- 1Department of Thyroid and Breast Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Pathology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Breast malignant adenomyoepithelioma (MAME) after breast augmentation has never been reported.
Case summary: We reported a case of a 55-year-old woman who was diagnosed with breast MAME 16 years after breast augmentation. Breast augmentation was performed on the patient with two 200 ml round textured prostheses in the subpectoral plane through axillary incisions in 2004. However, a breast ultrasound in 2020 revealed a suspicious malignant lump in the right breast, which was finally confirmed as MAME by pathology. Skin-sparing modified radical mastectomy and immediate breast reconstruction with expander implantation were performed. Subsequently, the patient received three cycles of chemotherapy with the regimen of anthracycline and cyclophosphamide. In the following nearly 2 years of follow-up, no tumor recurrence and metastasis were found, and the overall treatment was satisfactory for the patient.
Conclusion: Here, we present a unique case in which a patient was diagnosed with breast MAME after breast augmentation. Skin-sparing modified radical mastectomy and immediate breast reconstruction with expander implantation are feasible approaches that yield at least short-term oncological safety and acceptable aesthetic results. However, whether there is a potential relationship between MAME and breast implants remains to be further explored. Meanwhile, due to the rarity of breast MAME, more authoritative strategies considering both oncological safety and aesthetics to seek better long-term therapeutic effects are needed.
Background
Breast augmentation is the most commonly performed aesthetic surgical procedure. Silicone breast implants are used in nearly 300,000 breast augmentation and 100,000 breast reconstruction operations annually in the United States (1). Although several epidemiologic pieces of evidence show no link between implants and the risk of developing breast cancer (2), the research on breast cancer after augmentation mammoplasty is still attracting much attention. Early research suggested that cosmetic breast implants adversely affect breast cancer-specific survival following the diagnosis of such disease (3). Some authors have reported that breast cancers in augmented women present at a later stage are more aggressive tumors than those arising in nonaugmented women (4). It has also been reported that women with submuscular implants have a higher incidence of breast cancer than those with subglandular implants (5). In addition, there have been reports that women with textured breast implants are more likely to be diagnosed with anaplastic large cell lymphoma (ALCL) (6). All these studies remind a potential relationship between breast implants and breast tumors.
Adenomyoepithelioma (AME) is a rare tumor that can be seen in salivary glands, skin appendages, lungs, and breasts. Among them, AME of the breast was first reported by Hamperl in 1970 (7), which was considered to be a benign tumor formed by the biphasic proliferation of epithelial and myoepithelial cells. In the classification of breast tumors published by the World Health Organization in 2019, this kind of disease is clearly defined as breast epithelial–myoepithelial lesions. Two types of lesions composed of epithelial and myoepithelial cells in mammary ducts and/or tubules are seen under a microscope. Breast malignant adenomyoepithelioma (MAME) is a rare double-cell group lesion of mammary epithelial cells and myoepithelial cells, which means that one or both of them have malignant characteristics. So far, breast MAME after breast augmentation with implants has never been reported.
Case description
History of illness and physical examination
A 55-year-old female patient presented with a right breast lump by palpation without pain and nipple discharge. A medical history confirmed that the patient was implanted with two 200 ml round textured prostheses in the subpectoral plane through axillary incisions in 2004. There were no obvious adverse reactions such as infection and seroma after the breast augmentation surgery. No breast lump was detected before breast augmentation, and the family history of breast cancer was denied.
Physical examination
Physical examination showed that bilateral breasts were symmetrical, with no nipple deviation and depression, no nipple bleeding or discharge, and no orange peel sign or dimple sign. In addition, the prosthesis could be reached in both breasts. A lump of about 3 cm could be reached under the right nipple with hard texture, unclear boundary, low mobility, and no obvious adhesion to the skin. There was no obvious mass in the left breast or enlarged lymph nodes in the bilateral axilla.
Imaging examination
The breast ultrasound on August 21, 2020 (Figures 1A,B), showed a lobulated and spiculated hypoechoic solid neoplasm of 28.8*24.5*13.9 mm in the inner lower quadrant of the right breast adjacent to the nipple. The neoplasm was measured about 4.8 mm away from the body surface. According to the Breast Imaging Reporting and Data System (BI-RADS), the tumor was classified as grade 5, which meant a malignant possibility. In addition, no obvious abnormality was found in bilateral axillary lymph and the bilateral prosthesis capsule was intact.
