- 1Department of Vascular Surgery, The First Hospital of Nanchang, Nanchang, China
- 2Second Clinical Medical College, Nanchang University Medical School, Nanchang, China
- 3Vascular and Endovascular Surgery, the PLA General Hospital, Beijing, China
Objective: This study aims to evaluate the clinical efficacy of collagen dressing for patients with chronic wounds.
Materials and methods: Relevant randomized controlled trials were searched from the databases such as PubMed, EMBASE, and the Cochrane library as of January 2022. For dichotomous outcomes and continuous outcomes, risk ratio and mean difference were calculated, respectively. Subgroup analysis was performed according to the type of chronic ulcer and follow-up. In addition, trial sequential analysis (TSA) was performed to further verify the results. Jadad score was used to assess the quality of trials. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was utilized to assess the level of evidence for outcomes.
Results: In 11 studies, a total of 961 patients of whom 485 were in the collagen group. Compared with standard of care (SOC) alone, the group that added an extra collagen dressing achieved a higher wound healing rate (Risk Ratio = 1.53; 95% CI, 1.33–1.77). The collagen group also showed a higher healing velocity than the SOC group (Mean Difference, 2.69; 95% CI, 0.87–4.51). In addition, the adverse events related to dressing between the two groups were similar (Risk Ratio = 0.67; 95% CI, 0.44–1.01).
Conclusion: Collagen dressing increases the wound healing rate and may be an effective and safe treatment for chronic wound management. However, more extensive research shall be conducted to substantiate these results.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=245728, identifier: CRD42021245728.
Introduction
Skin injuries can be divided into acute and chronic wounds, according to the required time of healing. According to the definition, chronic wounds generally are wounds that cannot heal in 6 weeks (1). Chronic wound management was a challenging health issue. The most common typical chronic ulcers seen in lower limbs are venous/arterial leg ulcers and diabetic foot ulcers (DFUs) (2). Affected by a variety of factors, chronic wounds are highly lethal, with diabetic foot even higher than many cancer cases. At the same time, they have a massive impact on the quality of life, which is also an important reason why more attention should be paid. Furthermore, chronic wounds also have imposed a huge financial burden. In the US alone, more than 6 million patients suffer from chronic trauma annually, and it is estimated that the cost of the healthcare system is ranges from $20 to $25 billion (3).
Collagen is the most abundant and widely distributed functional protein in the body and contributes to wound healing. In the hemorrhagic coagulation phase, collagen has the role of hemostat due to three-dimensional scaffolds. After interacting with fibronectin and growth factors, collagen stimulates the chemotaxis of monocytes and fibroblasts, and then granulation tissue is produced (4). As a potential treatment, collagen dressings are applied in many fields such as chronic wounds, and reconstructive, separately or together with other materials (5). Meanwhile, Tiago et al. also demonstrated that collagen was the most efficient treatment for skin wounds (6). Therefore, collagen is in a dominant position in wound healing. For example, it can improve the ability of wound macrophages, accelerate epithelial cell formation, and promote angiogenesis (7–9).
At present, the role of collagen in wound healing is becoming more widely recognized. However, the effect of collagen dressing on chronic wounds was inconclusive (10). The goal of this article was to summarize the current evidence to evaluate whether superior outcomes can be obtained by adding extra collagen dressing to chronic wounds compared with standard wound care alone.
Methods
The study protocol was prospectively registered at PROSPERO (registration no.: CRD42021245728) (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=245728) and followed Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidance (11).
Search
The articles were systematically reviewed by searching in the PubMed, EMBASE, and the Cochrane library databases as of January 2022, which compared collagen dressing and standard of care with standard of care alone for chronic wounds. Additional references from eligible studies were systematically searched to determine any supplemental publications. Taking the PubMed database as an example, the keywords of search strategy are as follows: “collagen”, “dressing”, “chronic”, “wound”, “diabetic foot ulcer”, and “venous leg ulcers (VLCs)”.
Eligibility
The inclusion criteria were as follows: (1) studies [published randomized controlled trials (RCTs)]; (2) participants (adult patients with typical chronic ulcers, such as VLUs, pressure ulcers, and DFUs); and (3) interventions (collagen-containing dressing is used in the experimental group, while conventional dressing, such as saline-moistened dressing, is used in the control group). The exclusion criteria were as follows: (1) studies that did not include original data; (2) studies that the type of article was review articles, editorials or conference abstracts, letters to the editor, and preclinical studies; (3) studies that did not report on previously defined outcomes; and (4) non-English text.
Outcome parameters and definition
The primary outcome was wound healing rate because this was the most consistently reported outcome. The formula used to calculate wound healing rate is the number of patients who achieved wound healing during follow-up/the total number of observed patients. Wound healing was defined as complete closure as 100% re-epithelialization of the wound. Healing velocity, recurrence of ulceration, and adverse events (related to dressing) were the second outcomes. Healing velocity in follow-up duration was defined as the percentage of wound area per week that was reduced. Adverse events included amputations, skin sensitization, pain, discomfort, immunological rejection, infections, and off-odor et al.
Data collection and extraction
Two independent researchers (HS, ZX) searched the database according to the search strategy designed previously and screened the retrieved studies as per the inclusion and exclusion criteria, and recorded the reasons for exclusion. If a dispute arose between the two researchers, it would be resolved by a panel discussion. Data extracted included the authors of the studies, country, year of publication, study design, population baseline, intervention, wound duration, wound area, and wound type.
Critical appraisal
Based on Cochrane Collaboration's tool, the risk of bias 2.0 (RoB 2.0) was assessed by two researchers (HS, ZX) independently (12), and any dispute was resolved by a group discussion. To describe the quality of trials, the Jadad score was used, which was composed of randomization appropriateness, blinded outcome assessment, and a complete description of loss to follow-up (13). Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to evaluate the overall level of evidence for each endpoint (14).
Data synthesis
Data synthesis was performed by using STATA 17.0 software (StataCorp, College Station, TX). I2 statistical and Chi-square tests were used to calculate heterogeneity, and it was defined as high heterogeneity when I2 was greater than 50% and if it is less than 50% it was defined as low heterogeneity. In case of high heterogeneity, a random-effect model was used for the pooled results; otherwise, a fixed-effect model was performed on the pooled data. Forest plots were conducted to visualize the effect. It was defined that P < 0.05 was considered statistically significant. For dichotomous outcomes and continuous outcomes, the risk ratio (RR) and mean difference (MD) were calculated, respectively. To evaluate the stability of the meta-analysis results, sensitivity analysis was performed by removing one study from the analysis each time. Egger's and Begg’s tests were performed to further probe publication bias (15, 16). To find the source bias of data, a subgroup analysis of relevant studies was performed according to the types of wounds and the time of follow-up.
