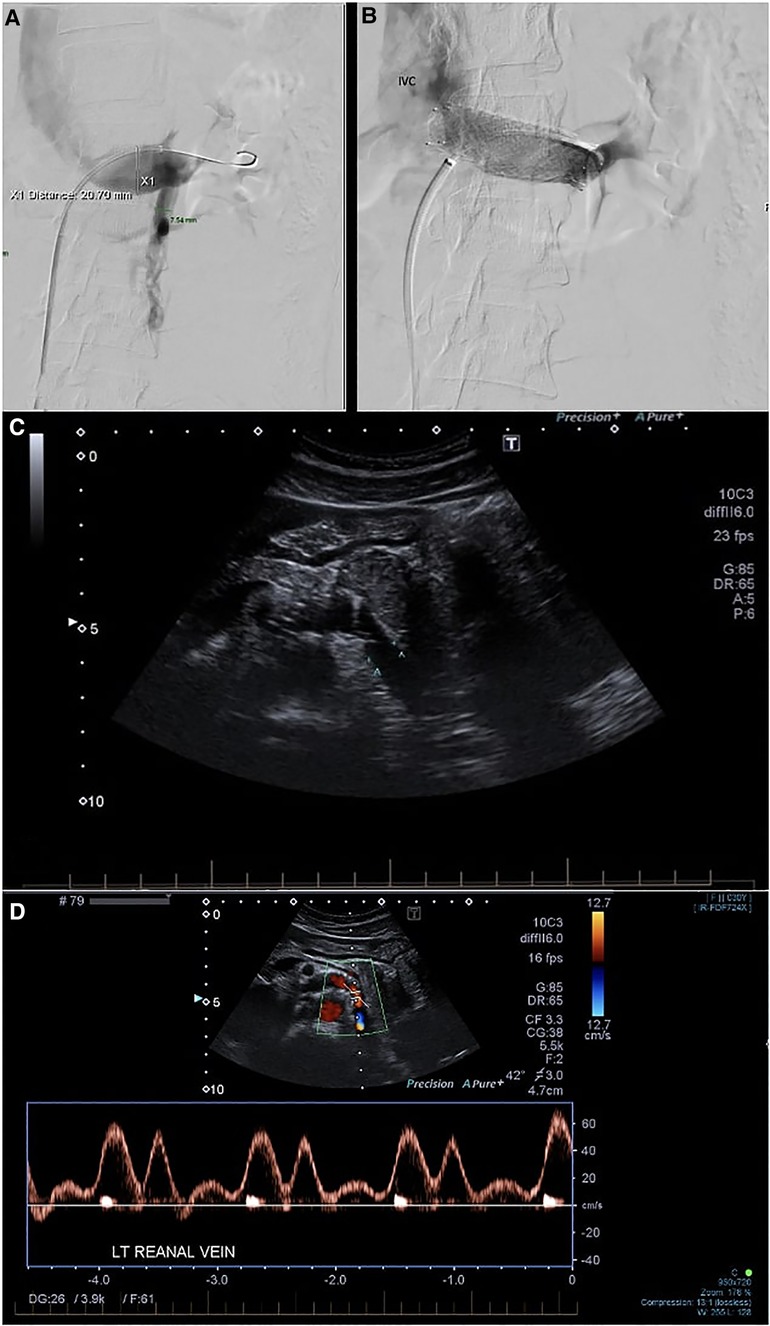
Figure 2. (A) Phlebography demonstrating stenosis of the LRV with reflux into the LOV. (B) Venogram after stent deployment. Reflux into the left ovarian vein is no longer seen. The distal part of the stent is somewhat constrained, but the proximal part prolapses into the IVC. (C) Follow-up duplex ultrasound at 3 months. (D) Post-procedure LRV duplex velocities and assessment of velocity ratio. IVC, inferior vena cava; LRV, left renal vein; LOV, left ovarian vein.
A 16 mm × 40 mm Zilver Vena self-expanding stent (Cook Medical, Bloomington, IN, United States) was deployed. No post-stent angioplasty with balloon was performed as there were already concerns regarding the oversizing of the stent. Disappointingly, the deployment was associated with an immediate recurrence of significant pain. Checking angiograms (Figure 2A) showed resolution of the stenosis and the absence of reflux into the ovarian vein. The pain improved over the course of the next few hours and was completely settled by the second postoperative day. She was discharged on 2 months of apixaban 5 mg b.i.d. and long-term aspirin 100 mg daily. She was symptom-free at 3-month follow-up with a duplex ultrasound demonstrating resolution of stenosis (Figure 2C). On further follow-up at 35 months, the patient continued to be symptom-free with no complications.