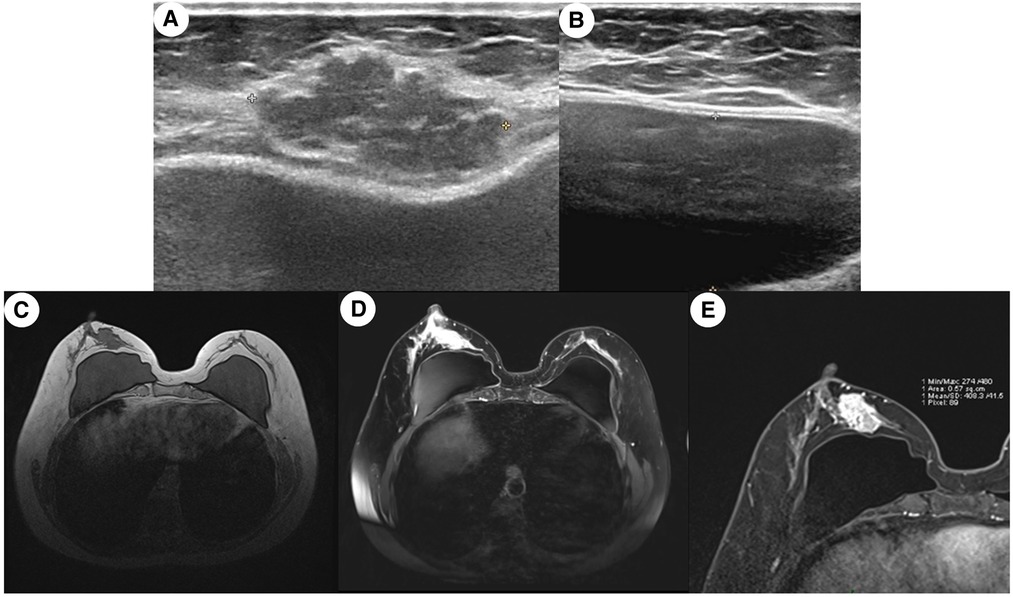
Figure 1. Medical imaging presentation of the breast mass. (A) Ultrasound image of a mixed-density multilobulated mass subcutaneous to the breast of an irregular shape and uneven margin; (B) ultrasound image of the pocket of breast prosthesis; (C) MRI image of the breast showing a lobulated mass in the right inner quadrant was adjacent to the margin of the prosthesis, and the vessels were increased and thickened; (D) contrast-enhanced MRI imaging showing that several early enhanced small nodules were seen in the upper posterior area of the tumor and the diffusion was limited, which seems like the satellite lesion of the tumor; (E) enlargement of the image in (D). The capsule of the prosthesis was wrinkled, and the edge of the prosthesis presented the change of chronic inflammation.
The MRI on August 24, 2020 (Figures 1C–E), confirmed the existence of phyllodes lump and thickening and increase of blood vessels, suspected as a malignant tumor with a grade of 4C of BI-RADS. At the same time, several early enhanced small nodules, which were suspected as the satellite lesions of the tumor, were found in the superior external area of the lump. Moreover, chronic inflammation-like changes were reported in the surrounding tissues of the prosthesis under the pectoralis major.
Treatment and final diagnosis
On September 2, 2020, considering the patients’ aesthetic requirements, we attempted to remove the lump completely with a negative incisal margin. Unfortunately, the intraoperative pathological examination indicated the breast MAME and posterior margin of the nipple showed cancer involvement. Therefore, we had to excise the nipple–areola complex and perform the skin-sparing modified radical mastectomy. Although there was no established uniform recommendation for the time interval of replacing implants, most plastic surgeons recommend routine replacement no more than 15 years after initial placement (8). At the same time, considering the possibility of subsequent radiotherapy and chemotherapy for breast cancer, the prosthesis was removed, and immediate breast reconstruction with expander implantation was performed. As intraoperative pathological examination demonstrated that no cancer metastasis was found in four sentinel lymph nodes, therefore, the patient did not undergo axillary lymph node dissection. The pathological results are shown in Figures 2, 3. Immunohistochemistry shows the following tumor cells: ER (−), PR (+, about 5%, weak to moderate intensity), HER2 (1+), GATA-3 (+), AR (+, 40%, moderate intensity), E-cadherin (+), EMA (+), GCDFP-15 (−), CD-117 (focal +), Syn (−), SOX10, CK5/6, p63, S-100 and SMM-HC (myoepithelial +), and Ki67 (Li: about 30%). Because of a bidirectional differentiation of glandular and myoepithelium, it was diagnosed as AME; at the same time, due to obvious cell atypia, pathological mitosis, infiltration at the tumor edge, etc., it was diagnosed as MAME.
