- 1Department of Obstetrics and Gynecology, School of Medicine, Laparoscopy Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Department of Gynecology and Obstetrics, Clinique Gynécologique et Obstétrical, Rouen University Hospital, Rouen, France
- 3Department of Obstetrics and Gynecology, School of Medicine, Infertility Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 4Department of Obstetrics and Gynecology, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Cardiologist, Student Research Center, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 6Master of Biostatistics in Clinical Research Development Center of Nemazee Hospital, Department of Statistics, Shiraz University of Medical Sciences, Shiraz, Iran
Objective: The present work aimed to investigate the feasibility, complications, recurrence rate, and infertility outcomes of the radical and conservative surgical methods for colorectal endometriosis in short- and long-term follow-ups.
Methods: In this prospective study, the patients with confirmed diagnosis of colorectal DIE were included from March 2015 to March 2021, who were referred to an Endometriosis Surgery Center affiliated with Shiraz University of Medical Sciences (SUMS). Information on demographics, surgical approaches, intra-operative, and post-operative findings as well as complications were collected and compared. Six- and 12-month interviews were conducted to evaluate the functional outcomes of all the procedures.
Results: Out of 3,111 patients who underwent endometriosis surgery, 837 (28.19%) with the average age of 34.2 ± 5.9 years and average ASRM score of 102.1 ± 36.8 had rectosigmoid endometriosis. Laparoscopic rectal shaving was performed in 263(30.0%) patients while 326 (37.2%) underwent segmental bowel resection, and 248 (28.30%) were treated with disc excision. Prophylactic ileostomy was performed in six (0.68%) patients and peritonitis was reported in four (0.45%). Five (0.58%) subjects developed rectovaginal fistula and one (0.11%) was diagnosed with bladder atonia. The recurrence rate was 3.8%, 1.2%, and 0.3% in rectal shaving, disc, and segmental bowel resection techniques, respectively. Dysmenorrhea, dyspareunia, and dyschezia were improved after surgery by 7.3, 9.4, and 12.5 times, respectively. We observed 25.2% of total pregnancy following the operation, the majority of which occurred in the first year after the surgery.
Conclusion: There were very few short-term or long-term complications in the three different techniques when the choice was correct.
Introduction
The prevalence of endometriosis is about 10% in the reproductive age. The prevalence of colorectal endometriosis was reported to be 8%–12% with 90% of the lesions seen in the recto-sigmoid region (1–5). According to ASRM (American Society for Reproductive Medicine) classification, rectal endometriosis often presents with severe forms of endometriosis. Symptoms like dysmenorrhea, dyschezia, dyspareunia, constipation, and tenesmus are associated with colorectal endometriosis, all of which can significantly affect a person's quality of life (6).
In patients with any symptoms of intestinal obstruction, or those who do not respond to medical therapy, surgical treatment is carried out through laparoscopic or robotic methods (7–9). Currently, the three suggested surgical treatment methods for rectal endometriosis include the shaving technique, disc excision (DR), which is considered a conservative method, segmental bowel resection (SR), and re-anastomosis, which is an intense method. Based on recent studies, the existing methods are different in terms of short- and long-term complications, the risk of recurrence, and functional outcomes. Additionally, there is still no clear agreement among gynecologist surgeons on the optimal patient management for these lesions (10–13).
Today, radiological methods have made it possible to determine the nodule size, its location and multifocality, and the percentage of lumen stenosis prior to surgery. Based on this information, we are able to determine which patients will benefit from radical surgery (14, 15).
Currently, there is scarce research on the feasibility, complications, recurrence rate, and infertility outcomes of the three available surgical methods in short- and long-term follow-ups. The present study included a large number of colorectal endometriosis surgery cases so that all these three surgical procedures could be compared in terms of all the short- and long-term outcomes.
Materials and methods
Study population
This was a prospective study performed on the patients referred to an Endometriosis Surgery Center affiliated with Shiraz University of Medical Sciences (SUMS) with a suspicious history of endometriosis. The patients diagnosed with colorectal endometriosis using imaging techniques were recruited from March 2015 to March 2021. SUMS Institutional Review informed consent was taken from all the subjects before participation.
The inclusion criteria were (1) the age range of 18 to 50 years old and one of the followings: (2) endometriosis-related pain without response to medication, (3) involvement of tubes in case of infertility, (4) complete family planning according to decision making by couples, (5) conditions where using hormonal drugs is not possible, (6) unexplained infertility with AMH 1–2 ng/ml, (7) unilateral OMA with AMH >2.5, (8) more than two unsuccessful IVF attempts, (9) the need for pathology samples, and (10) patient's unwillingness to receive medical treatment. All the surgeries, as well as all IVF procedures, were performed by the first author. The exclusion criteria were (1) all the patients with cardiovascular diseases, (2) insulin-dependent diabetes mellitus, (3) BMI (Body Mass Index) >35 kg/m2, and (4) a history of pelvic organ malignancy. The endometriosis-related pain symptoms, such as dysmenorrhea, dyspareunia, dyschezia, mid cyclic or non-cyclic pain, constipation, as well as other GI troubles (Gastro-Intestinal) were recorded before the operation and every 6 months afterwards based on VAS score (Visual Analogue Scale) (16).
The recorded demographic information and medical history of the patients included age, BMI, parity, history of previous operation and endometriosis surgeries, pre- and post-operation pain symptoms related to endometriosis, intra- and post-operation complications according to Cliven–Dindo classification, stage of endometriosis disease according to ASRM classification (17, 18), histo-pathologic report, recurrence rate of disease, pregnancy rate, pregnancy outcome, and the method of pregnancy. These data were compared between the three different surgical procedures performed on colorectal endometriosis lesion, namely shaving, disc resection and segmental bowel resection, and re-anastomosis.