Trial sequential analysis
In the present study, TSA v0.9.5.10 Beta Software was used to perform the trial sequential analysis (TSA). The required information size (RIS) is evaluated by setting relative risk reduction = 20%, the first type of error α = 0.05, and power = 80%. If the cumulative Z-curve crosses the RIS threshold, it means that the sample size of the accumulated evidence is sufficient. However, if the cumulative Z-curve does not cross the RIS threshold, it means that the sample size is not sufficient. Moreover, the result shall be confirmed by more studies. The TSA boundary is set based on the RIS. When the cumulative Z-curve crosses the TSA boundary, the conclusions are considered statistically significant.
Results
Literature search and selection
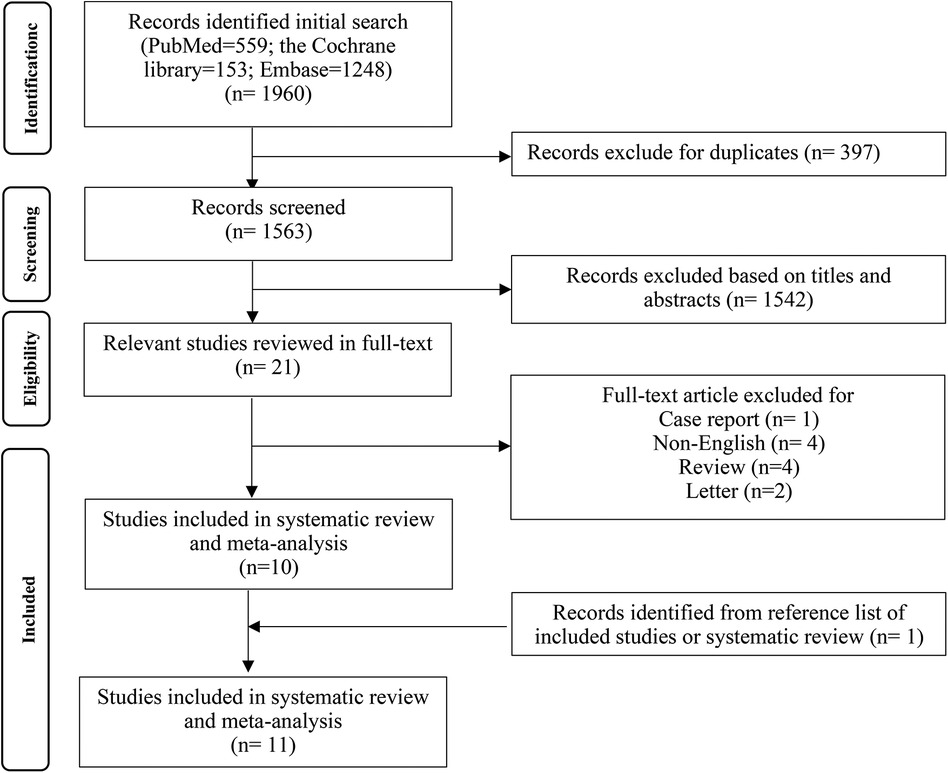
After searching according to PICOS principles, 1,960 studies were initially retrieved, of which 21 were defined as potentially eligible. Among these 21 studies, one study was excluded for case report, four studies were excluded for non-English, four studies were excluded for review, and two studies were excluded for letter. The literature search and selection processes are described in Figure 1. In the end, 11 original studies were included in the current meta-analysis.
