- 1Department of Medical, Surgical Sciences and Advanced Technologies G.F. Ingrassia, University of Catania, Catania, Italy
- 2Department of Experimental Oncology, Mediterranean institute of Oncology, Viagrande, Catania, Italy
- 3Fondazione Mediterranea G.B. Morgagni, Catania, Italy
A fistula that connects the bowel to other organs, such as the urinary bladder or small intestine, is a relatively frequent complication, often associated with inflammatory diseases such as diverticulitis, Crohn's disease, colorectal cancer, or lymphoma. Splenocolic fistula is an extremely rare condition described in the literature. It can occur in cases of splenic tumors, including splenic diffuse large B cell lymphoma. We report the case of an 82-year-old man who presented with melaena, worsening asthenia, hypotension, and abdominal pain in the left flank and the ipsilateral lumbar region. Ultrasound and computed tomography documented splenomegaly, thickening of the splenic flexure of the colon, and the presence of a fistulous passage between the colon and the splenic hilum. The diagnosis of lymphoma was made following laparotomy and caudal splenopancreatectomy. Due to the aggressive clinical behavior of this type of lymphoma, splenectomy is the main treatment in patients with splenomegaly, abdominal pain, and tumor expansion.
Introduction
A colonic fistula is an abnormal tunnel that connects the colon to the skin or an internal organ. When abnormal communication occurs between the colon and spleen, a splenocolic fistula develops, a rare condition described in the literature (1). It has been described in association with malignancy (2), Crohn's disease (3), and pancreatitis (4).
Diffuse large B-cell lymphomas (DLBCLs) are lymphoid neoplasms characterized by rapid, aggressive growth, making up about 40% of B-cell malignancies (5). They are the most frequently diagnosed form in Western countries. The average age of onset is around 65 years, with a preference for the male sex. These neoplastic forms are often associated with a 3q27 translocation affecting the BCL6 gene, but translocations involving the BCL2 or MYC genes can also occur (6). Patients with DLBCLs are stratified into prognostic groups based on the International Prognostic Index (IPI), calculated in relation to predictor factors such as age, lactate dehydrogenase (LDH), sites of involvement, Ann Arbor stage, and ECOG performance status (7).
DLBCLs are characterized by extensive lymph node involvement, systemic symptoms, and, in 40% of cases, an extranodal localization (more often affecting the gastrointestinal system). Clinically, they determine palpable splenomegaly, abdominal pain, and signs of tumor expansion in cases of hepatic and splenic involvement (8). In the last decade, a remarkable improvement in long-term disease control and overall survival has been observed, with more than 80% of patients in remission 5 years after the treatment. The standard treatment of advanced DLBCL is a combination of chemotherapy plus immunotherapy. Usually, complete remission is observed after first-line treatment (9). Most relapses occur during the first 2 years after finishing treatment, and often, they are associated with a poor prognosis.
Intra-abdominal fistula formation is frequent. It is often due to inflammatory gastrointestinal diseases or malignant tumors. It is known that fistulas occur mainly in various kinds of lymphoma rather than carcinomas (10). We report here a rare case of splenocolic fistula in a patient with diffuse large B-cell lymphoma, highlighting the nonspecific clinical picture that can lead to a diagnostic and therapeutic delay.
Case report
An 82-year-old man presented with worsening asthenia, hypotension, abdominal pain in the left flank and ipsilateral lumbar region, and melaena. He had been reporting for about 1 year the onset of physical discomfort, anemia, abdominal pain in the left quadrants, and tendency to be constipated. He had already undergone an esophagogastroduodenoscopy and a pancolonoscopy, which had not shown any significant pathologies. On physical examination, he was afebrile and had a tender abdomen on deep palpation on both the left side and the ipsilateral lumbar region. Laboratory tests were significant for leukocytosis (17,700 white blood cells per cubic millimeter), anemia (hemoglobin 10.50 mg/dl), and thrombocytosis (901,000 platelets per cubic millimeter). The patient was given fluid resuscitation and broad-spectrum antibiotics. Ultrasonography of the abdomen showed splenomegaly (DT 15.32 cm), presence of a hypoechoic area with a pyramidal shape (base to the splenic hilum and apex to the subcapsular site), and intralesional air, indicating the possible presence of a fistula. A thickening of the splenic flexure of the colon with a fistulous tract that infiltrated the hilum of the spleen was evident.
