
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 06 September 2022
Sec. Thoracic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.973954
Background: The subxiphoid approach has been widely used recently. However, there is little data focusing on neurological outcomes in patients with thymomatous myasthenia gravis (MG) who underwent subxiphoid thoracoscopic thymectomy. The purpose of this study was to compare the neurological outcomes of patients with thymomatous MG who underwent extended thymectomy with a subxiphoid or transthoracic approach 1 year postoperatively.
Methods: The records of patients with Masaoka stage I and II thymomas who underwent extended thymectomy from January 2019 to December 2020 with tumor size less than 5 cm and thymomatous MG were retrospectively reviewed and evaluated. Neurological outcomes were measured by a quantitative myasthenia gravis score (QMGS), with a 2.3-point reduction in QMGS associated with improvement in clinical MG status. The clinical efficacy and variables affecting the outcomes were assessed using the Kaplan–Meier method and Cox proportional hazard regression analysis.
Results: A total of 89 patients were included in the analysis, of which 44 had a subxiphoid approach and 45 had a trans-sternal approach. Mean QMGS decreased from 12 at initial diagnosis to 8.7 preoperatively and 5.6 at 12 months postoperatively in the subxiphoid group and from 12.1 to 8.9 to 6.0 in the transthoracic group. Thirteen patients (28.9%) who underwent the trans-sternal approach and 10 (22.7%) who underwent the subxiphoid approach did not have an improved clinical status compared with their preoperative status. The median time to clinical improvement was 3 months (95% CI, 2.15–3.85) for the subxiphoid approach and 6 months (95% CI, 5.54–6.46) for the trans-sternal approach. Univariate results showed that the subxiphoid approach was associated with a faster improvement in clinical status (HR = 1.701, 95% CI, 1.044–2.773, P < 0.05), and age ≦48 was associated with a faster improvement in clinical status (HR = 1.709, 95% CI, 1.044–2.799, P < 0.05). The multivariate model including age ≦48 (HR = 1.837, 95% CI, 1.093–3.086, P = 0.022) and the subxiphoid approach (HR = 1.892, 95% CI, 1.127–3.177, P = 0.016) was significantly associated with a faster improvement in clinical status.
Conclusions: In patients with Masaoka stage I and II thymoma who underwent thymectomy, with tumor size less than 5 cm and thymomatous MG, age ≦48 years and the subxiphoid approach were associated with a rapid improvement in clinical status.
Extended thymectomy has been the mainstay of treatment for thymoma combined with myasthenia gravis (MG) (1, 2). The traditional method of surgical treatment is median sternotomy (3). Recently, minimally invasive techniques, including thoracoscopic and robot-assisted thoracic surgery, have become the standard of care for early-stage thymic tumors (4–6). However, the appropriate surgical approach remains controversial due to the recommendation to remove all thymus glands, both encapsulated and extracapsular tissues, during surgery, resulting in a better outcome for patients with MG. Several studies have shown that minimally invasive thymectomy can reduce intraoperative blood loss, postoperative pain, and postoperative complications (7–9). However, the neurological outcomes of MG after minimally invasive thymectomy are debatable. Few studies have focused on whether minimally invasive surgery is effective in improving neurological outcomes in patients with MG-affected thymoma. Franca Melfi suggested that robotic surgery for patients with thymoma and concomitant MG could be effective in improving neurological prognosis (10).
The subxiphoid approach has been used successfully in many centers in the last decade. Our center has been using the subxiphoid and subcostal arch approaches since 2017. In contrast to the unilateral approach, which makes it challenging to visualize the contralateral phrenic clearly, the subxiphoid approach allows adequate visualization of the entire anterior mediastinal space (11, 12). Furthermore, it was reported that subxiphoid and subcostal arch thoracoscopic thymectomy appeared to be a safe and feasible procedure for early-stage thymoma and MG (10, 13). However, they did not explore the neurological outcomes of MG under this approach.
QMGS may be more sensitive than other methods in detecting differences in neurological outcomes in MG (14, 15). A randomized trial comparing thymectomy plus prednisone with prednisone alone for MG used QMGS to assess outcomes and reported improved clinical outcomes for thymectomy compared with those for prednisone alone for 3 years. The results of the trial showed that a 2.3-point reduction in QMGS was associated with an improvement in the clinical status of MG, and the time to achieve a 2.3-point reduction was approximately 3 months (1).