Study characteristics
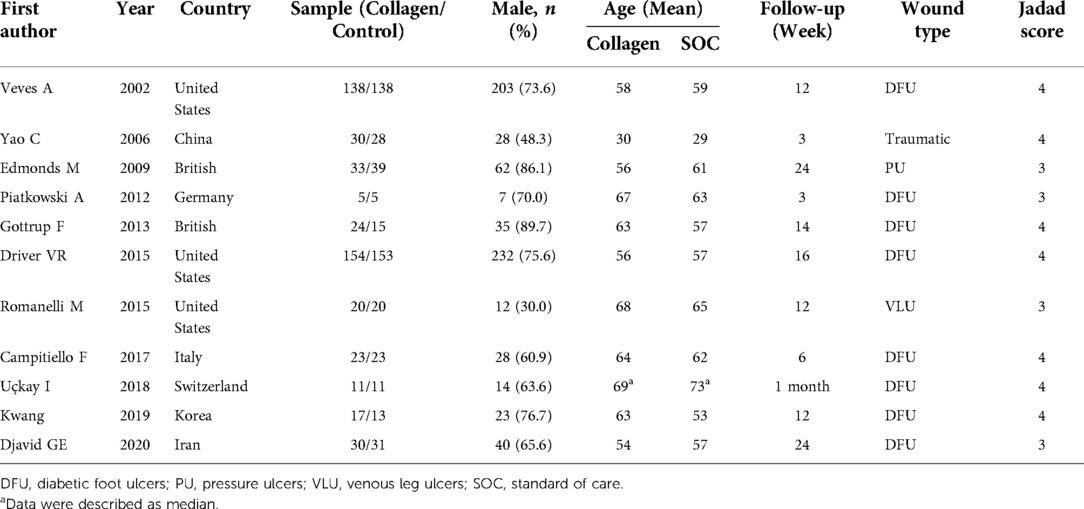
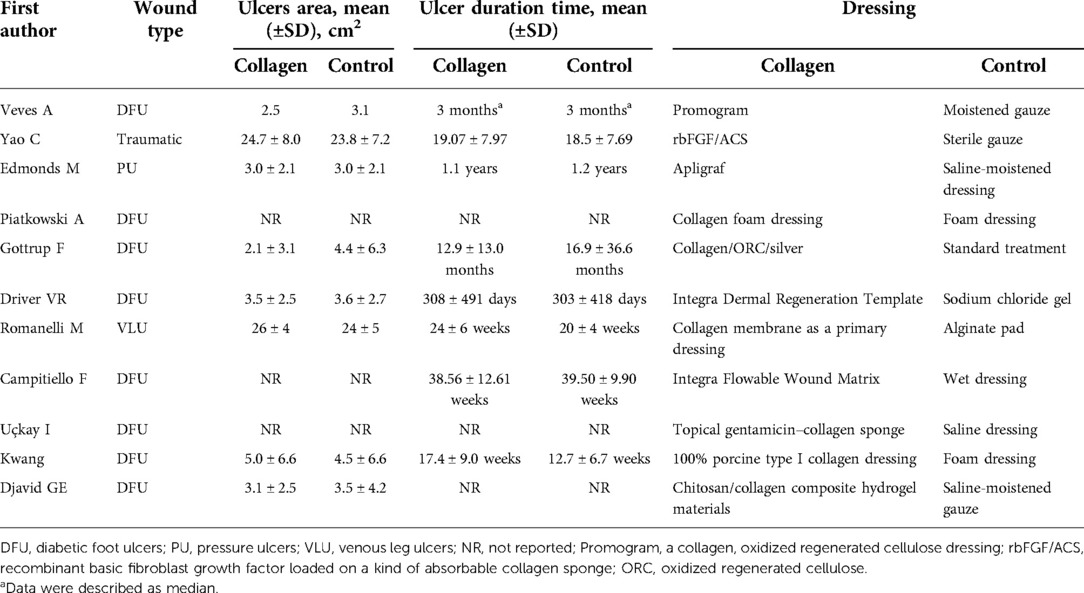
The basic characteristics of the included studies are described in Table 1, and the wound characteristics are described in Table 2 (17–27). A total of 961 patients, of whom 485 were in the collagen group, were enrolled in this study. Patients in the collagen group received collagen dressing and standard of care (SOC), while the control group underwent SOC alone. All these patients suffered from at least one chronic wound. Figures 2 and 3 show the risk of bias in the included studies. The results of the Cochrane RoB 2.0 tool indicated that two studies were considered high risk, and nine studies were considered moderate risk.
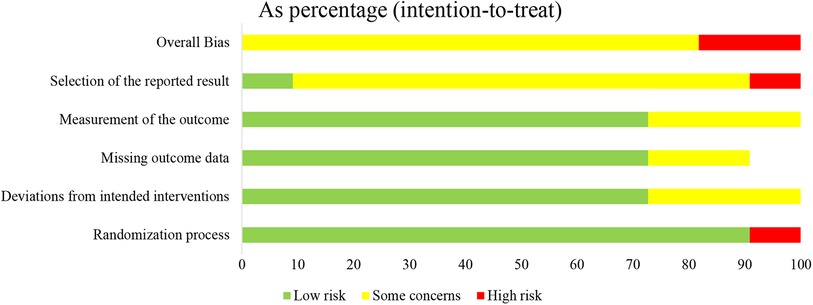
Figure 2. Methodologic quality diagram showing review authors’ judgments about each methodologic quality item presented as percentages across all included studies.
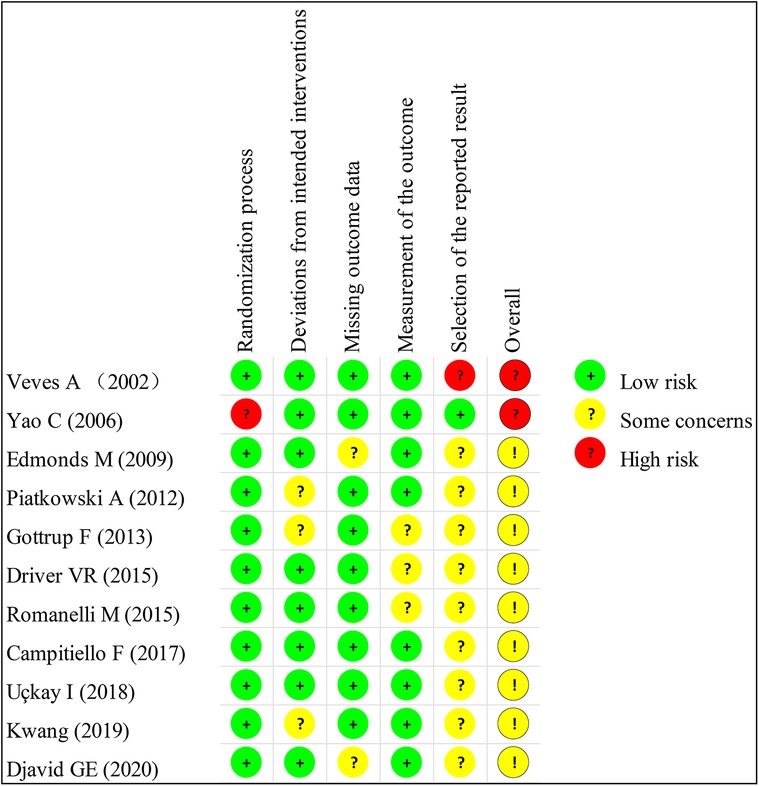
Figure 3. Methodologic quality summary showing review authors’ judgments about each methodologic quality item for each included study.