The patients were followed for 6 months after the surgery and then annually. We used histopathology specimens as the gold standard of diagnosis for rectal endometriosis.
All the surgeries were performed laparoscopically and according to the guidelines with nerve and vascular sparing techniques. In our work, superficial DIE lesions of bowel were removed by shaving, deep lesions between 1 cm–3 cm were operated by disc excision, and multiple and large (>3 cm) lesions with >50% involvement of bowel loop circumference were removed by segmental resection (2, 16, 18). None of the surgeries were converted to laparotomy except for two cases of internal iliac injury.
Statistical analysis
All the data were entered into a database and further analyzed using the statistical package for social sciences (SPSS Inc. version 24.0). Data are presented as mean ± SD or proportions. Parametric variables with normal distribution were compared using one-way analysis of variance (ANOVA) with Tukey, as the post hoc test. The non-parametric variables were compared using Kruskal-Wallis test. The groups were compared with t test (for continued variables) and chi-square for the categoric variables. A two-sided P-value of less than 0.05 was considered to be statistically significant.
Results
Out of 3,111 patients referred with endometriosis to our center in the study period, 837 underwent rectal endometriosis surgery. Out of this population, 263 (30.0%) underwent shaving, 248 (28.3%) DR, and 326 (37.2%) SR. Among all the patients, we had only six cases of cecal involvement. Two cases of small bowel involvement were reported, who previously had laparotomy endometriosis pelvic surgery.
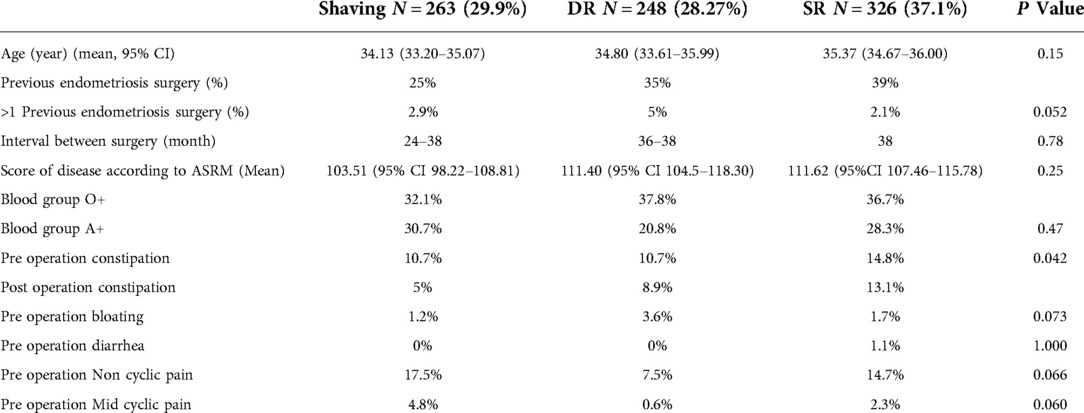
The patients’ demographics, medical history and endometriosis-related pains for each group are summarized in Table 1.
The most common symptoms reported by the patients were dysmenorrhea (94.1%). All their initial symptoms and percentage of recovery after the surgery, based on the type of operation, are listed Figure 1. Twelve months following the operation, the intensity of dysmenorrhea decreased from 77.46% down to 11.34%, the dyspareunia declined from 85.99% to 7.25, and dyschezia decreased from 88.8% to 2.81% in all the patients, compared to the pre-operative period.
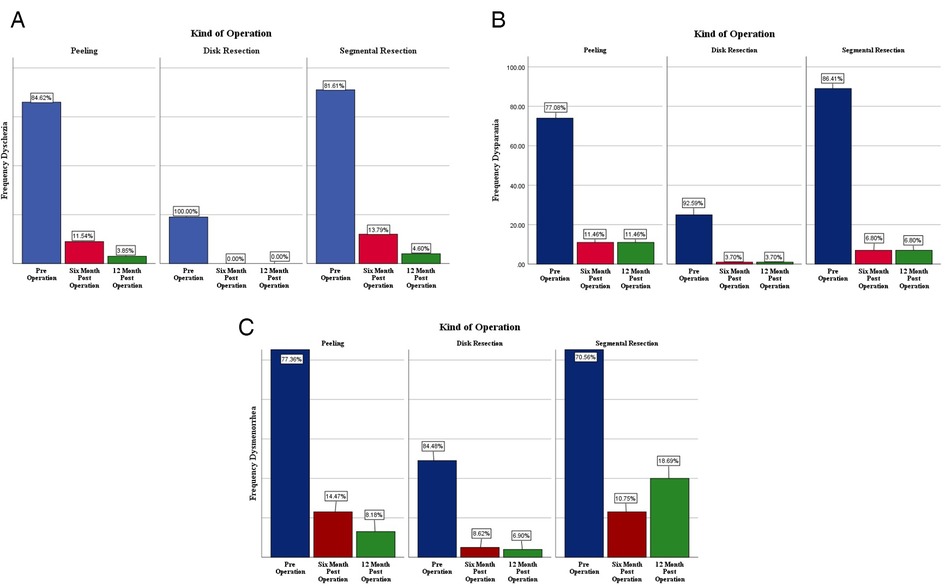
Figure 1. Comparison of dyschezia (A), dyspareunia (B) and dysmenorrhea (C), before surgery, six and 12 months after surgery based on the type of colorectal endometriosis surgeries.