A retrospective study was conducted to evaluate the clinical and neurological outcomes of extended thymectomy under video-assisted thoracoscopic surgery (VATS) through the subxiphoid and subcostal arches and compared with median sternotomy in patients diagnosed with early phrenic thymoma with thymomatous MG.
This study was approved by the Clinical Research Committee of the First Affiliated Hospital of Sun Yat-sen University. Because of the retrospective nature of the study, the requirement for informed patient consent was waived. At our hospital, a team of experienced neurologists and thoracic surgeons worked together to treat patients diagnosed with thymoma and thymomatous MG.
Clinical data were collected from January 2019 to December 2020 from all consecutive patients with Masaoka stage I and II thymoma and thymomatous MG who underwent thoracoscopic thymectomy via subxiphoid and subcostal arch or open sternotomy. The diagnosis of thymoma was based on computed tomography (CT) imaging of the chest. Tumors less than 5 cm in size with well-defined borders were considered suitable for the VATS approach under the subxiphoid and subcostal arches. The staging of thymoma was evaluated according to Masaoka's staging system (16). Masaoka stage I and II thymomas with tumor size less than 5 cm were collected. The severity of MG was assessed according to the clinical classification of the Myasthenia Gravis Foundation of America (MGFA) (17). Before surgery, MG was initially treated to control symptoms, as decided by neurologists. Thymectomy was performed when MG symptoms improved significantly in MGFA classes less than IIB, and the daily dosage of prednisone was <20 mg/day.
All patients who underwent surgery were evaluated by thoracic surgeons, neurologists, and anesthetists prior to surgery. Informed consent was obtained from all patients before surgery. A routine preoperative evaluation was performed to rule out distant metastases and evaluate cardiac and pulmonary functions. Extensive thymectomy was performed by thoracic surgeons by open sternotomy or under VATS using subxiphoid and subcostal arch approaches.
All surgical specimens were subjected to histopathological examination. Thymomas were classified according to the new World Health Organization classification. The pathological staging of thymomas was performed according to the Masaoka staging system. Only patients with thymoma confirmed by histopathological examination and not type C (thymic carcinoma) were included in the analysis. The patients were seen in the neurology clinic at 1, 3, 6, and 12 months postoperatively to evaluate their neurological status using QMG scores, which were recorded at the time of preoperative confirmation of the initial diagnosis and the day before surgery. In this study, the QMG score was used as an outcome measure, which was also used in our study. The study showed that a 2.3-point reduction in score was associated with an improvement in clinical status. The doses of medications were gradually reduced according to the improvement in the symptoms of MG. Only patients with at least 1 year of follow-up and complete follow-up data for neurological outcomes were included in the analysis.
All patients were supine on the operating table with the surgeon standing between their legs. The assistant was on the right side of the patient. A 3-cm incision was made at the lower edge of the xiphoid to set up the thoracoscope. The rectus abdominis muscle was dissected, and the posterior sternum space was created by finger dissection. Under the guidance of the operator's finger, two 5 mm extrapleural thoracic ports were created at the intersection of the midclavicular line and bilateral costal arches to introduce thoracoscopic grasping forceps and a harmonic scalpel. A 30° oblique 10-mm thoracoscope was introduced through a subxiphoid incision. A pneumomediastinum was created by an 8-cm H2O positive pressure carbon dioxide (CO2) insufflation to enlarge the retrosternal space and facilitate tumor dissection. During the operation, both the right and the left mediastinal pleura were opened. The entire thymus and thymoma and all associated adipose tissue were excised en-bloc. The prepericardial fat and the fat pads near the cardiac–diaphragmatic angles, the aortopulmonary window, and the lower poles of the thyroid gland were carefully dissected and excised. The superior vena cava, both the innominate veins and the aorta, were skeletonized. All samples were placed in a plastic bag and removed from the mediastinum through the subxiphoid port. An 18-F drainage tube was inserted into the mediastinum through the left costal arch port (13, 18). Patients with converted open sternotomy intraoperative were excluded from the analysis.