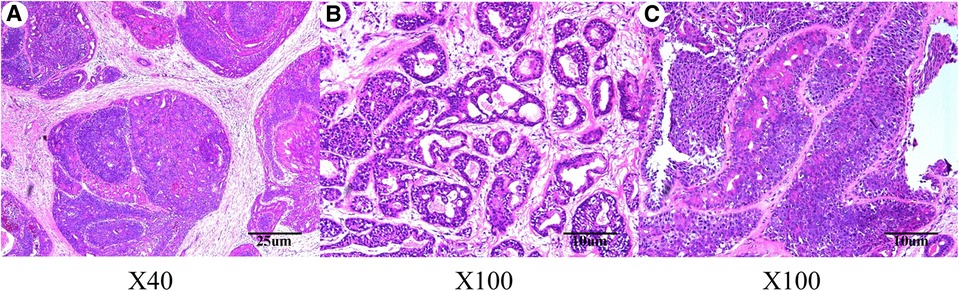
Figure 2. Hematoxylin–eosin staining indicated malignant adenomyoepithelioma of the breast lump. (A) Magnification of the main body of the lump (original magnification ×40); (B) magnification of the main body of the lump (original magnification ×100); (C) magnification of the different areas of the lump body (original magnification ×100).
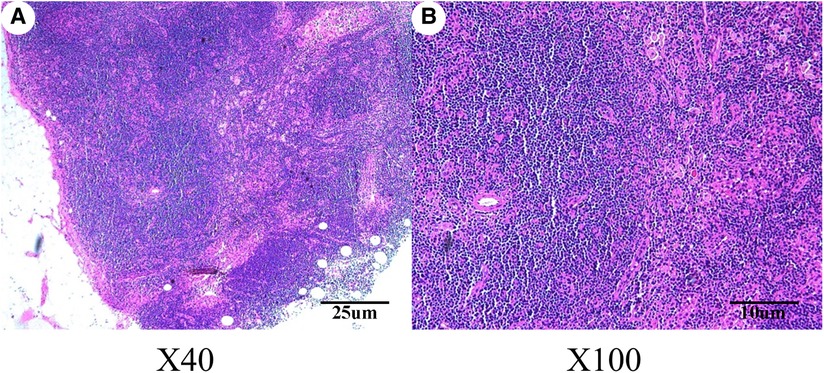
Figure 3. Hematoxylin–eosin staining revealed that no metastasis was observed in the sentinel lymph nodes of the right breast. (A) Magnification of the main body of the lymph nodes (original magnification ×40); (B) magnification of the main body of the lymph nodes (original magnification ×100).
Outcomes and follow-up
The patient recovered well after the operation with no complications and was discharged on September 10, 2020. Since November 11, 2020, she has received three cycles of chemotherapy in another hospital with the AC*4 regimen (anthracycline combined with cyclophosphamide) and then gave up on her own. There was no recurrence or metastasis in nearly 2 years of follow-up after the operation. This study was reported in agreement with the principles of the CARE guidelines (9).
Discussion
The potential carcinogenicity of breast implants has always been a common concern for both doctors and patients. Although extensive data negate any link between the implants and an increased incidence of breast cancer (1, 10), a more rigorous recognition is that the evidence remains inconclusive about any association between silicone gel implants and long-term health outcomes (10). Moreover, the largest study of breast implants based on the long-term results of 9,9993 patients explicitly claimed that silicone implants are associated with an increased risk of certain rare harm (11). Meanwhile, Pan et al. suggested that the incidence of breast cancer for women with submuscular implants was higher than that for those with subglandular implants (5). All these views seem to support the presentation of this study to some extent. In addition, some scholars believe that cosmetic breast implants adversely affect breast cancer-specific survival following the diagnosis of such disease (3). However, some studies found that patients with implants were more likely to develop a cancer diagnosis compared with the general population. However, the data do not support breast implants being responsible for these findings. At the same time, some research studies indicated that women with breast implants have different patient demographics and lifestyles from the general population, which may also explain the finding. As for ALCL, reports from the scientific community have suggested a possible link between the disease and breast implants (6). Therefore, this study concludes that more authoritative, large-scale studies are needed to carefully elucidate the relationship between breast implants and long-term health outcomes. Also, whether there is a potential relationship between MAME and breast implants remains to be further explored.