The frequency of pelvic lesions and surgeries, based on the method of operation and histopathologic findings are shown in Tables 2, 3. Rectal endometriosis lesions were the most prevalent in the presence of the uterine sacral ligaments DIE as well as cases with obliteration of the posterior cul-de-sac.
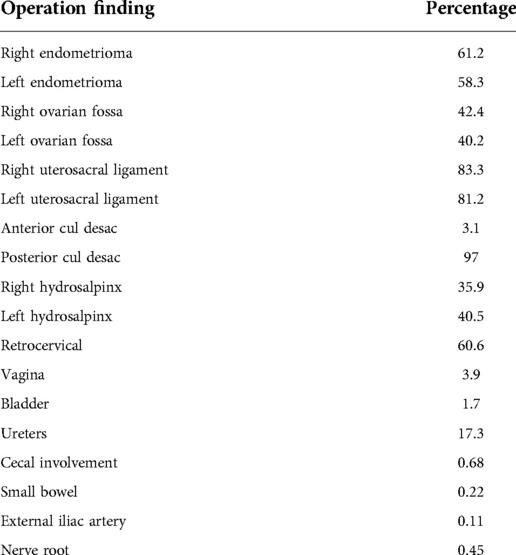
Table 2. Percentage of endometriosis involvement in different parts of the pelvis simultaneously with colorectal endometriosis according to histopathologic findings.
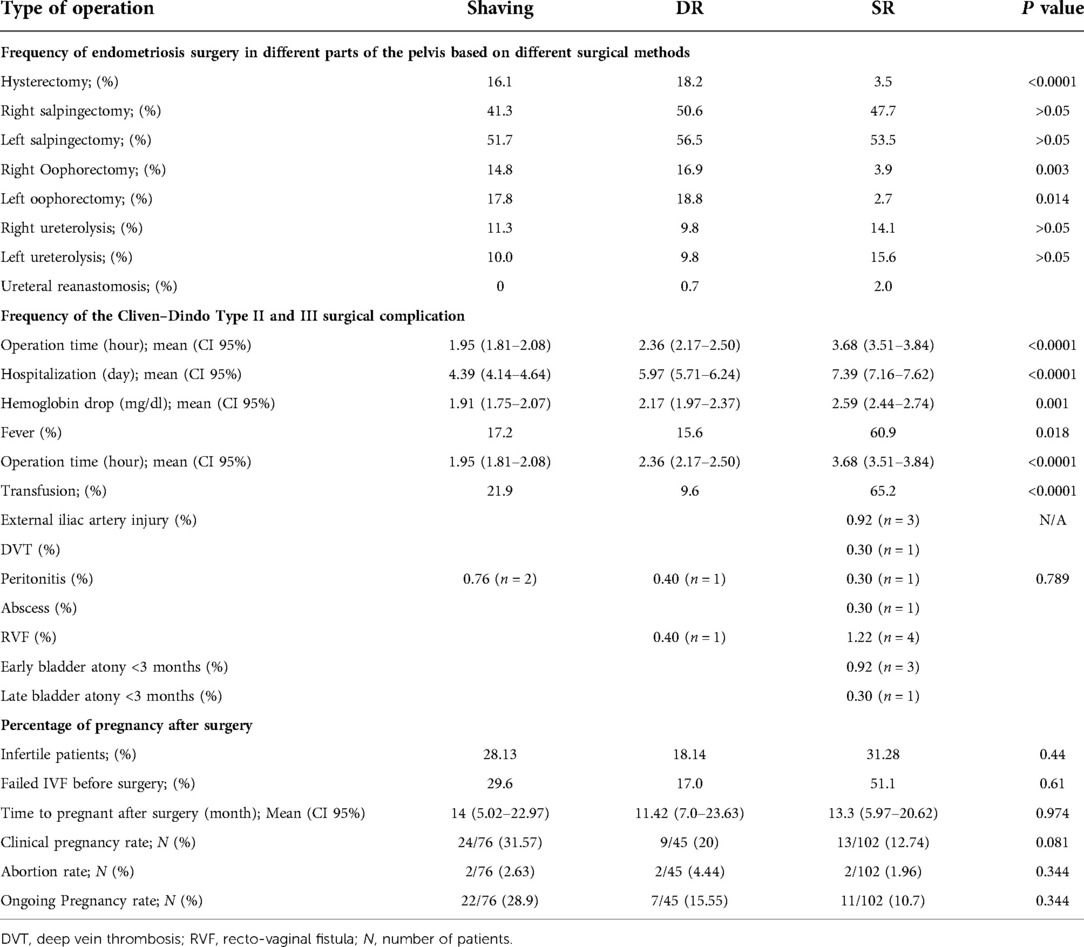
Table 3. Frequency of endometriosis surgery in different parts of the pelvis, the Cliven–Dindo Type II and III surgical complication and percentage of pregnancy after surgery based on different types of colorectal endometriosis surgeries.
All the patients were in stage 3 and 4 of the disease according to ASRM classification (3.97 ± 0.18).
In the segmental resection group, rectal involvement was deeper compared to that in the other groups (P = 0.000).
Table 3 also represents the post-operation complications according to Cliven–Dindo classification.
Fever and the need for blood transfusion were 17.2, 15.6, 60.9%, and 21.9, 9.6, 65.2% in the peeling, DR, and SR groups, respectively (P < 0.01). Certain complications, such as peritonitis and rectovaginal fistula, were 0.76, 0.40, 0.40% and 0.0, 0.4, 1.22% in the peeling, DR, and SR groups. Other complications, like abscess, external iliac artery injury, early and late bladder atony, and DVT, were seen in 2.7% of the patients and only in the segmental group.