Pooled results
Wound healing rate
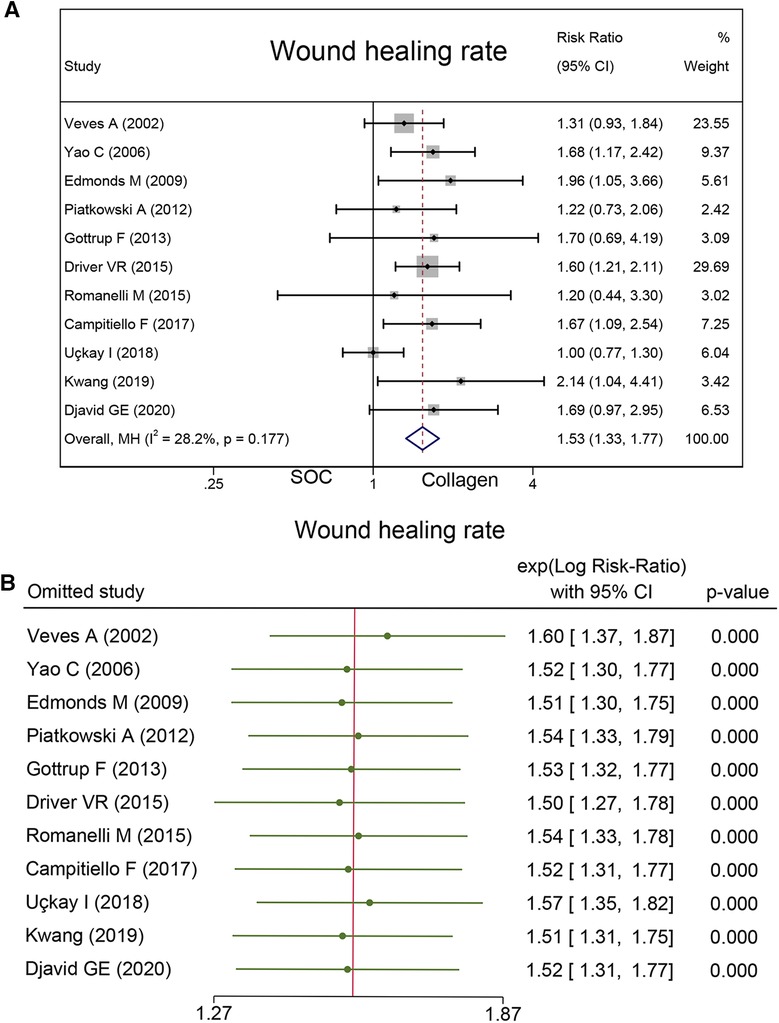
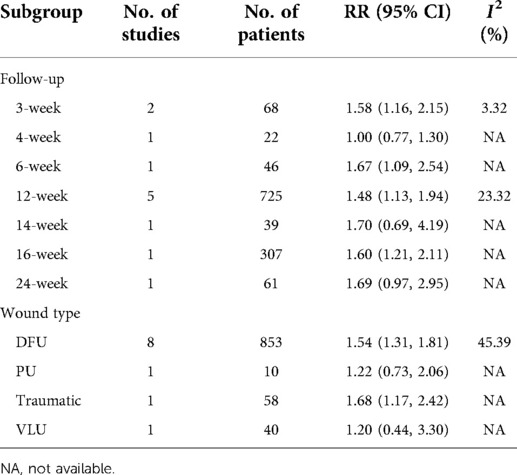
A total of 961 cases reported wound healing rate. In this endpoint, 53.4% (259/485) wounds in the collagen group and 34.50% (164/476) wounds in the SOC group achieved complete healing. Statistical analysis indicated that the wound healing rate in the collagen group is significantly higher than that in the control group (RR = 1.53; 95% CI, 1.33–1.77) (Figure 4A). No substantial heterogeneity was found as estimated by using the I2 statistic (I2 = 28.2%). No significant publication biases were found by Egger's (P = 0.14) and Begg's tests (P = 0.64). As Table 3 shows the subgroup analysis that was conducted based on follow-up and wound type. The source of heterogeneity may be explained by those factors.
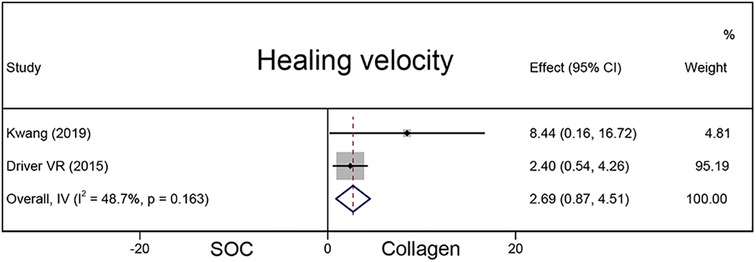
Healing velocity
A total of 337 chronic wounds in two studies were included to compare the healing velocity between the collagen and SOC groups (Figure 5). Compared with the SOC group, the collagen group had a higher average wound area reduction (MD = 2.69; 95% CI, 0.87–4.51).
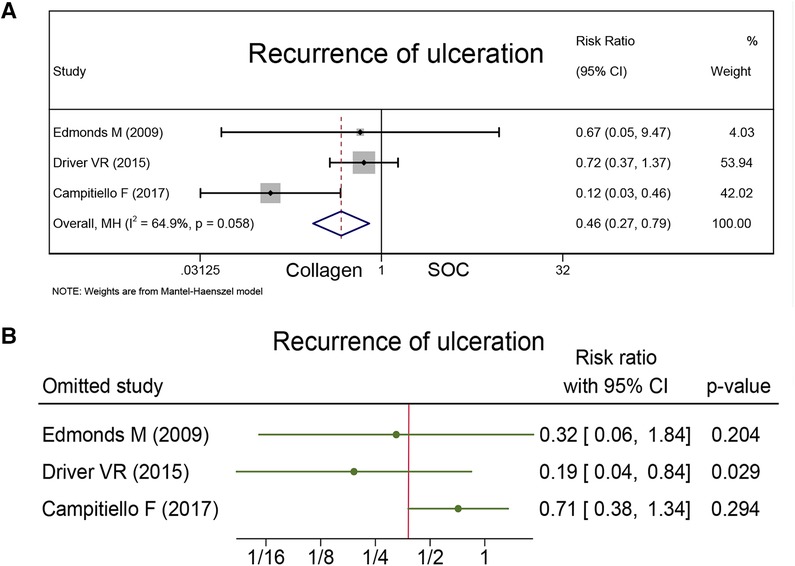
Recurrence of ulceration
The recurrence of ulceration was reported as a safety outcome in three studies together with 172 healing wounds. Campitiello et al. indicated that the number of wounds healed in the collagen and control groups during the 6-week observation period was 20 and 12, respectively, and the rate of recurrence was 8.69% for the collagen group and 43.47% for the SOC group. Driver et al. and Edmonds et al. indicated that the rate of recurrence was 19% for the collagen group and 26% for the SOC group (P = 0.32), and 7% for the collagen group and 10% for the SOC group during 12-week treatment (P = 1.00). As Figure 6A presents that the collagen group had a less recurrence rate than the control group (RR = 0.46; 95% CI, 0.27–0.79).