Breast AME is a very rare breast tumor. Most of the relevant literature works are in the form of case reports. According to the statistics of AME cases reported in the literature since 2010, the age of onset of breast AME ranged from 27 to 83 years, with an average of about 60 years. Most of the cases were found in females, although male breast AME cases were also reported. The vast majority of breast AME cases are benign, and the most common site is the external superior quadrant of the breast; the longest course of the disease is 14 years, and the shortest is 4 days. The metastatic sites of AME include lymph nodes, lungs, brain, bone, thyroid, liver, kidneys, thoracic wall, soft tissue, and axillary lymph nodes (12). In the cases with metastasis, the number of distant metastases was significantly more than that of axillary lymph node metastases, so we believe that hematogenous metastasis is more common than lymph node metastasis in breast AME.
Breast AME is difficult to diagnose because of its low incidence rate. According to the clinical data, the most common clinical manifestation was a painless breast lump. Physical examination showed that the lump was medium or hard; most had no nipple discharge, but there were also records of bloody discharge. The most common ultrasound result was round or oval hypoechoic or mixed echo solid lobulated nodules with clear boundaries. Most of the breast molybdenum showed lobulated, irregular, fuzzy boundary, isodense, or slightly high-density lumps, and some of them had small central flake calcification. The clinical manifestations and ultrasound, mammography, and MRI findings of this disease are difficult to distinguish from other breast tumors, among which the most easily misdiagnosed is breast fibroadenoma. Therefore, the main method of diagnosis is surgical resection and pathological examination: pathological diagnosis includes morphological, biological, and immunohistochemical examination. Breast AME is easy to be misdiagnosed clinically, and it should be carefully differentiated from breast fibroadenoma, benign myoepithelioma, pleomorphic adenoma, adenoid cystic carcinoma, myoepithelial carcinoma, etc.
Meanwhile, it is difficult to distinguish between benign and malignant breast AMEs. The malignant features reported in the literature that may lead to recurrence and metastasis included cellular pleomorphism, increased mitotic activity, nuclear pleomorphism, prominent nucleoli, hyperchromasia, and necrosis. The above indexes should be considered comprehensively; otherwise, it is easy to be misdiagnosed. For MAME, the biological behavior seems to be related to the degree of malignant component and tumor size (13). The two components of AME (mammary epithelium and myoepithelium) can be malignant transformation and distant metastasis. Most of the time, it is the malignant transformation of only one of the components, but cases of two components malignant transformation at the same time have also been reported (14). Immunohistochemistry showed that the tumor was bipolar; in most cases, SMA, actin, p63, CK5/6, and S-100 were positive in myoepithelial cells, while ER/PR was negative. Some think that the combination of p63 and actin/troponin immunostaining is the most suitable method for visualizing myoepithelial cells (15). Most cases are often triple negative (ER/PR/HER-2 negative) (16), so endocrine therapy and anti-Her-2 therapy are often ineffective, which also leads to the difficulty of treatment. Tavassoli (17) divided breast AMEs into three types: (1) spindle cell type, in which the lesions were mainly composed of proliferative spindle myoepithelial cells mixed with a small number of epithelial cells; (2) tubular type, in which the lesions are composed of myoepithelial cells and glandular epithelial cells around the duct, similar to sclerosing papilloma, tubular adenoma, and glandular tubular adenoma; and (3) tubular type, in which the proliferative myoepithelial cells are arranged in a solid, nest-like arrangement, and some of them are like plasma cells, the cytoplasm is dense, transparent, eosinophilic, and the nucleus moves around. Additionally, recently, breast AMEs were characterized as PIK3CA, AKT1, and HRAS mutations (18).