Type II of the Cliven–Dindo operation-associated complications was significantly higher in the SR group (P = 0.037).
In case of multifocal rectal involvement, two separated nodules were reported in 50 cases (5.97%), three-point involvement in 12 cases (1.43%), and four-point in two cases, all in the SR group (0.23%). We had no reports of malignancy in our study.
Prophylactic iliostomy was performed in only five cases in the SR group, whose rectal lesion was very low (below 8 cm from the anal verge) or whose hysterectomy or vaginal lesion was removed at the same time as the rectal lesion excision.
The recurrence rate, based on the follow-up ultrasound and symptoms recurrence, was 3.8% (n = 10) in the peeling method, 1.2% (n = 3) in the DR, and 0.3% (n = 1) in the SR methods; this rate was significantly lower in the SR method (P = 0.008).
Herein, 276 out of 837 subjects were categorized as an infertile group, out of whom 17 were over the age of 42, 10 had undergone bilateral salpingo-ophorectomy, and two had severe male factor infertility. Twenty-four patients were lost to the follow-up. In total, out of 223 patients, 24 (24/76) in the peeling group, nine (9/45) in the disc group, and 13 (13/102) in the segmental group were infertile. Table 3 depicts the clinical pregnancy, abortion, and ongoing pregnancy rate and other information about pregnancy outcomes. The majority of the spontaneous pregnancies were seen in the peeling group (n = 21, P = 0.059); 72% of them became pregnant in the first year after the surgery and the rest got pregnant up to 60 months afterwards. There were no significant differences between the groups in terms of IVF failure before the surgery (P = 0.61). Comparing the pregnancy rate before and after the surgery, our results showed a significant improvement only in the peeling group (P = 0.005).
Discussion
Given the fact that the proper surgical treatment for rectal lesions still remains controversial in different patients, having a large database with a large sample size will undoubtedly help surgeons to make a better decision about each patient individually (19–24). In this study with a large sample size, we examined all the short- and long-term outcomes as well as fertility results and draw comparisons between the two radical and conservative surgical methods. We showed that by choosing the right patient for each technique, all these methods are feasible and could be accompanied by excellent and acceptable outcomes.
Regarding the weakness of our study, we could mention the lack of comparison of hormone therapy after the surgery in the three groups and its effect on disease recurrence in addition to the lack of comparison of the lifestyle and behavior of the patients in the management of complications after the surgery. There are some other weaknesses, including loss of patients in the follow-up, which impacts the recurrences and pregnancy rate, unbalanced and discontinuous hormonal therapy intake, unbalanced length of the follow-up, the lack of randomization between the three techniques, leading to incomparable three subpopulations.
This study had several strengths, including having more colorectal endometriosis surgeries per year compared to most similar studies (146/year). Additionally, all the ultrasound imagings, surgeries, IVF procedures, examinations of patients in terms of disease recurrence, and surgical treatments of complications were often done by the first author himself. In this study, all the short- and long-term complications of the surgery were collected and reported based on the three different surgical methods. Despite the large number of cases and not having performed prophylactic ileostomy, we observed fewer surgical complications and lower recurrence rate compared to similar studies in this field although the follow-up period in our study was longer (up to 6 years).
In the current work, the history of previous endometriosis surgeries was higher in the SR group (P = 0.06). This may indicate incomplete initial surgery and the use of conservative methods in young patients, which may lead to recurrence of lesions and re-operation during the reproductive age. Abrão et al. recommended the use of further radical methods in younger patients and reserved the conservative methods for pre-menopausal women (25). In a study by Afros et al., patients with a nodule >3 cm had an RR of 2.5 (95% CI, 1.66–3.99) of the requiring bowel resection so that the recurrence of lesions would be reduced (26).
All the endometriosis-related pain symptoms, showed a significant reduction after the surgery in the present study. The symptoms did not show any significant increase or decrease when the patients were followed-up for at least 12 months compared to the first 6 months after the surgery. Similarly, Roman et al. reported a significant reduction in endometriosis-related pain symptoms immediately after the surgery (27). Turco et al. showed that after segmental resection of colorectal endometriosis lesions, most of the pain symptoms related to endometriosis, on top of the quality of life of patients according to the EHP-30 questionnaire, had a considerable improvement that was more evident in patients with multinodular rectal lesions (28). Thus, it can be concluded that with complete surgery, patients' pain will be significantly reduced and their quality of life will increase and remain constant in long-term follow-ups (29, 30).
In the present work, despite the higher number of Cliven–Dindo II complications in the patients with radical surgery, type IIIa and IIIb complications were less common compared to those in other studies (18).
Our results (Table 3) were in accordance with those reported by Benifallah et al. systematic review and meta-analysis, where the mean operation times of 203 and 258.7 min for DR and SR and the mean hospital stay of 5 and 7 days were reported. Although they observed better results for disc than segmental resection in the terms of operation time and hospitalization days, the differences between the two groups were not statistically significant (P = 0.99, I2 = 71%) (31). According to previous papers, since rectal shaving is less invasive, it is associated with a lower risk of immediate pre- and post-operative complications compared to the other two methods (8, 12, 32). However, this should not be considered as a reason to suggest rectal shaving for all patients regardless of other consequences, such as the need for more incidence of reoperation and recurrence rate of disease.