Demographic data, as well as intraoperative and postoperative results, were collected and analyzed. A formal pain assessment for the study was also performed using visual analog scales at 24 and 72 h after surgery. The purpose of this study was to compare the neurological outcomes of the two approaches 1 year after the operation.
Continuous variables were reported as mean ± standard deviation (SD) and median (range), whereas categorical variables were presented as numbers and percentages. To investigate the association between independent variables and improved clinical status, univariate and multivariate Cox proportional hazards regression models were used. Significant variables in the univariate results were entered into the multivariate model, and significant variables in the multivariate results were recognized as factors associated with the improvement in clinical status. Associated factors were further used as group factors for Kaplan–Meier survival analysis and the log-rank test to observe changes in the rates of achieving clinical status improvement during postoperative follow-up. All analyses were performed using IBM SPSS version 25 software (SPSS Statistics V25, IBM Corporation, Somers, NY, USA). In all analyses, a two-tailed value of P < 0.05 was considered to indicate statistical significance.
In total, 265 consecutive patients were enrolled from January 2019 to December 2020. As shown in Figure 1, 89 patients were included in the analysis, 44 of whom had a subxiphoid approach, and 45 had a trans-sternal approach. The mean age of these 89 patients was 48.8 years. The characteristics of the clinical and pathological data between the subxiphoid approach and the trans-sternal approach groups are given in Table 1. There were no statistical differences between the two groups in terms of gender, age, MGFA clinical classification, WHO histological type, maximum tumor size, and Masaoka–Koga stage. Pain scores were significantly higher at 24 and 72 h after surgery in patients with the trans-sternal approach. The duration of operation and blood loss differed significantly between the two approaches, and we did not compare these two variables in Table 1. However, the mean operative duration for the subxiphoid approach was 98 min, and the mean blood loss was 29.2 ml.
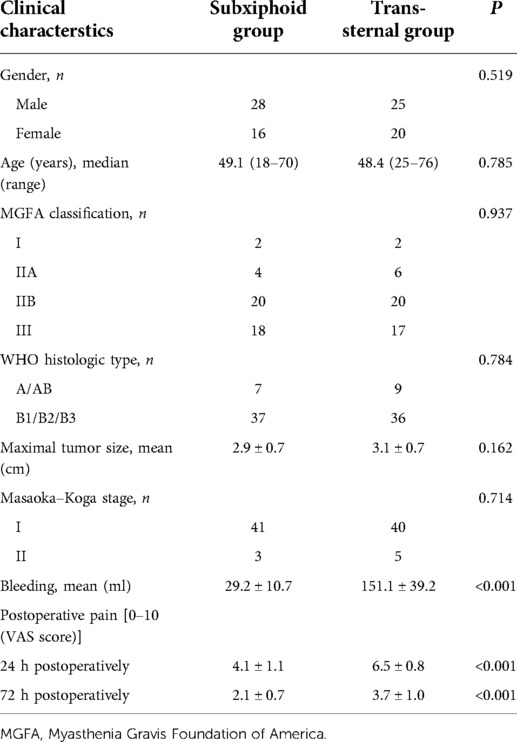
Table 1. Characteristics of clinical and pathological data between subxiphoid approach and trans-sternal approach groups.
The comparative data on neurological outcomes between the subxiphoid and the trans-sternal approach groups are presented in Table 2. There were no statistical differences between the two groups in terms of preoperative administration of pyridostigmine, steroid administration, immunosuppressive medications, or perioperative immunoglobulin infusion. There were two cases of postoperative MG crisis in the subxiphoid group and four cases in the trans-sternal group.
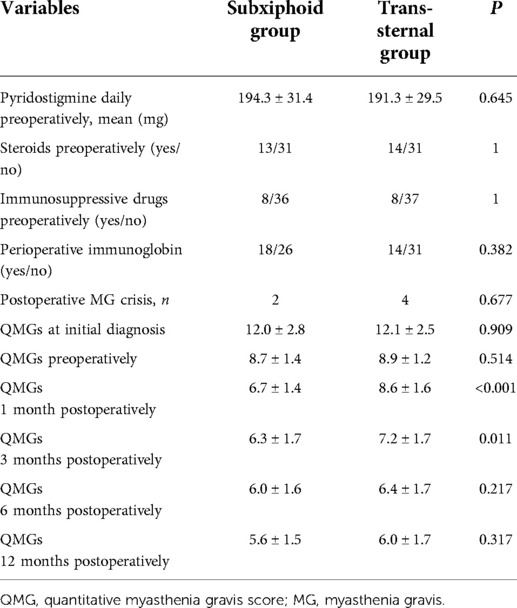
Table 2. Comparison of neurological outcomes between subxiphoid approach and trans-sternal approach groups.