Discussion
Management options for nutcracker syndrome range from conservative observation to endovascular stenting (EVS) or open surgery with the approach guided by the severity of symptoms and local experience (1). Endovenous intervention offers a minimally invasive approach and is becoming increasingly popular as primary management for symptomatic cases. This trend is motivated in part by promising results in the treatment of iliac vein compression such as May–Thurner syndrome, perhaps the closest clinicoanatomical analogy to NCS.
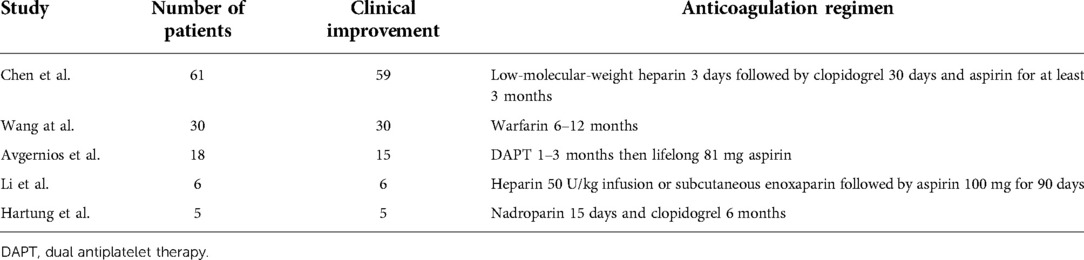
The medium-term results of EVS are promising. The six largest retrospective studies detail a total of 192 patients treated with EVS (7–12). Of these, five studies describe symptoms after the procedure as seen in Table 1. Complete or partial symptomatic improvement was reported in majority if not all patients across these studies, as demonstrated in Table 1 (7–12). Of the two patients described by Avgernios et al. (9) who had no response to treatment, one was later diagnosed with endometriosis and one remained symptomatic (and undiagnosed) despite a kidney autotransplant.
In-stent restenosis appears to be uncommon. Avgernios et al. (9) reported three examples of restenosis requiring interventions, two of which were in patients who had previous renal vein transpositions. No other examples of in-stent restenosis and no stent fracture are recorded in these studies, although recompression associated with stent migration is described in three cases (7, 11, 13).
Postoperative selection of antithrombotic therapy is also variable, both based on choice of an agent and duration of the treatment. Dual antiplatelet therapy (DAPT) varies from 30 days to 3 months (9, 14–17). Avgernios et al. (9) commenced their patients on DAPT for 1–3 months and then on ‘baby’ aspirin (81 mg) for life. Wang et al. (8) commenced all 28 patients on warfarin therapy 6–12 months after the procedure. For our patient, we prescribed 2 months of apixaban, after which anticoagulation was ceased with no complications; however, antiplatelet therapy with 100 mg aspirin was continued.
The most feared complication of EVS is stent migration. Wu et al. (13) described stent migration in 5 out of a cohort of 75 patients (6.7%), during a mean of 55 months follow-up. Of these migrations, two stents (10 mm × 40 mm and 14 mm × 40 mm, SMART Control) moved to the right atrium and were retrieved by open cardiac surgery. One stent (10 mm × 40 mm, SMART Control) migrated to the left and was treated expectantly; one (10 mm × 14 mm, SMART Control) migrated to the right, partially prolapsing into the inferior vena cava (IVC) and was also treated expectantly,; and one (14 mm × 40 mm, Wallstent) completely prolapsed into the IVC and was removed with open surgery.
In a description of early experience of five patients, Hartung et al. (11) describe perioperative stent migration into the IVC in one case (20 mm × 60 mm, Wallstent), which required further endovascular intervention, and late migration of two stents (both 16 mm × 40 mm, Wallstent) to the right, prolapsing into the IVC and allowing recompression of the LRV and recurrence of symptoms. Chen et al. (7) (n = 61) described three cases of stent migration: one example of postoperative migration of a stent (10 mm × 40 mm, Wallstent) to the right atrium, requiring an open operative retrieval; one example of migration to the left (12 mm × 40 mm, Wallstent); and one example of migration to the right (10 mm × 40 mm, Wallstent). Wang et al. (8) (n = 30, 12–80 months follow-up), Avgernios et al. (9) (n = 18), and Li et al. (10) (n = 6) described no instances of migration.