Leakage and peritonitis were reported in only four cases, with 0.7% in the peeling group, 0.4% in the DR, and 0.3% in the SR group (P = 0.78). In a systematic review by Meuleman et al., who included 49 articles with 2,036 patients, the chance of anastomosis leakage was reported to be about 1.3% (14). However, in line with our results, in the systematic review by Bendifallah et al., the chance of leakage followed by peritonitis was 0.2 in peeling, 1.0% in disc, and 1.9% in segmental bowel resection, with no differences between the three groups (P = 0.32) (31). In a multi-center study on 4,721 patients, the chance of early anastomosis leakage was 0.4%, emphasizing that the prevalence of radical surgeries in that study was very low (3.8% underwent SR and 58% peeling) (33). Nisolle et al. studied 177 cases of segmental and peeling patients and compared them in terms of Cliven–Dindo IIIb. Only one case of anastomosis leakage was reported in the segmental group (2%), which was caused by tension on the anastomosis. It seems as if by maintaining vascularity and pelvic nerves during surgery and proper mobilization of the anastomosis and checking the integrity of the anastomosis before the end of the surgery, certain complications, such as anastomotic leakage, can be prevented (34). In order to investigate vascular damage and subsequent ischemia that leads to fistula formation at the site of rectal or ureteral anastomosis, Ianieri et al., in a systematic review based on the results of eight studies, concluded that indocyanin during surgery is a useful tool for evaluating suitable blood supply of intestinal anastomosis. In their study, Hernández et al. qualitatively confirmed the value of intraoperative indocyanine use to predict fistula formation in full-thickness intestinal resection and re-anastomosis due to insufficient vascularization of the anastomosis site, with a sensitivity and specificity of 100%. However, the obtained results in this field are still inconsistent and there is a need for larger studies with a larger sample size (35, 36).
All our five RVF cases, four of whom required surgical intervention, had a simultaneous colpotomy incision.
The reason behind the occurrence of the RVF is the simultaneous colpotomy incisions at the time of rectal lesions removal. Our RVF rate (0.4%) was obviously lower than that reported in similar articles despite not having performed prophylaxis iliostomy. The risk of RVF following colorectal endometriosis surgery in a systematic review by Balla et al. was reported to be about 2.4% (37). In a recently published systematic review, the chance of an RVF in segmental resection of bowel was reported to be about 2.7%, which is associated with low rectal lesions and simultaneous clopotomy incisions (32). In a study by Bendifallah et al., there was no difference in the risk of RVF between disc and segmental resection (P = 0.76, I2 = 0) (31). Meanwhile, in our study, although not statistically significant, most cases of fistula were reported in the segmental group.
To prevent RVF in patients with colorectal endometriosis, it is necessary to pay attention to the following points: (1) preservation of the serosal surface of pelvic organs; the lower rectal lesions, which lack the serosal surface and whose resection requires extensive dissection of the pararectal space, are associated with a higher chance of RVF; (2) patients with positive air leakage test who require more manipulation and suturing during operation; (3) avoiding simultaneous surgery on rectum and vagina; (4) surgeon's experience (38).
About the other complications in our study, in the SR group, we found one case of late bladder atonia (0.1%) which improved within 3 months of self-catheterization.
In a recently published systematic review, the rate of late bladder atony has been reported to be 6.6% in SR, 4.1% in DR, and 0.4% in peeling groups (39). The majority of the reports in other studies on the prevalence of urological morbidities was shown to be low and unknown, which is due to the short follow-up time (40). As we know, about 72% of these complications will be resolved during the follow-up period (13). Only in a few case series, post-operative urinary problems were reported to be between 0% and 5% among rectal endometriosis surgery (22, 41, 42). In the research by Hernandez et al., over a period of 38 to 48 months, comparing 76 patients in the SR group, 20 in the DR group, and 47 in the shaving group, only 5.2% showed urinary incontinency and 2.6% presented fecal incontinency only in the SR group (43).
With regards to the LARS symptoms and examining certain symptoms, including pre- and post-operation constipation, post-operation bloating and diarrhea, and incomplete emptying of the rectum or fecal incontinency, in our study, only post-operative constipation did not significantly improve in the segmental group (from 14.8% into 13.1%) despite the improvement in the peeling (10.7% into 5.0%) and disc resection groups (10.7% into 8.9%) (P = 0.042).
LARS symptoms are more prevalent in case of removing very low rectal lesions (<9 centimeters distance from anal verge). As reported by Bafort et al., the length of removed rectosigmoid was not found to be related to post-operative LARS symptoms, and the symptoms of the intestine and bladder after the surgery were not significantly different between disc and segmental resection groups (44). Roman et al. suggested that conservative surgery, such as disc resection with trans anal stapler, can reduce the length of rectum removed, leading to less damage to the pelvic vasculature and nerves. As a result, post-operative LARS symptoms can be reduced as well (45). Despite accepting Roman's theory, which was conducive to removing rectal lesions over the recent years, our study concluded that segmental resection has no more complications than other conservative methods, which should not be neglected by an experienced surgeon.