An abdomen CT scan showed splenomegaly and concentric wall thickening of the descending colon near the splenic flexure, with fistulous communication between the colon and the medial surface of the spleen. A hypodense area with air bubbles was also evident (Figure 1). Because the mass infiltrated the splenic parenchyma at the hilum and the peripancreatic adipose tissue, the patient underwent splenectomy, distal pancreatectomy, and splenic flexure resection. A side-to-side anastomosis and an ileostomy were then fashioned in the right iliac fossa. The ileostomy was to protect the integrity of the side-to-side colo-colic anastomosis, which was performed under suboptimal conditions. Macroscopic examination showed a spleen measuring 18 × 11 × 8 cm. In the dome of the spleen, a 2 cm ulcerated whitish nodule was present. A solid whitish neoformation of 7 × 6 × 4 cm at the level of the splenic flexure was also evident (Figure 2). Histopathological examination of tissue samples confirmed that DLBCLextended to the splenic hilum, diffusely infiltrating the organ, the transverse mesocolon, the left flexure, and the adipose tissue of the pancreas tail (Figure 3).
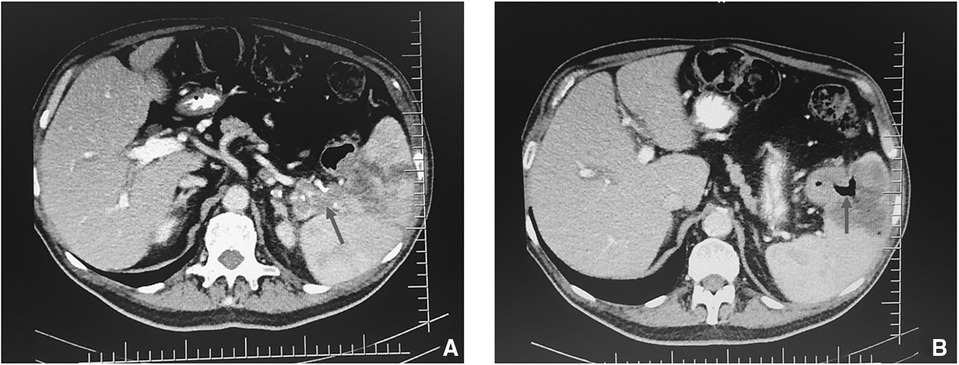
Figure 1. CT scan of the thorax and abdomen showing (A) suspicious fistulous tract between colon and spleen (arrow) and (B) air in the spleen (arrow).
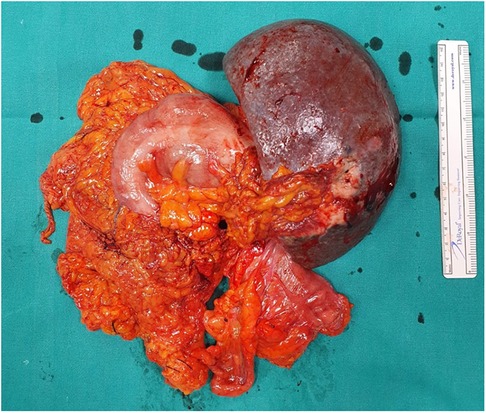
Figure 2. Surgical specimens containing splenic flexure, descending colon, and peripancreatic adipose tissue adhered and in close relationship with the medial surface of the spleen.