The changes in QMG during the 12-month postoperative follow-up are shown in Figure 2. As shown in Table 2, the mean QMG decreased from 12 at initial diagnosis to 8.7 preoperatively and 5.6 at 12 months postoperatively in the subxiphoid group and from 12.1 to 8.9 and 6.0 in the trans-sternal group. There were no statistical differences in mean QMGS between the two groups at initial diagnosis, preoperative time, 6 months postoperatively, and 12 months postoperatively. Mean QMGS in the subxiphoid group was significantly lower at 1 month and 3 months postoperatively. The curves for the two groups separate only at 1 and 3 months postoperatively but coincide at 6 months postoperatively.
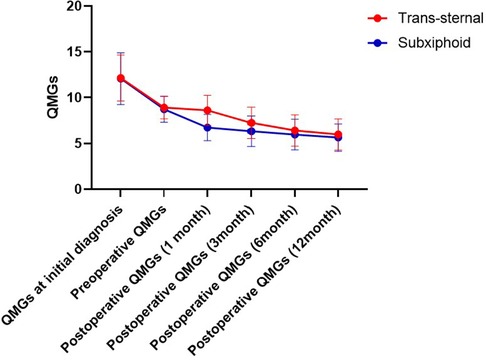
Figure 2. Change of QMGs from the initial diagnosis to 12 months follow-up. QMG, quantitative myasthenia gravis score.
To investigate the impact of the surgical approach on postoperative neurological outcomes, a 2.3-point reduction in the QMG score prior to surgery was associated with an improvement in clinical status. Kaplan–Meier analysis indicated that the median time to achieve improved clinical status was 3 months (95% CI, 2.15–3.85) for the subxiphoid approach and 6 months (95% CI, 5.54–6.46) for the trans-sternal approach. Thirteen patients (28.9%) underwent the trans-sternal approach, and 10 (22.7%) who underwent the subxiphoid approach did not improve their clinical status compared with the preoperative period.
Univariate and multivariate analyses were used to explore the independent variables associated with a faster improvement in clinical status. The results are given in Table 3. Since the event was defined as a reduction of 2.3 QMGS, which implies an improvement in clinical status, an estimated hazard ratio (HR) <1 indicates a slower improvement in clinical status, whereas a HR >1 indicates a faster improvement in clinical status. Univariate results showed that the subxiphoid approach was associated with a faster improvement in clinical status (HR = 1.701, 95% CI, 1.044–2.773, P < 0.05), whereas age ≦48 was associated with a faster improvement in clinical status (HR = 1.709, 95% CI, 1.044–2.799, P < 0.05). Age ≦48 (HR = 1.837, 95% CI, 1.093–3.086, P = 0.022) and the subxiphoid approach (HR = 1.892, 95% CI, 1.127–3.177, P = 0.016) remained significant in the multivariate model.
Thoracoscopic subxiphoid and subcostal arch extended thymectomy is a feasible intervention for patients with early-stage thymoma and thymomatous MG (10, 13). For early-stage thymomas with a tumor size of less than 5 cm, the subxiphoid approach can provide adequate visualization of the entire anterior mediastinum space during operation. The use of this approach allows for extended thymectomy and adipose tissue dissection. According to the ITMIG guidelines, after identification of the right and left phrenic nerves, the anterior mediastinal adipose tissue must be removed to ensure oncological and neurological radicality, followed by dissection from the jugular to the anterior pericardiophrenic angle (19). Trans-sternal thymectomy has long represented the standard surgical approach for treating patients with thymoma and thymomatous MG with excellent visualization of the anterior mediastinum to ensure appropriate control of nerves and vessels (4). The trans-sternal approach was adopted as a control group to assess the neurological outcomes of thymomatous MG after extended thymectomy with a subxiphoid approach.