In our study, the recurrence rate was significantly lower in the SR method (P = 0.008). Hernandez et al. reported a 12.7% recurrence rate in the peeling group, 5% in the DR, and 1.3% in the SR group (P = 0.052). The chance of recurrence was the highest in the peeling group, which is in agreement with our study (43). In a systematic review by Bendifallah et al., similar to ours, the risk of recurrence in the segmental group was lower compared with rectal shaving (I2 = 0%, P = 0.001); nonetheless, the recurrence rate did not differ between disc (n = 106) and segmental (n = 229) resection techniques (I2 = 0%, P = 0.11). However, these results were obtained only from three out of the 41 articles included in their study. In this paper, the recurrence time was reported between 12 and 94 months after the operation (39). In agreement with our observations, in a randomized clinical trial by Roman et al., although the chance of recurrence in the DR was reported to be slightly higher than that of the SR, the quality of life was the same in both groups (27). In the systematic review by Giampaolino et al., the temporarily suspension of both ovaries to the abdominal wall after surgery of stage 3 and 4 of endometriosis was shown to prevent moderate and severe adhesions after the surgery. Nevertheless, in the case of colorectal endometriosis surgeries, there is insufficient information on colorectal endometriosis surgeries for the necessity of placing anti-adhesion devices at the end of the operation or temporary suspension of both ovaries to the anterior abdominal wall in order to reduce the adhesion formation and complications of re-operation in these patients in case of recurrence of the disease (46). Multifocal lesions are sometimes far from the primary lesion without any adhesion to the first lesion and can be missed easily without intestinal palpation during surgery. Such lesions might require the en-block removal of a big part of intestine (47, 48). Bowel palpation for finding multiple bowel lesions during laparoscopy is a step that should not be neglected.
In conclusion, if there is a surgical indication for removal of the endometriosis lesions, complete and definitive resection of the lesions is recommended. All the removal procedures for colorectal endometriosis, if performed with the appropriate indication and in the appropriate individual, are associated with improved endometriosis-related symptoms, reduced recurrence rate, and very few complications.
In order to choose the best treatment method for rectal endometriosis lesions with a few surgical complications, further investigation is needed with the introduction of new surgical techniques as well as a larger sample size.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by ethics committee of Shiraz University of Medical Sciences (Code: IR.SUMS.REC.1395.S404). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SA: Conception, design of study and data revising; HR: design of study / final approach; EA: Data interpretation / manuscript preparation, TP: Patient recruitment / data collection; MHS: Patient recruitment, drafting / design; SA: Data revising and editing; AKHA: Data analysis and interpretation; EHNK: Patient recruitment, drafting / design. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank all the staff members of our surgical and laboratory units for their expert assistance in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saridogan E, Becker CM, Feki A, Grimbizis GF, Hummelshoj L, Keckstein J, et al. Recommendations for the surgical treatment of endometriosis. Part 1: ovarian endometrioma. Hum Reprod Open. (2017) 2017(4):hox016. doi: 10.1093/hropen/hox016
2. Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. (2015) 21(3):329–39. doi: 10.1093/humupd/dmv003
3. Abrão MS, Borrelli GM, Clarizia R, Kho RM, Ceccaroni M. Strategies for management of colorectal endometriosis. Semin Reprod Med. (2017) 35(1):65–71. doi: 10.1055/s-0036-1597307
4. Nezhat C, Li A, Falik R, Copeland D, Razavi G, Shakib A, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. (2018) 218(6):549–62. doi: 10.1016/j.ajog.2017.09.023
5. Ruffo G, Scopelliti F, Manzoni A, Sartori A, Rossini R, Ceccaroni M, et al. Long-term outcome after laparoscopic bowel resections for deep infiltrating endometriosis: a single-center experience after 900 cases. Biomed Res Int. (2014) 2014:463058. doi: 10.1155/2014/463058
6. Rossini R, Lisi G, Pesci A, Ceccaroni M, Zamboni G, Gentile I, et al. Depth of intestinal wall infiltration and clinical presentation of deep infiltrating endometriosis: evaluation of 553 consecutive cases. J Laparoendosc Adv Surg Tech Part A. (2018) 28(2):152–6. doi: 10.1089/lap.2017.0440
7. Roman H, Tuech JJ. New disc excision procedure for low and mid rectal endometriosis nodules using combined transanal and laparoscopic approach. Colorectal Dis. (2014) 16(7):O253–6. doi: 10.1111/codi.12605
8. Donnez O, Roman H. Choosing the right surgical technique for deep endometriosis: shaving, disc excision, or bowel resection? Fertil Steril. (2017) 108(6):931–42. doi: 10.1016/j.fertnstert.2017.09.006
9. Ceccaroni M, Ceccarello M, Caleffi G, Clarizia R, Scarperi S, Pastorello M, et al. Total laparoscopic ureteroneocystostomy for ureteral endometriosis: a single -center experience of 160 consecutive patients. J Minim Invasive Gynecol. (2018) 26:78–8. doi: 10.1016/j.jmig.2018.03.031
10. Kondo W, Bourdel N, Tamburro S, Cavoli D, Jardon K, Rabischong B, et al. Complications after surgery for deeply infiltrating pelvic endometriosis. BJOG. (2011) 118(3):292–8. doi: 10.1111/j.1471-0528.2010.02774.x
11. Jayot A, Nyangoh Timoh K, Bendifallah S, Ballester M, Darai E. Comparison of laparoscopic discoid resection and segmental resection for colorectal endometriosis using a propensity score matching analysis. J Minim Invasive Gynecol. (2018) 25(3):440–6. doi: 10.1016/j.jmig.2017.09.019
12. Abo C, Moatassim S, Marty N, Saint Ghislain M, Huet E, Bridoux V, et al. Postoperative complications after bowel endometriosis surgery by shaving, disc excision, or segmental resection: a three-arm comparative analysis of 364 consecutive cases. Fertil Steril. (2018) 109(1):172–8.e1. doi: 10.1016/j.fertnstert.2017.10.001
13. Roman H, Bubenheim M, Huet E, Bridoux V, Zacharopoulou C, Daraï E, et al. Conservative surgery versus colorectal resection in deep endometriosis infiltrating the rectum: a randomized trial. Hum Reprod. (2018) 33(1):47–57. doi: 10.1093/humrep/dex336
14. Meuleman C, Tomassetti C, D'Hoore A, Van Cleynenbreugel B, Penninckx F, Vergote I, et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update. (2011) 17(3):311–26. doi: 10.1093/humupd/dmq057
15. Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. (2017) 108(6):886–94. doi: 10.1016/j.fertnstert.2017.10.026
16. Alborzi S, Rasekhi A, Shomali Z, Madadi G, Alborzi M, Kazemi M, et al. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine (Baltimore). (2018) 97(8):e9536. doi: 10.1097/md.0000000000009536
17. Revised American society for reproductive medicine classification of endometriosis: 1996. Fertil Steril. (1997) 67(5):817–21. doi: 10.1016/s0015-0282(97)81391-x
18. Dindo D. The Clavien–Dindo classification of surgical complications. In: Cuesta M, Bonjer H, editors. Treatment of postoperative complications after digestive surgery. London: Springer (2014). p. 13–7.