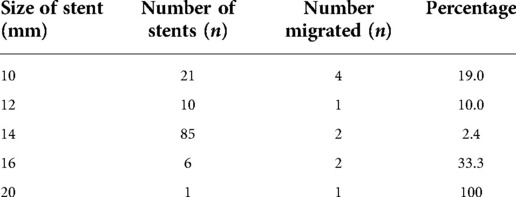
It would seem likely that the size and type of the stent would affect the risk of migration. Of the 192 stents deployed (7–12), 146 were SMART Control, 31 were Wallstent, 15 Protégé Everflex, and 1 each of Zilver and Palmaz stents. As seen in Table 2, size data are available for 122 of the stents, with the distribution being 10 mm (n = 21), 12 mm (n = 10), 14 mm (n = 85), and 16 mm (n = 6). Hartung et al. (11) deployed a 20-mm diameter Wallstent, but this was later retrieved after migration. For stents that migrated, the size distribution was 10 mm (n = 4), 12 mm (n = 1), 14 mm (n = 2), 16 mm (n = 2), and 20 mm (n = 1). Of the stents subject to migration, six were Wallstent and four were SMART Control stents.
The left renal vein diameter typically measures 12.0 ± 2.0 mm in cadaveric studies (16–19) but is known to expand during the Valsalva maneuver (20). The distal portion is commonly dilated in the presence of outflow obstruction. With our patient, the distal LRV measured up to 12 mm on the preoperative CT scan but appeared larger on the catheter venograms (Figure 2). To aid with LRV stent sizing accuracy, intravascular ultrasound (IVUS) is becoming an increasingly common mode of imaging utilized during angiography. It can aid with the sizing of the LRV stent and has the potential to reduce the risk of oversizing or undersizing the stent (12). However, further evidence is required to support the benefits of IVUS in LRV stenting.
The stent chosen was a 16 mm × 60 mm Zilver Vena stent with the primary concern of preventing migration. The Zilver Vena stent is a dedicated venous stent and although limited experience in the renal vein is described, it has shown good safety and efficacy in iliocaval use (21–23) It was disappointing that the deployment of the stent was associated with a recurrence of pain that had been entirely absent with simple balloon inflation. We interpreted this as caused by the intrinsic stretch of the LRV by the stent. Fortunately, the pain subsided after the procedure and had completely resolved by the second postoperative day.
Conclusion
NCS is a rare condition requiring a high index of suspicion for diagnosis. Treatment options vary based on severity; however, considering EVS is becoming an increasingly common and minimally invasive solution, it seems particularly efficacious for the treatment of venous stenosis or compression which contributes to flank pain in such cases. In this case, EVS has demonstrated effective results at follow-up with no long-term consequences. The sizing of stents remains a matter for judgment, and intravascular ultrasound could be considered to aid planning. Although larger stents may reduce the risk of migration, oversizing is not without consequences. Further data will assist development of optimal treatment algorithms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RK and DA: Writing and editing of manuscript. Both authors contributed to the article and approved the submitted version.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kurlinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. (2010) 85(6):552–9. doi: 10.4065/mcp.2009.0586
2. Velasquez CA, Saeyeldin A, Zafar MA, Brownstein AJ, Erben Y. A systematic review on management of nutcracker syndrome. J Vasc Srug Venous Lymphat Disord. (2018) 6:271–8. doi: 10.1016/j.jvsv.2017.11.005
3. Rudloff U, Holmes RJ, Prem JT, Faust GR, Moldwin R, Siegel D. Mesoaortic compression of the left renal vein (nutcracker syndrome): case reports and review of the literature. Ann Vasc Surg. (2006) 20(1):120–9. doi: 10.1007/s10016-005-5016-8
4. Ananthan K, Onida S, Davies AH. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. (2017) 53:886–94. doi: 10.1016/j.ejvs.2017.02.015
5. Siddiqui WJ, Bakar A, Aslam M, Arif H, Bianco BA, Trebelev AE, et al. Left renal vein compression syndrome: cracking the nut of clinical dilemmas—three cases and review of literature. Am J Case Rep. (2017) 18:754–9. doi: 10.12659/AJCR.905324