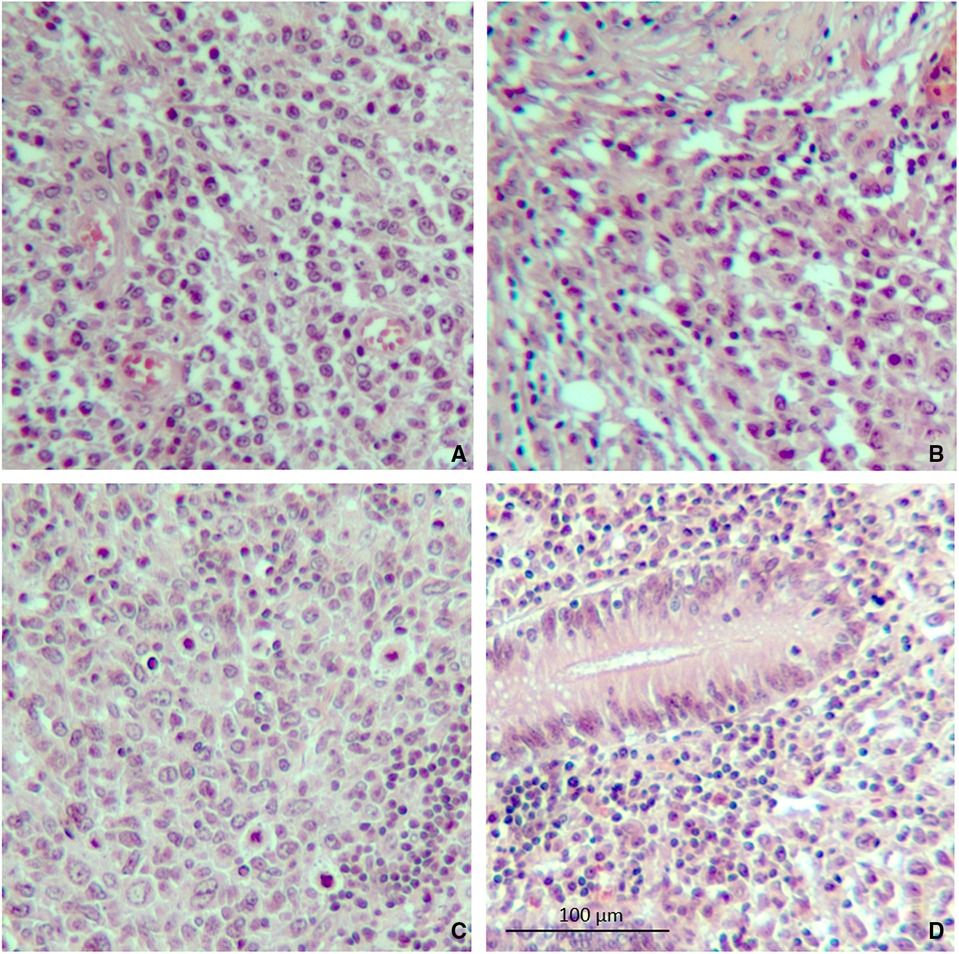
Figure 3. Microscopic specimen of the spleen and colon. (A) Atypical lymphoma cells were found in the spleen on hematoxylin–eosin stain. (B) Evidence of capsular invasion by lymphoma cells. (C) Splenic hilum lymph node containing lymphoma cells. (D) Lymphoma cells found in the colon wall near the splenocolic fistula (10× magnification).
Immunohistochemical analysis revealed CD20, CD43 (Figure 4), CD22, PAX5, CD45, and VIM (data not shown) protein expression. The postoperative course was uneventful. A follow-up chest x-ray showed left-sided pleural effusion. The patient was submitted to pleural drainage, and the pleural fluid culture was positive for Klebsiella pneumoniae. Specific antibiotics were then administered. Once the infection had cleared, the patient left the clinical center and began chemotherapy in association with immunotherapy for cancer treatment, according to the Clinical Practice Guidelines. The regimen is called R-CHOP (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone, administered every 21 days for 6–8 cycles). The patient is still being treated. Three months later, an ileostomy closure surgery was done. The patient underwent laboratory tests and radiological examinations every 3/6 month for 1 year and is still undergoing cancer therapy.
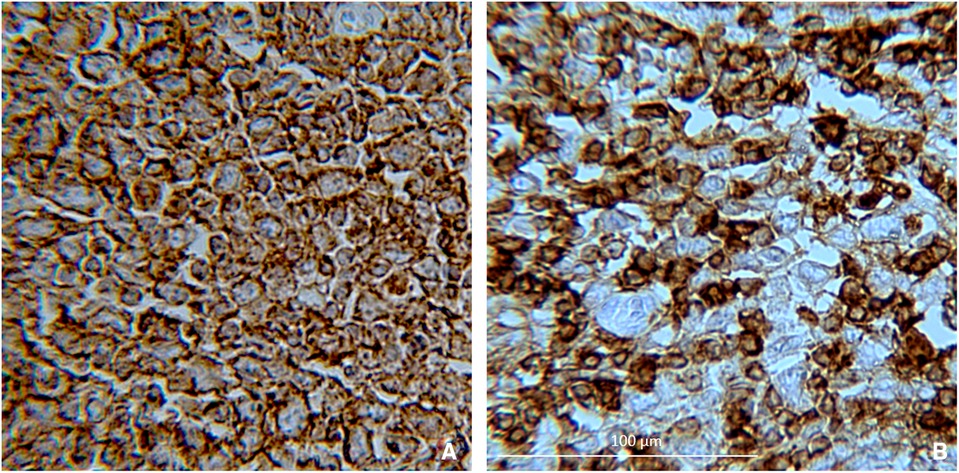
Figure 4. Positivity for (A) CD20 and (B) CD43, indicating that it is large diffuse B-cell lymphoma (20× magnification).