At present, the treatment for breast AME has not been unified, but there are literature (19) records that breast AME has the risk of recurrence and metastasis. Therefore, for benign breast AME, we recommended local lump resection and keeping a safe incisal margin, while for malignant breast AME, we recommended modified radical mastectomy to ensure a negative margin; sentinel lymph node biopsy was used to decide whether axillary lymph node dissection is necessary. There is no clear data to support the effectiveness of chemotherapy, radiation therapy, and endocrine therapy. Whether patients with MAME should receive chemotherapy and radiotherapy is still controversial. We believe that it should be considered comprehensively from the tumor’s biological behavior and morphological behavior, invasion degree of surrounding tissues, lymph node metastasis, and patients’ will. Two cases of chemotherapy and one case of radiotherapy were recorded: one received TC (paclitaxel liposome and cyclophosphamide) regimens with four cycles; one received one cycle of TE regimen (docetaxel and epirubicin) before operation and another cycle of TE regimen after operation; one received radiotherapy [gray (Gy) 50 total dose plus a boost of Gy 10 to the tumor bed] (20). For metastatic breast AME, there is no definite reported treatment method, and its prognosis is very poor (21). No literature proves that radiotherapy and chemotherapy have therapeutic effects on metastatic breast AME, but some scholars (22) believe that eribulin may be beneficial to patients with metastatic breast AME.
Since there is no literature on MAME after augmentation mammoplasty, this first reported study may provide experience for managing such patients. Some limitations were present in this study. First, more details of augmentation mammoplasty were unknown; Second, the follow-up time was too short to assess the long-term outcomes. Third, we are unable to present preoperative and postoperative surgical photos of the patient due to the patient’s concern about privacy protection.
Conclusion
Here, we present a unique case of a patient diagnosed with breast MAME after breast augmentation. Skin-sparing modified radical mastectomy and immediate breast reconstruction with expander implantation is a feasible approach that yields at least short-term oncological safety and acceptable aesthetic results. However, whether there is a potential relationship between MAME and breast implants remains to be further explored. Meanwhile, due to the rarity of breast MAME, more authoritative strategies considering both oncological safety and aesthetics to seek better long-term therapeutic effects are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conception and design: all authors; administrative support: XQ and LM; provision of study materials or patients: ZcY and KjB; collection and assembly of data: LH and BQ; data analysis and interpretation: LH and BQ; pathological diagnosis: ZY; manuscript writing: all authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rohrich RJ, Kaplan J, Dayan E. Silicone implant illness: science versus myth? Plast Reconstr Surg. (2019) 144:98–109. doi: 10.1097/PRS.0000000000005710
2. Cho EH, Shammas RL, Phillips BT, Greenup RA, Hwang ES, Hollenbeck ST. Breast cancer after augmentation: oncologic and reconstructive considerations among women undergoing mastectomy. Plast Reconstr Surg. (2017) 139:1240e–9e. doi: 10.1097/PRS.0000000000003342
3. Lavigne E, Holowaty EJ, Pan SY, Villeneuve PJ, Johnson KC, Fergusson DA, et al. Breast cancer detection and survival among women with cosmetic breast implants: systematic review and meta-analysis of observational studies. Br Med J. (2013) 346:f2399. doi: 10.1136/bmj.f2399
4. Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, et al. Breast cancer after augmentation mammoplasty. Ann Surg Oncol. (2001) 8:138–44. doi: 10.1007/s10434-001-0138-x
5. Pan SY, Lavigne E, Holowaty EJ, Villeneuve PJ, Xie L, Morrison H, et al. Canadian breast implant cohort: extended follow-up of cancer incidence. Int J Cancer. (2012) 131:E1148–57. doi: 10.1002/ijc.27603
6. Leberfinger AN, Behar BJ, Williams NC, Rakszawski KL, Potochny JD, Mackay DR, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. (2017) 152:1161–8. doi: 10.1001/jamasurg.2017.4026
7. Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. (1970) 53:161–220. doi: 10.1007/978-3-662-30514-0_3