19. de Almeida A, Fernandes LF, Averbach M, Abrão MS. Disc resection is the first option in the management of rectal endometriosis for unifocal lesions with less than 3 centimeters of longitudinal diameter. Surg Technol Int. (2014) 24:243–8. PMID: 24526427
20. Fanfani F, Fagotti A, Gagliardi ML, Ruffo G, Ceccaroni M, Scambia G, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. (2010) 94(2):444–9. doi: 10.1016/j.fertnstert.2009.03.066
21. Roman H, Abo C, Huet E, Bridoux V, Auber M, Oden S, et al. Full-thickness disc excision in deep endometriotic nodules of the rectum: a prospective cohort. Dis Colon Rectum. (2015) 58(10):957–66. doi: 10.1097/dcr.0000000000000447
22. Roman H, Darwish B, Bridoux V, Chati R, Kermiche S, Coget J, et al. Functional outcomes after disc excision in deep endometriosis of the rectum using transanal staplers: a series of 111 consecutive patients. Fertil Steril. (2017) 107(4):977–86.e2. doi: 10.1016/j.fertnstert.2016.12.030
23. Oliveira MA, Crispi CP, Oliveira FM, Junior PS, Raymundo TS, Pereira TD. Double circular stapler technique for bowel resection in rectosigmoid endometriosis. J Minim Invasive Gynecol. (2014) 21(1):136–41. doi: 10.1016/j.jmig.2013.07.022
24. Kondo W, Ribeiro R, Zomer MT, Hayashi R. Laparoscopic double discoid resection with a circular stapler for bowel endometriosis. J Minim Invasive Gynecol. (2015) 22(6):929–31. doi: 10.1016/j.jmig.2015.04.021
25. Abrão MS, Andres MP, Barbosa RN, Bassi MA, Kho RM. Optimizing perioperative outcomes with selective bowel resection following an algorithm based on preoperative imaging for bowel endometriosis. J Minim Invasive Gynecol. (2020) 27(4):883–91. doi: 10.1016/j.jmig.2019.06.010
26. Afors K, Centini G, Fernandes R, Murtada R, Zupi E, Akladios C, et al. Segmental and discoid resection are preferential to bowel shaving for medium-term symptomatic relief in patients with bowel endometriosis. J Minim Invasive Gynecol. (2016) 23(7):1123–9. doi: 10.1016/j.jmig.2016.08.813
27. Roman H, Tuech JJ, Huet E, Bridoux V, Khalil H, Hennetier C, et al. Excision versus colorectal resection in deep endometriosis infiltrating the rectum: 5-year follow-up of patients enrolled in a randomized controlled trial. Hum Reprod. (2019) 34(12):2362–71. doi: 10.1093/humrep/dez217
28. Turco LC, Scaldaferri F, Chiantera V, Cianci S, Ercoli A, Fagotti A, et al. Long-term evaluation of quality of life and gastrointestinal well-being after segmental colo-rectal resection for deep infiltrating endometriosis (endo-resect Qol). Arch Gynecol Obstet. (2020) 301(1):217–28. doi: 10.1007/s00404-019-05382-8
29. Ceccaroni M, Ceccarello M, Clarizia R, Fusco E, Roviglione G, Mautone D, et al. Nerve-sparing laparoscopic disc excision of deep endometriosis involving the bowel: a single-center experience on 371 consecutives cases. Surg Endosc. (2021) 35(11):5991–6000. doi: 10.1007/s00464-020-08084-4
30. Alborzi S, Hosseini-Nohadani A, Poordast T, Shomali Z. Surgical outcomes of laparoscopic endometriosis surgery: a 6 year experience. Curr Med Res Opin. (2017) 33(12):2229–34. doi: 10.1080/03007995.2017.1362377
31. Bendifallah S, Puchar A, Vesale E, Moawad G, Daraï E, Roman H. Surgical outcomes after colorectal surgery for endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. (2021) 28(3):453–66. doi: 10.1016/j.jmig.2020.08.015
32. Roman H. A national snapshot of the surgical management of deep infiltrating endometriosis of the rectum and colon in France in 2015: a multicenter series of 1135 cases. J Gynecol Obstet Hum Reprod. (2017) 46(2):159–65. doi: 10.1016/j.jogoh.2016.09.004
33. Byrne D, Curnow T, Smith P, Cutner A, Saridogan E, Clark TJ. Laparoscopic excision of deep rectovaginal endometriosis in bsge endometriosis centres: a multicentre prospective cohort study. BMJ Open. (2018) 8(4):e018924. doi: 10.1136/bmjopen-2017-018924
34. Nisolle M, Brichant G, Tebache L. Choosing the right technique for deep endometriosis. Best Pract Res Clin Obstet Gynaecol. (2019) 59:56–65. doi: 10.1016/j.bpobgyn.2019.01.010
35. Ianieri MM, Della Corte L, Campolo F, Cosentino F, Catena U, Bifulco G, et al. Indocyanine green in the surgical management of endometriosis: a systematic review. Acta Obstet Gynecol Scand. (2021) 100(2):189–99. doi: 10.1111/aogs.13971