Several studies have confirmed the safety of the subxiphoid approach when applied to anterior mediastinal lesions (18, 20, 21). The approach via subxiphoid and subcostal arches was adopted in our center in 2017. During the early learning process, the procedure was converted to open sternotomy due to hemorrhage. After a 5–10 case learning curve, only patients with extensive invasion required conversion to an open sternotomy. The mean operative time in the subxiphoid group was 98 min, similar to other studies (13, 18, 21, 22) with a range of 95–147 min. The blood loss in our study was 29.2 ml, which was also similar to their results, with a range of 25.5–73.8 ml. The subxiphoid approach was less invasive and had a faster postoperative recovery. Most patients were discharged on the third or fourth postoperative day.
The postoperative myasthenia crisis after thymectomy was reported to be between 6% and 34% (23). However, this crisis was reported in the results of several studies (24). The causes of the myasthenia crisis were complicated. In the present study, we considered pain to play an important role in the postoperative myasthenia crisis. The subxiphoid group had lower VAS scores, contributing to less respiratory failure and better recovery. The postoperative myasthenia crisis was also less frequent in the subxiphoid group. Furthermore, a comparison of the perioperative and follow-up outcomes of patients with MG who underwent subxiphoid-subcostal or unilateral thoracoscopic thymectomy showed that only pain scores were significantly lower in the subxiphoid group postoperatively. However, the myasthenia crisis was less frequent, and the neurological results were better (21, 22).
It is well known that the course of MG is different in patients diagnosed with thymoma or benign disease (25). Studies have shown that thymoma is significantly associated with the inability to achieve complete stable remission (CSR) at long-term follow-up (26). CSR was not adopted as an evaluation indicator in this study due to the short follow-up period of 1 year. QMGS is a continuous variable and a favorable measure. Furthermore, there was an association between myasthenia symptoms and more aggressive forms of thymoma (27). However, the patients in this study were diagnosed with Masaoka stage I and II thymoma. The mean QMG decreased from 8.7 preoperatively to 5.6 at 12 months postoperatively in the subxiphoid group and from 8.9 to 6.0 in the trans-sternal group. Approximately 71.1% of the patients in the open group and 78.3% in the subxiphoid group had better neurological status. This is comparable to the results of other studies (22, 28, 29).
Patients in the subxiphoid group could see an early improvement in neurological status within 3 months, while those in the open group improved within 6 months postoperatively. The mean QMG score decreased rapidly from preoperative to postoperative, indicating a statistically significant improvement in clinical neurological status. However, the mean QMG scores were similar at 6 months, implying no statistically significant differences in long-term neurological outcomes between the two groups. Viet Anh Le reported that when they compared left-sided and right-sided VATS, as well as VATS and open surgery, there was no association between MG results and VATS sites between the two surgical approaches (30). In addition, another study showed no significant correlation between surgical approach and neurological outcomes (22). However, robotic surgical treatment for patients with thymoma and concomitant MG effectively improved neurological outcomes (10).
In patients with Masaoka stage I and II thymoma who underwent thymectomy, with tumor size less than 5 cm and thymomatous MG, age 48 years and the subxiphoid approach were associated with a faster improvement in clinical status. Our previous study also showed that younger patients could have better neurological outcomes (31).