Discussion
DLBCL is classified within the non-Hodgkin lymphomas (NHLs), accounting for about 40% of all NHL cases worldwide (11, 12). It is also the most often diagnosed form in Western countries. The disease occurs more frequently in whites, young or old adults, with a male preponderance. It usually starts as a mass that grows quickly in a lymph node deep inside the body, such as in the chest or abdomen, or in a lymph node near the body's surface, such as in the neck or armpit. It can also start at a site other than a lymph node, such as bones, brain or spinal cord, and the intestines. It is characterized by the absence of generalized lymphadenopathy and bone marrow involvement. The diagnosis is based on the combined results of biopsy, processing, and microscopic examination to evaluate the morphology. Immunophenotyping, along with staining for B-cell markers, is usually performed (13). Clinically, DLBCL is characterized by rapid symptomatology with splenomegaly, dyspepsia, pain, and sometimes symptoms of intestinal obstruction. In cases of extranodal involvement, symptoms and signs can vary, depending on which organs or tissues are affected. They reflect the presence of a rapidly expanding tumor or infiltrate that produces symptoms specific to the organ of involvement, such as increased size, pain, and/or dysfunction. Treatment can be a combination of radiation and chemotherapy. Surgery is performed when there is damage to the liver and spleen.
The definition of primary extranodal lymphoma is controversial, especially in those patients who have both nodal and extranodal involvement. According to Krol et al. (14), primary NHL is considered extranodal when it originates at an extranodal site, even in the presence of disseminated disease, as long as the extranodal component is clinically dominant.
NHL can start anywhere in the body where lymph tissue is found, and for this reason, the digestive tract, where many organs have lymph tissue, is often involved. This is particularly true for DLBCL.
Intra-abdominal fistula formation is a rare event. Fistulas are usually the result of intra-abdominal inflammation or infection or the consequence of malignant disease, or they develop as a complication of abdominal surgery or trauma. Our search of the literature led to the finding of a previous report of colonic fistula formation, a gastrocolic fistula (15), and, subsequently, two more recent cases of fistula connecting the colon and spleen (16, 17). Many studies documented the tendency of the colon to fistulize to contiguous organs, such as the bladder (colovesical fistula), vagina (colovaginal fistula), and intestinal loops (coloenteric fistula). Other organs, such as the uterus, gallbladder, and spleen, are rarely involved (18–21).
Fistula formation can be a consequence of different pathologies, such as Crohn's disease (22), diverticular disease (23), colorectal cancer (4, 24), and lymphoma (25).
It is known that a fistulization is a rare event in malignancies such as gastric or colonic cancer (26). The development of fistulas in association with gastrointestinal lymphoma, although rare, is more common than in carcinoma because of the absence of a desmoplastic reaction (27). Splenic involvement rarely occurs. Treatment usually consists of the resection of the segment of the colon and the other affected organs. Unlike colovesical and colovaginal fistulas, splenocolic fistula is a rare clinical entity, with few cases reported in the literature. As for the most common forms, it can be secondary to various inflammatory and neoplastic conditions, including complicated diverticular disease, locally advanced colorectal cancer, lymphoma (10), or pancreatitis (28). Fistulas between the colon and spleen, even if rare, are often associated with severe Crohn's disease (3, 29).
Both in splenocolic fistula and other cases of colic fistulas associated with malignancies, necrosis is often thought to be the ultimate cause of fistula formation. Necrosis is accompanied by ulceration and extensive coagulation. In most cases, surgery, after preoperative imaging, allows removal of the fistulous tract by resecting the organs, or the affected portions of them, involved in the pathological process.
In our case, the patient presented with a rare splenocolic fistula. Surgical intervention was urgent and particularly useful to diagnose the fistula associated with DLBCL and to confirm the splenic localization. Similar cases have been reported in the literature. In particular, Katayama et al. described a case of a fistula of the colon in non-Hodgkin's lymphoma of the spleen (30). Natschitz et al. described a colosplenic fistula that complicated immunoblastic lymphoma.