8. Fardo D, Sequeira Campos M, Pensler JM. Breast augmentation, Treasure Island (FL): StatPearls (2022).
9. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE Guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
10. Balk EM, Earley A, Avendano EA, Raman G. Long-term health outcomes in women with silicone gel breast implants: a systematic review. Ann Intern Med. (2016) 164:164–75. doi: 10.7326/M15-1169
11. Coroneos CJ, Selber JC, Offodile AC 2nd, Butler CE, Clemens MW. US FDA breast implant postapproval studies: long-term outcomes in 99,993 patients. Ann Surg. (2019) 269:30–6. doi: 10.1097/SLA.0000000000002990
12. Xu J, Tang X, Iida Y, Fuchinoue F, Kusumi T, Yagihashi N, et al. Adenomyoepithelioma with carcinoma of the breast: a report of two cases and a review of the literature. Pathol Res Pract. (2016) 212:130–4. doi: 10.1016/j.prp.2015.09.008
13. Van Dorpe J, De Pauw A, Moerman P. Adenoid cystic carcinoma arising in an adenomyoepithelioma of the breast. Virchows Arch. (1998) 432:119–22. doi: 10.1007/s004280050144
14. Hungermann D, Buerger H, Oehlschlegel C, Herbst H, Boecker W. Adenomyoepithelial tumours and myoepithelial carcinomas of the breast–a spectrum of monophasic and biphasic tumours dominated by immature myoepithelial cells. BMC Cancer. (2005) 5:92. doi: 10.1186/1471-2407-5-92
15. Díaz Del Arco C, Estrada Muñoz L, Pascual Martín A, Pelayo Alarcón A, de Pablo Velasco D, Ortega Medina L. Adenomyoepithelioma of the breast: report of four cases and literature review. Rev Esp Patol. (2018) 51:55–60. doi: 10.1016/j.patol.2016.12.001
16. Delteil C, Jalaguier Coudray A, Charafe Jauffret E, Thomassin Piana J. (Adenomyoepithelioma with dominant myoepithelial contingent of the breast: a case report and literature review). Ann Pathol. (2015) 35:449–53. doi: 10.1016/j.annpat.2015.05.006
17. Tavassoli FA. Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. (1991) 15:554–68. doi: 10.1097/00000478-199106000-00004
18. Lubin D, Toorens E, Zhang PJ, Jaffer S, Baraban E, Bleiweiss IJ, et al. Adenomyoepitheliomas of the breast frequently harbor recurrent hotspot mutations in PIK3-AKT pathway-related genes and a subset show genetic similarity to salivary gland epithelial-myoepithelial carcinoma. Am J Surg Pathol. (2019) 43:1005–13. doi: 10.1097/PAS.0000000000001275
19. Hayes MM. Adenomyoepithelioma of the breast: a review stressing its propensity for malignant transformation. J Clin Pathol. (2011) 64:477–84. doi: 10.1136/jcp.2010.087718
20. Petrozza V, Pasciuti G, Pacchiarotti A, Tomao F, Zoratto F, Rossi L, et al. Breast adenomyoepithelioma: a case report with malignant proliferation of epithelial and myoepithelial elements. World J Surg Oncol. (2013) 11:285. doi: 10.1186/1477-7819-11-285
21. Kihara M, Yokomise H, Irie A, Kobayashi S, Kushida Y, Yamauchi A. Malignant adenomyoepithelioma of the breast with lung metastases: report of a case. Surg Today. (2001) 31:899–903. doi: 10.1007/s005950170031
Keywords: breast augmentation, malignant adenomyoepithelioma, MAME, prosthetic implantation, oncological safety, breast reconstruction
Citation: Hu L, Qian B, Yan Z, Bing K, Mei L and Qu X (2022) Case report and literature review: Malignant adenomyoepithelioma after breast augmentation. Front. Surg. 9:981045. doi: 10.3389/fsurg.2022.981045
Received: 29 June 2022; Accepted: 15 September 2022;
Published: 13 October 2022.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Anastasios Papanastasiou, University of West Attica, GreeceCenap Dener, Breast and Endocrine Surgery, Turkey
Maria Sotiropoulou, Evaggelismos General Hospital, Greece
© 2022 Hu, Qian, Yan, Bing, Mei and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Mei NTIzMDM2OTIxQHFxLmNvbQ== Xincai Qu UXV4YzIwMDhAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Longqing Hu
Longqing Hu Bei Qian
Bei Qian Zhecheng Yan2
Zhecheng Yan2 Xincai Qu
Xincai Qu