This monocentric retrospective study had several limitations. Due to the retrospective nature of the study, randomization was absent, and selection bias could not be eliminated. The number of patients and the duration of the follow-up period should be increased to confirm the neurological and oncological results. A prospective multicenter comparative study with other surgical techniques (unilateral thoracoscopic thymectomy) would be more appropriate.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Committee on Clinical Investigations of the First Affiliated Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
HZ, ZL, and XY are co-first authors and composed this article. CS and ZC directed this study. JZ, BZ, and XZ were in charge of data collection. All named authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, took responsibility for the integrity of the work as a whole, and gave final approval for the version to be published. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. (2016) 375(6):511–22. doi: 10.1056/NEJMoa1602489
2. Li F, Ismail M, Elsner A, Uluk D, Bauer G, Meisel A, et al. Surgical techniques for myasthenia gravis robotic-assisted thoracoscopic surgery. Thorac Surg Clin. (2019) 29:177–86. doi: 10.1016/j.thorsurg.2018.12.006
3. Pompeo E, Dauri M, Massa R, Peer M. Minimalist thoracoscopic resection of thymoma associated with myasthenia gravis. J Thorac Cardiovasc Surg. (2017) 154:1463–5. doi: 10.1016/j.jtcvs.2017.05.084
4. Meyer DM, Herbert MA, Sobhani NC, Tavakolian P, Duncan A, Bruns M, et al. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transsternal and minimally invasive approaches. Ann Thorac Surg. (2009) 87(2):385–90; discussion 390–1. doi: 10.1016/j.athoracsur.2008.11.040
5. Wagner AJ, Cortes RA, Strober J, Grethel EJ, Clifton MS, Harrison MR, et al. Long-term follow up after thymectomy for myasthenia gravis: thoracoscopic vs open. J Pediatr Surg. (2006) 41(1):50–4; discussion 50–4. doi: 10.1016/j.jpedsurg.2005.10.006
6. Nakagiri T, Inoue M, Shintani Y, Funaki S, Kawamura T, Minami M, et al. Improved procedures and comparative results for video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc. (2015) 29(9):2859–65. doi: 10.1007/s00464-014-3964-1
7. Zahid I, Sharif S, Routledge T, Scarci M. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg. (2011) 12(1):40–6. doi: 10.1510/icvts.2010.251041
8. Batirel HF. Minimally invasive techniques in thymic surgery: a worldwide perspective. J Vis Surg. (2018) 4(7). doi: 10.21037/jovs.2017.12.18
9. Batirel HF. Early stage thymoma: is VATS the new standard of care? J Thorac Dis. (2016) 8(7):1431–3. doi: 10.21037/jtd.2016.05.35
10. Romano G, Zirafa CC, Ceccarelli I, Guida M, Davini F, Maestri M, et al. Robotic thymectomy for thymoma in patients with myasthenia gravis: neurological and oncological outcomes. Eur J Cardiothorac Sur. (2021) 60(4):890–5. doi: 10.1093/ejcts/ezab253
11. Hsu C-P, Chuang C-Y, Hsu N-Y, Chen C-Y. Comparison between the right side and subxiphoid bilateral approaches in performing video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc. (2004) 18(5):821–4. doi: 10.1007/s00464-003-9146-1
12. Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg. (2013) 8:153. doi: 10.1186/1749-8090-8-153
13. Jiang L, Chen H, Hou Z, Qiu Y, Depypere L, Li J, et al. Subxiphoid versus unilateral VATS thymectomy for thymomas: a propensity score-matching analysis. Ann Thorac Surg. (2022) 113(5):1656–62. doi: 10.1016/j.athoracsur.2021.05.011
14. Bedlack RS, Simel DL, Bosworth H, Samsa G, Tucker-Lipscomb B, Sanders DB. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology. (2005) 64(11):1968–70. doi: 10.1212/01.WNL.0000163988.28892.79
15. Sharshar T, Chevret S, Mazighi M, Chillet P, Huberfeld G, Berreotta C, et al. Validity and reliability of two muscle strength scores commonly used as endpoints in assessing treatment of myasthenia gravis. J Neurol. (2000) 247(4):286–90. doi: 10.1007/s004150050585
17. Jaretzki A 3rd, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, et al., Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg. (2000) 70(1):327–34. doi: 10.1016/s0003-4975(00)01595-2