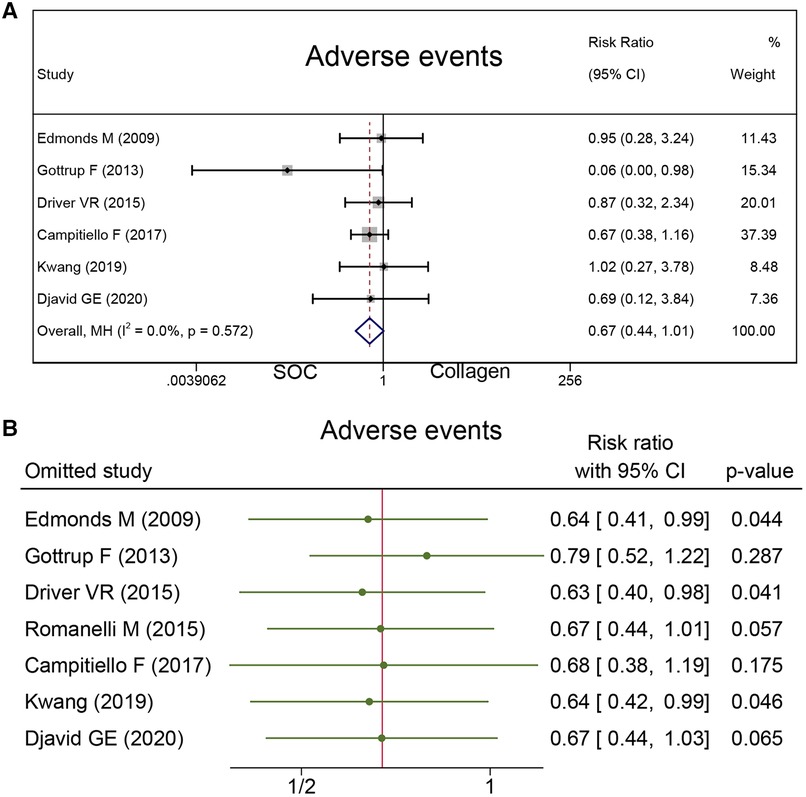
Adverse events
To measure the safety of collagen dressing, adverse events were reported in seven studies with 556 cases. There was no difference in the risk of adverse events between the collagen group and SOC group (RR = 0.67; 95% CI, 0.44–1.01), as presented in Figure 7A.
Sensitivity analysis and GRADE assessment
Sensitivity analysis was conducted, and the results are presented in Figures 4B, 6B, and 7B. The sensitivity analysis demonstrated that the results were stable. Table 4 shows the overall evidence of each outcome. The certainty level of wound healing rate and recurrence of ulceration was moderate, meanwhile, the evidence level of healing velocity and adverse events were high.
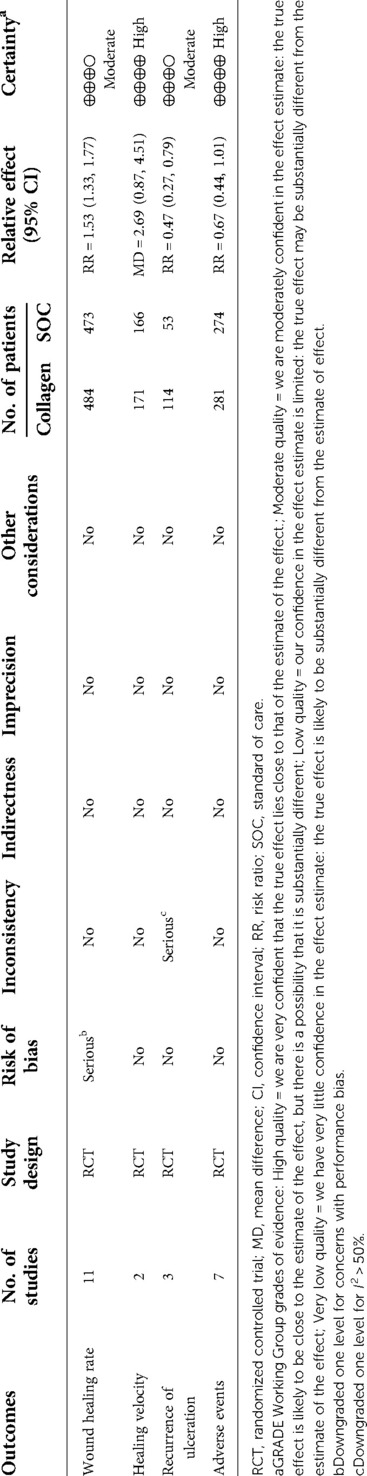
Table 4. GRADE assessment for outcomes reported in RCTs on collagen vs. standard of care for chronic wounds.
TSA results
TSA was carried out to reduce the risk of Type I error and to evaluate the RIS by keeping the overall 5% risk of Type I error and the relative risk reduction of 20% (power of 80%). For the primary outcome, the result suggested that the Z-curve crossed the TSA boundary, and the positive conclusions were obtained in advance, which was consistent with the above meta-analysis results. Hence, it can be asserted that collagen dressing was more effective in the treatment of chronic wounds, and the result was reliable (Figure 8).
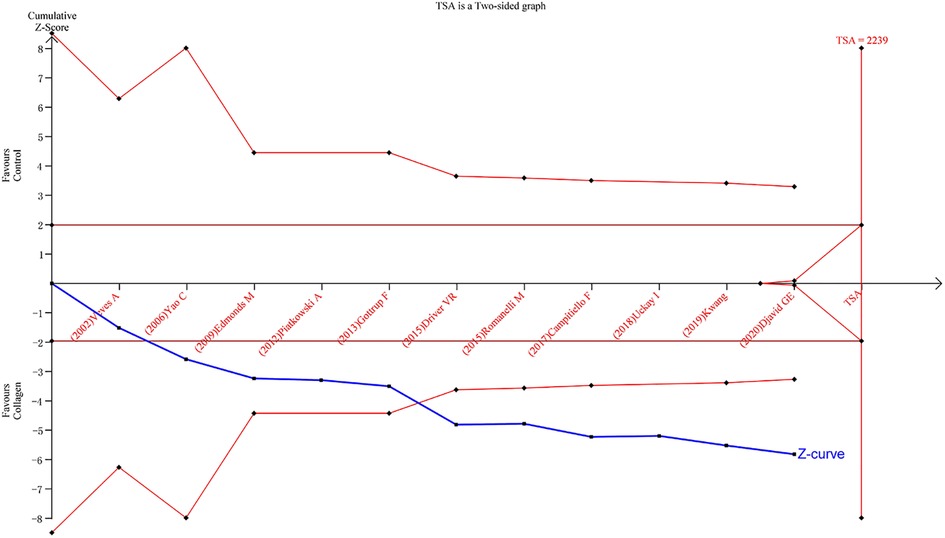
Figure 8. Results of TSA with the primary outcome. The required information size was calculated based on a two-side α = 5%, power 80%, and a relative risk reduction of 20%.