In conclusion, we report an exceedingly rare condition in which the striking feature was the presence of a splenocolic fistula associated with DLBCL in an elderly patient. Our findings suggest that lymphoma cells had infiltrated from the spleen to the colon. As with any severe chronic pathology, early detection and recognition of the disease process were necessary to prevent complications. It is crucial to consider splenocolic in the differential diagnosis when a patient present with sepsis, splenomegaly, abdominal pain, and tumor expansion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with national legislation and institutional requirements.
Author contributions
TL: Data curation and acquisition, writing and revising the work. MS: Conception and writing the work. OC, EL, GC, and PF: Data curation and acquisition and writing. SC: Methodology, investigation, resources, supervision, drafting the work, final approval of the version to be published. All authors critically revised the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Goldberg JB, Moses RA, Holubar SD. Colosplenic fistula: a highly unusual colonic fistula. J Gastrointest Surg. (2012) 16:2338–40. doi: 10.1007/s11605-012-2033-0
2. Chun ES, Demos TC, Gaynor ER. Case report. Colosplenic fistula in a patient treated with interleukin-2 for malignant melanoma. J Comput Assist Tomogr. (1997) 21(4):674–6. doi: 10.1097/00004728-199707000-00031
3. Pappalardo E, Ricci A, Dray X, Marteau P, Valleur P. Splenic abscess secondary to a colosplenic fistula in Crohn’s disease. Acta Chir Belg. (2007) 107(3):323–4. doi: 10.1016/j.ijscr.2017.07.053
4. Goldsmith PJ, Pine JK, Smith AM. An unusual case of rectal bleeding: colosplenic fistula complicating pancreatitis. Pancreas. (2011) 40(2):316–7. doi: 10.1097/MPA.0b013e3181fe4ae3
5. Solimando AG, Annese T, Tamma R, Ingravallo G, Maiorano E, Vacca A, et al. New insights into diffuse large B-cell lymphoma pathobiology. Cancers (Basel). (2020) 12(7):1869. doi: 10.3390/cancers12071869
6. Zhang YX, Wang H, Ren C, Yu H, Fang W, Zhang N, et al. Correlation between C-MYC, BCL-2, and BCL-6 protein expression and gene translocation as biomarkers in diagnosis and prognosis of diffuse large B-cell lymphoma. Front Pharmacol. (2018) 9:1497. doi: 10.3389/fphar.2018.01497
7. Zhou Z, Sehn LH, Rademaker AW, Gordon LI, LaCasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. (2014) 123(6):837–42. doi: 10.1182/blood-2013-09-524108
8. Bairey O, Shvidel L, Perry C, Dann EJ, Ruchlemer R, Tadmor T, et al. Characteristics of primary splenic diffuse large B-cell lymphoma and role of splenectomy in improving survival. Cancer. (2015) 121(17):2909–16. doi: 10.1002/cncr.29487
9. Non-Hodgkin’s Lymphoma: Diagnosis and Management. NICE Guideline, No. 52. National Guideline Alliance (UK). London: National Institute for Health and Care Excellence (UK) (2016).
10. Naschitz JE, Yeshurun D, Horovitz IL, Dahaan A, Lazarov NB, Boss YE. Spontaneous colosplenic fistula complicating immunoblastic lymphoma. Dis Colon Rectum. (1986) 29(8):521–3. doi: 10.1007/BF02562610
11. Rai B, Astekar MS, Manjunatha BS, Sapra G. Diffuse large B cell lymphoma of hard palate: a case report with literature review. J Exp Ther Oncol. (2017) 11(2):101–5. doi: 10.4103/0973-029X.131927
12. [Database] Padala SA, Kallam A. Diffuse large B cell lymphoma. StatPearls. Treasure Island (FL): StatPearls Publishing (2020).
13. Crombie J. Classifying DLBCL subtypes for optimal treatment. Oncology. (2019) 33(10):686504.31661151
14. Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West populations-based NHL registry. Ann Oncol. (2003) 14:131–9. doi: 10.1093/annonc/mdg004
15. Murchison C. Cases of gastrocolic fistula with observations on its pathology, diagnosis, etc. Edin Med J. (1857) 3:4–21.