18. Lu Q, Zhao J, Wang J, Chen Z, Han Y, Huang L, et al. Subxiphoid and subcostal arch “three ports” thoracoscopic extended thymectomy for myasthenia gravis. J Thorac Dis. (2018) 10(3):1711–20. doi: 10.21037/jtd.2018.02.11
19. Ruffini E, Filosso PL, Guerrera F, Lausi P, Lyberis P, Oliaro A. Optimal surgical approach to thymic malignancies: new trends challenging old dogmas. Lung Cancer. (2018) 118:161–70. doi: 10.1016/j.lungcan.2018.01.025
20. Zielinski M, Czajkowski W, Gwozdz P, Nabialek T, Szlubowski A, Pankowski J. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg. (2013) 44(2):e113–9; discussion e119. doi: 10.1093/ejcts/ezt224
21. Cao P, Hua S, Qu W, Kong K, Han P, Yue J, et al. Subxiphoid-subcostal thoracoscopic thymectomy for seropositive myasthenia offers equivalent remission rates and potentially faster recovery. Interact Cardiovasc Thorac Surg. (2022) 34(4):576–83. doi: 10.1093/icvts/ivab294
22. Qiu Z, Chen L, Lin Q, Wu H, Sun H, Zhou X, et al. Perioperative outcomes and mid-term effects in performing video-assisted thoracoscopic extended thymectomy for myasthenia gravis: subxiphoid versus right thoracic approaches. J Thorac Dis. (2020) 12(4):1529–39. doi: 10.21037/jtd.2020.03.43
23. Kas J, Kiss D, Simon V, Svastics E, Major L, Szobor A. Decade-long experience with surgical therapy of myasthenia gravis: early complications of 324 transsternal thymectomies. Ann Thorac Surg. (2001) 72(5):1691–7. doi: 10.1016/s0003-4975(01)03080-6
24. Jiang L, Depypere L, Rocco G, Chen J-S, Liu J, Shao W, et al. Spontaneous ventilation thoracoscopic thymectomy without muscle relaxant for myasthenia gravis: comparison with “standard” thoracoscopic thymectomy. J Thorac Cardiovasc Surg. (2018) 155(4):1882–9. doi: 10.1016/j.jtcvs.2017.11.045
25. Keijzers M, de Baets M, Hochstenbag M, Abdul-Hamid M, Hausen AZ, van der Linden M, et al. Robotic thymectomy in patients with myasthenia gravis: neurological and surgical outcomes. Eur J Cardiothorac Surg. (2015) 48(1):40–5. doi: 10.1093/ejcts/ezu352
26. Kaufman AJ, Palatt J, Sivak M, Raimondi P, Lee DS, Wolf A, et al. Thymectomy for myasthenia gravis: complete stable remission and associated prognostic factors in over 1000 cases. Semin Thorac Cardiovasc Surg. (2016) 28(2):561–8. doi: 10.1053/j.semtcvs.2016.04.002
27. Lin Q, Zhang Y, Yang L. Single-center retrospective analysis of 162 cases with thymoma complicating myasthenia gravis. J Buon. (2017) 22(3):741–5. ISSN: 1107-0625, online ISSN: 2241-6293. 28730784
28. Yu L, Zhang X-j, Ma S, Li F, Zhang Y-f. Thoracoscopic thymectomy for myasthenia gravis with and without thymoma: a single-center experience. Ann Thorac Surg. (2012) 93(1):240–4. doi: 10.1016/j.athoracsur.2011.04.043
29. Agasthian T, Lin SJ. Clinical outcome of video-assisted thymectomy for myasthenia gravis and thymoma. Asian Cardiovasc Thorac Ann. (2010) 18(3):234–9. doi: 10.1177/0218492310369017
30. Nguyen TG, Nguyen NT, Nguyen VN, Nguyen TK, Vu DT, Le VA. Video-assisted thoracoscopic surgery for myasthenia gravis with thymoma: a six-year single-center experience. Asian J Surg. (2021) 44(1):369–73. doi: 10.1016/j.asjsur.2020.10.006
31. Haoshuai Z, Jianyong Z, Lei Y, Bo Z, Xiao J, Zhang X, et al. Factors affecting improvement of neurologic status evaluated by Quantitative Myasthenia Gravis Score for patients with thymomatous myasthenia gravis after extended thymectomy. J Transl Med. (2021) 19(1):413. doi: 10.1186/s12967-021-03082-z
Keywords: thymoma, myasthenia gravis, subxiphoid approach, quantitative myasthenia gravis score, neurological outcome
Citation: Zhu H, Liu Z, Yao X, Zou J, Zeng B, Zhang X, Chen Z and Su C (2022) Neurological outcomes of extended thymectomy for thymomatous myasthenia gravis: Subxiphoid vs. trans-sternal approaches. Front. Surg. 9:973954. doi: 10.3389/fsurg.2022.973954
Received: 24 June 2022; Accepted: 27 July 2022;
Published: 6 September 2022.
Edited by:
Davide Tosi, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy© 2022 Zhu, Liu, Yao, Zou, Zeng, Zhang, Chen and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haoshuai Zhu emh1aHNoNkBtYWlsLnN5c3UuZWR1LmNu Chunhua Su c3VjaGhAbWFpbC5zeXN1LmVkdS5jbg==
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.