Discussion
This is the first meta-analysis that reviews the efficacy of collagen dressing on chronic wounds. In this article, available studies were combined, and a suitable methodology was used to analyze the clinical value of collagen dressing in the treatment of chronic wounds. The study design was mainly about RCTs, which enhanced the credibility of the results of this article. We specifically focused on four outcomes: wound healing rate, healing velocity, recurrence of ulceration, and adverse events. At the same time, subgroup analysis was also performed according to follow-up and wound types. In the current study, we found collagen dressing had an excellent effect on chronic wounds, which facilitated wound closure. At the same time, adverse events were similar in both groups and no immunological rejection was reported in those studies. Infections were recorded in the collagen group (8.3%) and the control group (15.0%), which suggested collagen dressing was safe and reliable.
The cost of mass production of dressings from human collagen is very high. In practice, recombinant human collagen (28) or animal collagen is used as raw materials. Most of the animal collagens are the Achilles tendon of horses and bovines or the skin of pigs or bovines (29). The process of collagen extraction is complex, a series of special gentle extraction and purification processes are applied to ensure the stability of the original collagen structure, reduce foreign body reactions and increase purity (30–32). Collagen dressings can be classified by the raw materials used in the production process, production method, and additional ingredients added to the collagen dressing (29). For example, the addition of oxidized regenerated cellulose (ORC) is effective in enhancing the management of wound exudate, while binding free radicals and metal ions to reduce protease levels (33, 34).
Collagen dressings are extremely advantageous in wound healing, which include the following: (1) it has low immune rejection in humans, which is based on the strongly preserved primary sequence and the helix structure (35, 36); (2) collagen binds platelets to trigger a coagulation cascade (37, 38) and is considered a mechanical haemostatic agent because it can slow blood flow and provide a clot-forming matrix (39); (3) due to the special three-dimensional structure of collagen, collagen dressings can absorb fluids many times their own weight, resulting in gelation and creating a moist wound environment, which is beneficial to dressing change; (4) as a competitive substrate for collagenase, it can reduce enzymatic degradation of tissues (40); (5) low pH treated collagen dressings reduce peri-wound pH, thus mitigating the risk of bacterial infection and inhibiting protease activity (41–43); (6) collagen breakdown products reduce the activity of type I collagenase (44); (7) the scaffold structure of collagen dressings chemotactic fibroblasts, macrophages and epithelial cells participates in wound healing (29); and (8) the collagen in the dressing promotes collagen deposition and angiogenesis for faster wound healing (45). A moist wound environment is also beneficial for dressing change, increases the healing rate, and improves the patient's quality of life (21). Due to the abovementioned advantages in wound management, collagen is also applied in skin substitutes (46). Apligraft is the first bio-engineered skin that was approved by FDA in 1998, which is composed of bovine collagen gel and neonatal keratinocytes (47). The successful application of Apligraft in wound management, such as DFUs, VLUs, acute wounds, and epidermolysis bullosa wounds was demonstrated (48, 49). Other skin substitutes that contribute to enhancing wound healing, such as Matriderm, Biobrane, Integra Dermal Regeneration Template, Nevelia, and OrCel also showed excellent clinical effects (46).
Although collagen dressings are costly in short term, the cost of collagen dressings can be offset by improved wound healing rate and accelerated wound healing (50, 51). Accelerating wound healing can be interpreted as reducing society's financial expenditure, as it can reduce the likelihood of incapacity. Simultaneously, it also cuts costs by declining the frequency of dressing changes (52). A previous study demonstrated adding a collagen-containing dressing for treatment DFU management could reduce cost by 22% (from £2,897 to £2,255 per patient) compared with standard care alone (53). Another study on VLU also held the same point of view on this, which reduced the cost of management by 40% (from £6,328 to £3,789 per patient) and improved quality of life (from 0.331 to 0.373 QALYs per patient) (54).
Chronic wound healing is a challenging problem. Tiago et al. (6) demonstrated that collagen has a positive effect on wound healing by reviewing 16 studies (14 studies for animals and only 2 studies for humans). However, a meta-analysis was not performed due to high heterogeneity and patients with diabetes mellitus were excluded whereas we performed a meta-analysis and included diabetes mellitus patients. Apart from typical wounds, collagen dressing is also successfully applied in atypical ulcers. Atypical ulcers, such as hydroxyurea-induced ulcers, ulcers caused by autoimmune diseases, and sickle cell anemia, were regarded as difficult to heal. In a retrospective cohort, Garwood et al. (55) found 30 of 71 atypical ulcers healing after applying bovine collagen matrix, which demonstrated that bovine collagen matrix may a successful treatment for atypical ulcers.
The current limitations of this study should be noted. First, only 11 original studies were included and 2 of them were reported as high risk. Second, the TSA analysis indicated that the number of cases included in this article was inadequate. Third, it also should be noted that the types of collagen dressings used in the included studies were inconsistent, which may contribute to heterogeneity and bias, although we tried to harmonies the dressing criteria as much as possible during the screening phase. Apart from inconsistencies in the type of dressing, there are also inconsistencies in the factors that can influence the results, including the method of measuring wound area and duration time. Fourth, immunological rejection was an issue that requested special concern. Unfortunately, limited by available data, the current systematic not pooled this endpoint. Therefore, future studies need to take these factors into account and more multicenter, randomized controlled clinical studies are needed to further demonstrate those views.