16. Al-Zahir AA, Meshikhes AW. Colonic lymphoma presenting acutely with perforated colo-splenic fistula. Int J Surg Case Rep. (2012) 3(8):368–71. doi: 10.1016/j.ijscr.2012.04.013
17. Ashouri M, Kuhi Z, Ataie-Ashtiani Z, Mohammadzadeh N. Colosplenic fistula presentation in the context of undiagnosed colon cancer: case report and review of literature. Int J Surg Case Rep. (2022) 92:106828. doi: 10.1016/j.ijscr.2022.106828
18. Lavery IC. Colonic fistulas. Surg Clin North Am. (1996) 76(5):1183–90. doi: 10.1016/s0039-6109(05)70506-5
19. Galanis I, Floros G, Theodoropoulos C, Metaxa M, Theodoropoulos P, Tsintavis P, et al. Colouterine and jejunouterine fistula secondary to chronic diverticulitis. Case Rep Gastrointest Med. (2021) 2021:5543505. doi: 10.1155/2021/5543505
20. Cao Y, Li C, Qiao Z, Zhu J. Laparoscopic diagnosis and treatment of cholecysto-colonic fistula: a case report. Asian J. Surg. (2021) 44(7):984–6. doi: 10.1016/j.asjsur.2021.04.019
21. Winter MW, Lee S. Colosplenopleural fistula: an unusual colonic fistula in a 44-year-old male with Crohn’s disease. Radiol Case Rep. (2015) 9(4):1028. doi: 10.2484/rcr.v9i4.1028
22. Annibali R, Pietri P. Fistulous complications of Crohn’s disease. Int Surg. (1992) 77(1):19–27.1577575
23. Elliott TB, Yego S, Irvin TT. Five-year audit of the acute complications of diverticular disease. Br J Surg. (1997) 84(4):535–9. doi: 10.1046/j.1365-2168.1997.02620.x
24. Gervaise A, De Saint Roman C, Sockeel P, Lapierre M, Darbois H, Rousset J, et al. Splenic abscess secondary to a colosplenic fistula as the presenting manifestation of colon cancer. J Radiol. (2010) 91(12 Pt1):1259–62. doi: 10.1016/s0221-0363(10)70184-0
25. Kang DH, Huh J, Lee JH, Jeong YK, Cha HJ. Gastrosplenic fistula occurring in lymphoma patients: systematic review with a new case of extranodal NK/T-cell lymphoma. World J Gastroenterol. (2017) 23(35):6491–9. doi: 10.3748/wjg.v23.i35.6491
26. MacMahon CE, Lund P. Gastrocolic fistulae of malignant origin. Am J Surg. (1963) 106(2):333–47. doi: 10.1016/0002-9610(63)90022-9
27. Allison JE. Gastrocolic fistula as a complication of gastric lymphoma. Am J Gustroenterol. (1973) 59(6):499–504.
28. Karpeh MS Jr, Hicks DG, Torosian MH. Colon invasion by primary splenic lymphoma: a case report and review of the literature. Surgery. (1992) 111(2):224–7.1736393
29. Rowell DL, Longstreth GF. Colosplenic fistula and splenic abscess complicating Crohn’s colitis. J Clin Gastroenterol. (1995) 21(1):74–5. doi: 10.1097/00004836-199507000-00016
Keywords: fistula, atypical presentation, splenomegaly, lymphoma diagnosis, diffuse large B-cell lymphoma
Citation: Luca T, Sergi M, Coco O, Leone E, Chisari G, Fontana P and Castorina S (2022) Splenocolic fistula in a patient with diffuse large B-cell lymphoma: A case report and review of literature. Front. Surg. 9:979463. doi: 10.3389/fsurg.2022.979463
Received: 27 June 2022; Accepted: 24 August 2022;
Published: 20 September 2022.
Edited by:
Stefano Pontone, Sapienza University of Rome, ItalyReviewed by:
Joao Ascensao, Washington DC VA Medical Center, United StatesAbdul Wahed Nasir Meshikhes, Alzahra General Hospital, Saudi Arabia
© 2022 Luca, Sergi, Coco, Leone, Chisari, Fontana and Castorina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Chisari Z2l1bGlhLmNoaXNhcmlAZ3J1cHBvc2FtZWQuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Tonia Luca1*†
Tonia Luca1*† Emanuela Leone
Emanuela Leone Giulia Chisari
Giulia Chisari Paolo Fontana
Paolo Fontana