36. Hernández A, de Zulueta PR, Spagnolo E, Soguero C, Cristobal I, Pascual I, et al. Deep learning to measure the intensity of indocyanine green in endometriosis surgeries with intestinal resection. J Pers Med. (2022) 12(6):982. doi: 10.3390/jpm12060982
37. Balla A, Quaresima S, Subiela JD, Shalaby M, Petrella G, Sileri P. Outcomes after rectosigmoid resection for endometriosis: a systematic literature review. Int J Colorectal Dis. (2018) 33(7):835–47. doi: 10.1007/s00384-018-3082-y
38. Vigueras Smith A, Sumak R, Cabrera R, Kondo W, Ferreira H. Bowel anastomosis leakage following endometriosis surgery: an evidence based analysis of risk factors and prevention techniques. Facts Views Vis Obgyn. (2020) 12(3):207–25. PMID: 33123696, PMCID: 7580259
39. Bendifallah S, Vesale E, Daraï E, Thomassin-Naggara I, Bazot M, Tuech JJ, et al. Recurrence after surgery for colorectal endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. (2020) 27(2):441–51.e2. doi: 10.1016/j.jmig.2019.09.791
40. Turco LC, Tortorella L, Tuscano A, Palumbo MA, Fagotti A, Uccella S, et al. Surgery-related complications and long-term functional morbidity after segmental colo-rectal resection for deep infiltrating endometriosis (endo-resect morb). Arch Gynecol Obstet. (2020) 302(4):983–93. doi: 10.1007/s00404-020-05694-0
41. Kovoor E, Nassif J, Miranda-Mendoza I, Lang-Avérous G, Wattiez A. Long-term urinary retention after laparoscopic surgery for deep endometriosis. Fertil Steril. (2011) 95(2):803.e9–12. doi: 10.1016/j.fertnstert.2010.07.1043
42. Vesale E, Roman H, Moawad G, Benoit L, Touboul C, Darai E, et al. Voiding dysfunction after colorectal surgery for endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol. (2020) 27(7):1490–502.e3. doi: 10.1016/j.jmig.2020.07.019
43. Hernández Gutiérrez A, Spagnolo E, Zapardiel I, Garcia-Abadillo Seivane R, López Carrasco A, Salas Bolívar P, et al. Post-operative complications and recurrence rate after treatment of bowel endometriosis: comparison of three techniques. Eur J Obstet Gynecol Reprod Biol X. (2019) 4:100083. doi: 10.1016/j.eurox.2019.100083
44. Bafort C, van Elst B, Neutens S, Meuleman C, Laenen A, d'Hoore A, et al. Outcome after surgery for deep endometriosis infiltrating the rectum. Fertil Steril. (2020) 113(6):1319–27.e3. doi: 10.1016/j.fertnstert.2020.02.108
45. Roman H, Vassilieff M, Tuech JJ, Huet E, Savoye G, Marpeau L, et al. Postoperative digestive function after radical versus conservative surgical philosophy for deep endometriosis infiltrating the rectum. Fertil Steril. (2013) 99(6):1695–704. doi: 10.1016/j.fertnstert.2013.01.131
46. Giampaolino P, Della Corte L, Saccone G, Vitagliano A, Bifulco G, Calagna G, et al. Role of ovarian suspension in preventing postsurgical ovarian adhesions in patients with stage III–IV pelvic endometriosis: a systematic review. J Minim Invasive Gynecol. (2019) 26(1):53–62. doi: 10.1016/j.jmig.2018.07.021
47. Millochau JC, Stochino-Loi E, Darwish B, Abo C, Coget J, Chati R, et al. Multiple nodule removal by disc excision and segmental resection in multifocal colorectal endometriosis. J Minim Invasive Gynecol. (2018) 25(1):139–46. doi: 10.1016/j.jmig.2017.09.007
Keywords: endometriosis, rectal endometriosis, Clavien-Dindo classification, infertility, rectovaginal fistula, painful menstruation
Citation: Alborzi S, Roman H, Askary E, Poordast T, Shahraki MH, Alborzi S, Hesam Abadi AK and Najar Kolaii EH (2022) Colorectal endometriosis: Diagnosis, surgical strategies and post-operative complications. Front. Surg. 9:978326. doi: 10.3389/fsurg.2022.978326
Received: 25 June 2022; Accepted: 29 August 2022;
Published: 4 October 2022.
Edited by:
Emanuela Spagnolo, University Hospital La Paz, SpainReviewed by:
Luigi Della Corte, University of Naples Federico II, ItalyLuigi Turco, Mater Olbia Hospital, Italy
© 2022 Alborzi, Roman, Askary, Poordast, Shahraki, Alborzi, Hesam Abadi and Najar Kolaii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elham Askary ZWxsaWFza2FyeV9tZEB5YWhvby5jb20=
Specialty Section: This article was submitted to Obstetrics and Gynecological Surgery, a section of the journal Frontiers in Surgery
 Saeed Alborzi1
Saeed Alborzi1 Elham Askary
Elham Askary