Conclusion
Collagen dressing combining SOC increases the rate of chronic wound healing, accelerates non-healing wound closure, and reduces ulcer recurrence compared with SOC alone. Therefore, adding extra collagen may be an effective potential wound management that holds the ability to facilitate chronic healing. However, more multicenter, randomized controlled clinical studies are needed to substantiate those points in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
SH conceived and wrote the article; XZ and WX searched databases and collected the data. LW analyzed the data. LW, LQ, ZZ, and XX performed the critical revision of the article. XQ revises manuscript. The authors did not receive any financial or non-financial support. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. van Koppen C, Hartmann R. Advances in the treatment of chronic wounds: a patent review. Expert Opin Ther Pat. (2015) 25(8):931–7. doi: 10.1517/13543776.2015.1045879
2. Morton L, Phillips T. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. (2016) 74(4):589–605; quiz-6. doi: 10.1016/j.jaad.2015.08.068
3. Richmond N, Lamel S, Davidson J, Martins-Green M, Sen C, Tomic-Canic M, et al. US-National Institutes of Health-funded research for cutaneous wounds in 2012. Wound Repair Regen. (2013) 21(6):789–92. doi: 10.1111/wrr.12099
4. Morimoto N, Yoshimura K, Niimi M, Ito T, Aya R, Fujitaka J, et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue Eng Part A. (2013) 19(17–18):1931–40. doi: 10.1089/ten.tea.2012.0634
5. Shekhter AB, Fayzullin AL, Vukolova MN, Rudenko TG, Osipycheva VD, Litvitsky PF. Medical applications of collagen and collagen-based materials. Curr Med Chem. (2019) 26(3):506–16. doi: 10.2174/0929867325666171205170339
6. Araujo TAT, Almeida MC, Avanzi I, Parisi J, Simon Sales AF, Na Y, et al. Collagen membranes for skin wound repair: a systematic review. J Biomater Appl. (2021) 36(1):95–112. doi: 10.1177/0885328220980278
7. Das A, Ghatak S, Sinha M, Chaffee S, Ahmed N, Parinandi N, et al. Correction of MFG-E8 resolves inflammation and promotes cutaneous wound healing in diabetes. J Immunol Res. (2016) 196(12):5089–100. doi: 10.4049/jimmunol.1502270
8. Elgharably H, Ganesh K, Dickerson J, Khanna S, Abas M, Ghatak P, et al. A modified collagen gel dressing promotes angiogenesis in a preclinical swine model of chronic ischemic wounds. (2014) 22(6):720–9. doi: 10.1111/wrr.12229
9. El Masry M, Chaffee S, Das Ghatak P, Mathew-Steiner S, Das A, Higuita-Castro N, et al. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. (2019) 33(2):2144–55. doi: 10.1096/fj.201800352R
10. Landsman A, Roukis TS, DeFronzo DJ, Agnew P, Petranto RD, Surprenant M. Living cells or collagen matrix: which is more beneficial in the treatment of diabetic foot ulcers? Wounds. (2008) 20(5):111–6. PMID: 25942411
11. Page M, Moher D, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. PRISMA 2020 Explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. (2021) 372:n160.33781993
12. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
13. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
14. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. (2011) 64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011
15. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50(4):1088–101. doi: 10.2307/2533446
16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
17. Park K, Kwon J, Park J, Shin J, Han S, Lee J, et al. Collagen dressing in the treatment of diabetic foot ulcer: a prospective, randomized, placebo-controlled, single-center study. Diabetes Res Clin Pract. (2019) 156:107861. doi: 10.1016/j.diabres.2019.107861
18. Romanelli M, Mulder G, Paggi B, Macchia M, Panduri S, Dini V. The use of a collagen matrix in hard-to-heal venous leg ulcers. J Wound Care. (2015) 24(11):543–4; 6–7. doi: 10.12968/jowc.2015.24.11.543
19. Djavid G, Tabaie S, Tajali S, Totounchi M, Farhoud A, Fateh M, et al. Application of a collagen matrix dressing on a neuropathic diabetic foot ulcer: a randomised control trial. J Wound Care. (2020) 29:S13–18. doi: 10.12968/jowc.2020.29.Sup3.S13
20. Campitiello F, Mancone M, Della Corte A, Guerniero R, Canonico S. To evaluate the efficacy of an acellular flowable matrix in comparison with a wet dressing for the treatment of patients with diabetic foot ulcers: a randomized clinical trial. Updates Surg. (2017) 69(4):523–9. doi: 10.1007/s13304-017-0461-9
21. Driver V, Lavery L, Reyzelman A, Dutra T, Dove C, Kotsis S, et al. A clinical trial of Integra template for diabetic foot ulcer treatment. Wound Repair Regen. (2015) 23(6):891–900. doi: 10.1111/wrr.12357
22. Edmonds M, European and Australian Apligraf Diabetic Foot Ulcer Study Group. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds. (2009) 8(1):11–8. doi: 10.1177/1534734609331597
23. Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. (2002) 137(7):822–7. doi: 10.1001/archsurg.137.7.822
24. Yao C, Yao P, Wu H, Zha Z. Acceleration of wound healing in traumatic ulcers by absorbable collagen sponge containing recombinant basic fibroblast growth factor. Biomed Mater. (2006) 1(1):33–7. doi: 10.1088/1748-6041/1/1/005
25. Piatkowski A, Ulrich D, Seidel D, Abel M, Pallua N, Andriessen A. Randomised, controlled pilot to compare collagen and foam in stagnating pressure ulcers. J Wound Care. (2012) 21(10):505–11. doi: 10.12968/jowc.2012.21.10.505
26. Gottrup F, Cullen BM, Karlsmark T, Bischoff-Mikkelsen M, Nisbet L, Gibson MC. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen. (2013) 21(2):216–25. doi: 10.1111/wrr.12020
27. Haycocks S, Chadwick P, Cutting KF. Collagen matrix wound dressings and the treatment of DFUs. J Wound Care. (2013) 22(7):369–70; 72–5. doi: 10.12968/jowc.2013.22.7.369