6. Shin JI, Lee JS, Kim MJ. The prevalence, physical characteristics and diagnosis of nutcracker syndrome [letter]. Eur J Vasc Endovasc Surg. (2006) 32(3):335–6. doi: 10.1016/j.ejvs.2006.04.030
7. Chen S, Zhang H, Shi H, Tian S, Jin W, Li M. Endovascular stenting for treatment of nutcracker syndrome: report of 61 cases with long term follow-up. J Urol. (2011) 186(2):570–5. doi: 10.1016/j.juro.2011.03.135
8. Wang X, Zhang Y, Li C, Zhang H. Results of endovascular treatment for patients with nutcracker syndrome. J Vasc Surg. (2012) 56(1):142–8. doi: 10.1016/j.jvs.2012.01.007
9. Avgerinos ED, Saadeddin Z, Humar R, Salem K, Singh M, Hager E, et al. Outcomes of left renal vein stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. (2019) 7(6):853–9. doi: 10.1016/j.jvsv.2019.06.016
10. Li H, Sun X, Liu G, Zhang Y, Chu J, Deng C, et al. Endovascular stent placement for nutcracker phenomenon. J Xray Sci Technol. (2013) 21:95–102. doi: 10.3233/XST-130356
11. Hartung O, Grisoli D, Boufi M, Marani I, Hakam Z, Barthelemy P, et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg. (2005) 42(2):275–80. doi: 10.1016/j.jvs.2005.03.052
12. Cherfan P, Averginos ED, Chaer RA. Left renal vein stenting in nutcracker syndrome: outcomes and implications. Vasc Endovasc Rev. (2020) 3:e17. doi: 10.15420/ver.2020.12
13. Wu Z, Zheng X, He Y, Fang X, Li D, Tian L, et al. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. (2016) 4(2):193–9. doi: 10.1016/j.jvsv.2015.10.005
14. Agle CG, Filho DSA, Almeida LC. Endovascular treatment of nutcracker syndrome: case report. J Vasc Bras. (2019) 18:e20180135. doi: 10.1590/1677-5449.180135
15. Baldi S, Rabellino M, Zander T, Gonzalez G, Maynar M. Endovascular treatment of the nutcracker syndrome: report of two cases. Minim Invasive Ther Allied Technol. (2011) 20(6):356–9. doi: 10.3109/13645706.2010.550303
16. Belczak SQ, Luz L, Paglia LB, Barbosa GPR, Freire AML, Miziara MA, et al. Endovascular treatment of a teenager with nutcracker syndrome: a case report. J Vasc Bras. (2020) 19:e20180126. doi: 10.1590/1677-5449.180126
17. Taneja M, Chua BSY, Daga K. Renal nutcracker syndrome in a young lady: unusual findings and endovascular management. BMJ Case Rep. (2018) 2018:bcr-2017-222880. doi: 10.1136/bcr-2017-222880
18. Satyapal KS, Rambiritch V, Pillai G. Morphometric analysis of renal veins. Anat Rec. (1995) 241(2):268–72. doi: 10.1002/ar.1092410213
19. Ballersteros LE, Saldarriaga V, Ramierez LM. Morphologic evaluation of the renal veins: a study with autopsy material from Colombian subjects. Rom J Morphol Embryol. (2014) 55(1):77–81.24715169
20. Syed MI, Yu B, Akhter T, Shaikh A. Renal vein stenting via the right internal jugular approach with a provocative Valsalva maneuver to reduce the risk of stent migration. Perspect Vasc Surg Endovasc Ther. (2011) 23:268–71. doi: 10.1177/1531003511409054
21. Salem AM, AboEINeel HA, Fakhr ME. Long-term outcomes of dedicated venous stents in the management of chronic iliofemoral obstruction. J Vasc Surge Venous Lymphat Disord. (2021) 10(1):52–9. doi: 10.1016/j.jvsv.2021.04.018
22. Williams ZF, Dillavou ED. A systematic review of venous stents for iliac and venacaval occlusive disease. J Vasc Surg Venous Lymphat Disord. (2020) 8:145–53. doi: 10.1016/j.jvsv.2019.08.015
Keywords: nutcracker syndrome (NC), endovascular treatment, endovascular stenting, literature review, left renal vein (LRV)
Citation: Kaur R and Airey D (2022) Nutcracker syndrome: A case report and review of the literature. Front. Surg. 9:984500. doi: 10.3389/fsurg.2022.984500
Received: 2 July 2022; Accepted: 9 November 2022;
Published: 23 December 2022.
Edited by:
Giulio Illuminati, Sapienza University of Rome, ItalyReviewed by:
Michele Greco, ClinicaVascolare, ItalyCecilia Gozzo, Humanitas Centro Catanese di Oncologia, Italy
Ryan M. McEnaney, VA Pittsburgh Healthcare System, United States
© 2022 Kaur and Airey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramandeep Kaur cmFtYW5kZWVwa2F1cjM0NjJAZ21haWwuY29t
Specialty Section: This article was submitted to Vascular Surgery, a section of the journal Frontiers in Surgery
 Ramandeep Kaur
Ramandeep Kaur David Airey
David Airey