28. Yang C, Hillas P, Báez J, Nokelainen M, Balan J, Tang J, et al. The application of recombinant human collagen in tissue engineering. Biodrugs. (2004) 18(2):103–19. doi: 10.2165/00063030-200418020-00004
29. Pallaske F, Pallaske A, Herklotz K, Boese-Landgraf J. The significance of collagen dressings in wound management: a review. J Wound Care. (2018) 27(10):692–702. doi: 10.12968/jowc.2018.27.10.692
30. Miyata T, Taira T, Noishiki Y. Collagen engineering for biomaterial use. Clin Mater. (1992) 9(3–4):139–48. doi: 10.1016/0267-6605(92)90093-9
31. Chvapil M. Collagen sponge: theory and practice of medical applications. J Biomed Mater Res. (1977) 11(5):721–41. doi: 10.1002/jbm.820110508
32. Chvapil M, Kronenthal L, Van Winkle W Jr. Medical and surgical applications of collagen. Int Rev Connect Tissue Res. (1973) 6:1–61. doi: 10.1016/B978-0-12-363706-2.50007-6
33. Fleck CA, Simman R. Modern collagen wound dressings: function and purpose. J Am Col Certif Wound Spec. (2010) 2(3):50–4. doi: 10.1016/j.jcws.2010.12.003
34. Cullen B, Watt PW, Lundqvist C, Silcock D, Schmidt RJ, Bogan D, et al. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. (2002) 34(12):1544–56. doi: 10.1016/S1357-2725(02)00054-7
35. Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv Drug Delivery Rev. (2003) 55(12):1595–611. doi: 10.1016/j.addr.2003.08.003
36. Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B. (2004) 71(2):343–54. doi: 10.1002/jbm.b.30096
37. Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. (2004) 2(4):561–73. doi: 10.1111/j.1538-7836.2004.00665.x
38. Kawamoto Y, Kaibara M. Procoagulant activity of collagen. Effect of difference in type and structure of collagen. Biochim Biophys Acta. (1990) 1035(3):361–8. doi: 10.1016/0304-4165(90)90101-2
39. Burks S, Spotnitz W. Safety and usability of hemostats, sealants, and adhesives. AORN J. (2014) 100(2):160–76. doi: 10.1016/j.aorn.2014.01.026
40. Metzmacher I, Ruth P, Abel M, Friess W. In vitro binding of matrix metalloproteinase-2 (MMP-2), MMP-9, and bacterial collagenase on collagenous wound dressings. Wound Repair Regen. (2007) 15(4):549–55. doi: 10.1111/j.1524-475X.2007.00263.x
41. Greener B, Hughes AA, Bannister NP, Douglass J. Proteases and pH in chronic wounds. J Wound Care. (2005) 14(2):59–61. doi: 10.12968/jowc.2005.14.2.26739
42. Schultz G, Mozingo D, Romanelli M, Claxton K. Wound healing and TIME; new concepts and scientific applications. Wound Repair Regen. (2005) 13(4 Suppl):S1–11. doi: 10.1111/j.1067-1927.2005.1304S1.x
43. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res. (2007) 298(9):413–20. doi: 10.1007/s00403-006-0713-x
44. Tam EM, Wu YI, Butler GS, Stack MS, Overall CM. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J Biol Chem. (2002) 277(41):39005–14. doi: 10.1074/jbc.M206874200
45. Wang X, You C, Hu X, Zheng Y, Li Q, Feng Z, et al. The roles of knitted mesh-reinforced collagen-chitosan hybrid scaffold in the one-step repair of full-thickness skin defects in rats. Acta Biomater. (2013) 9(8):7822–32. doi: 10.1016/j.actbio.2013.04.017
46. Dai C, Shih S, Khachemoune A. Skin substitutes for acute and chronic wound healing: an updated review. J Dermatolog Treat. (2020) 31(6):639–48. doi: 10.1080/09546634.2018.1530443
47. Trent JF, Kirsner RS. Tissue engineered skin: Apligraf, a bi-layered living skin equivalent. Int J Clin Pract. (1998) 52(6):408–13.9894378
48. Falabella AF, Valencia IC, Eaglstein WH, Schachner LA. Tissue-engineered skin (Apligraf) in the healing of patients with epidermolysis bullosa wounds. Arch Dermatol. (2000) 136(10):1225–30. doi: 10.1001/archderm.136.10.1225
49. Griffiths M, Ojeh N, Livingstone R, Price R, Navsaria H. Survival of Apligraf in acute human wounds. Tissue Eng. (2004) 10(7–8):1180–95. doi: 10.1089/ten.2004.10.1180
50. Ghatnekar O, Willis M, Persson U. Cost-effectiveness of treating deep diabetic foot ulcers with Promogran in four European countries. J Wound Care. (2002) 11(2):70–4. doi: 10.12968/jowc.2002.11.2.26675
51. Albert S. Cost-effective management of recalcitrant diabetic foot ulcers. Clin Podiatr Med Surg. (2002) 19(4):483–91. doi: 10.1016/S0891-8422(02)00018-6
52. Tiscar-González V, Menor-Rodríguez M, Rabadán-Sainz C, Fraile-Bravo M, Styche T, Valenzuela-Ocaña F, et al. Clinical and economic impact of wound care using a polyurethane foam multilayer dressing. Adv Skin Wound Care. (2021) 34(1):23–30. doi: 10.1097/01.ASW.0000722744.20511.71
53. Guest JF, Singh H, Vowden P. Potential cost-effectiveness of using a collagen-containing dressing in managing diabetic foot ulcers in the UK. J Wound Care. (2018) 27(3):136–44. doi: 10.12968/jowc.2018.27.3.136
54. Guest JF, Rana K, Singh H, Vowden P. Cost-effectiveness of using a collagen-containing dressing plus compression therapy in non-healing venous leg ulcers. J Wound Care. (2018) 27(2):68–78. doi: 10.12968/jowc.2018.27.2.68
Keywords: collagen dressing, chronic wound, literature review, meta-analysis, trial sequential analysis, wound healing
Citation: Shu H, Xia Z, Qin X, Wang X, Lu W, Luo Q, Zhang Z and Xiong X (2022) The clinical efficacy of collagen dressing on chronic wounds: A meta-analysis of 11 randomized controlled trials. Front. Surg. 9:978407. doi: 10.3389/fsurg.2022.978407
Received: 26 June 2022; Accepted: 10 August 2022;
Published: 31 August 2022.
Edited by:
Rami Kantar, Grossman School of Medicine, New York University, United StatesReviewed by:
Agata Janowska, University of Pisa, ItalySimona Ascanelli, University Hospital of Ferrara, Italy
© 2022 Shu, Xia, Qin, Wang, Lu, Luo, Zhang and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowei Xiong eGlvbmd4dzAzMTFAaG90bWFpbC5jb20=
Specialty Section: This article was submitted to Reconstructive and Plastic Surgery, a section of the journal Frontiers in Surgery
 Hongxin Shu
Hongxin Shu Zhiyu Xia
Zhiyu Xia Xuan Qin1
Xuan Qin1 Xiaowei Xiong
Xiaowei Xiong