
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg., 02 November 2022
Sec. Orthopedic Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.970537
Background: Smoking increases risk of several complications after total hip or knee arthroplasty (THA/TKA), so we systematically reviewed and meta-analyzed the literature to take into account all relevant evidence, particularly studies published since 2010.
Methods: The PubMed, Ovid Embase, Web of Science, and EBSCOHost databases were searched and studies were selected and analyzed according to MOOSE recommendations. Methodological quality of included studies was assessed using the Newcastle-Ottawa Scale. Data were qualitatively synthesized or meta-analyzed using a random-effects model.
Results: A total of 40 studies involving 3,037,683 cases were included. Qualitative analysis suggested that smoking is associated with worse patient-reported outcomes within one year after surgery, and meta-analysis showed that smoking significantly increased risk of the following outcomes: total complications (OR 1.41, 95% CI 1.01–1.98), wound complications (OR 1.77, 95% CI 1.50–2.10), prosthetic joint infection (OR 1.84, 95% CI 1.52–2.24), aseptic loosening (OR 1.62, 95% CI 1.12–2.34), revision (OR 2.12, 95% CI 1.46–3.08), cardiac arrest (OR 4.90, 95% CI 2.26–10.60), cerebrovascular accident (OR 2.22, 95% CI 1.01–4.85), pneumonia (OR 2.35, 95% CI 1.17–4.74), acute renal insufficiency (OR 2.01, 95% CI 1.48–2.73), sepsis (OR 4.35, 95% CI 1.35–14.00), inpatient mortality (OR 12.37, 95% CI 4.46–34.28), and persistent opioid consumption (OR 1.64, 95% CI 1.39–1.92).
Conclusion: Smoking patients undergoing THA and TKA are at increased risk of numerous complications, inpatient mortality, persistent opioid consumption, and worse 1-year patient-reported outcomes. Pre-surgical protocols for these outcomes should give special consideration to smoking patients.
Smoking increases the risk of various diseases, including cancer, stroke, as well as diseases affecting the heart, lungs and peripheral vasculature (1–5). A global epidemiological survey of 3 billion individuals indicated the rate of smoking to be 48.6% in men and 11.3% in women (6). As a result, smoking is a leading cause of preventable premature mortality worldwide (3). Preoperative smoking is common among patients undergoing elective surgery, and it increases risk of several postoperative complications, such as wound complications, pulmonary complications, and general infections (7, 8).
Total hip and knee arthroplasty (THA/TKA) are effective surgical procedures to improve function and reduce pain in patients with severe hip and knee joint disease. By 2030, 572,000 THA procedures and 3.48 million TKA procedures will likely be performed in the United States alone (9). It is reported that up to one-third of patients may experience various complications after THA/TKA, which has a negative impact on the rehabilitation and satisfaction of patients (9–13). National databases from several countries indicate a smoking prevalence of 10%–40% among THA/TKA patients (10–13). Numerous studies have examined the impact of smoking on postsurgical outcomes in these patients. Certain studies have concluded that smoking increases risk of systemic complications (14, 15), surgical complications (16–18), mortality (19), or readmission (20), or that it is associated with worse patient-reported outcomes (12). However, other studies have failed to find a correlation between smoking and these complications or poor outcomes (11, 21–24). A systematic review covering literature published up to 2010 concluded that smoking is associated with significantly higher risk of any postoperative complication and mortality following THA or TKA (25).
Since that review, more than 30 studies have been published that broadened and deepened our understanding of how smoking affects outcomes after THA and TKA. We therefore comprehensively assessed relevant studies published since 2000 through 2022, and we meta-analyzed or qualitatively synthesized data on 21 indices in seven outcomes: total complications, surgical complications, systemic complications, mortality, readmission, opioid consumption, and patient-reported outcomes.
This review was reported in line with MOOSE guidelines (26, 27). Two authors independently searched the databases of PubMed, Ovid Embase, Web of Science, and EBSCOHost from January 2000 to August 2022 using comprehensive search strategies (Search strategies applied to each database could be found in Supplementary Figure S1A–D). Reference lists of relevant articles were also reviewed to identify additional eligible studies. Language experts were contacted for translation of articles not written in English.
A study was considered eligible for inclusion if it (1) was a cohort study based on smoking status, or a case-control study that considered smoking as a possible risk factor; and (2) it reported data about the potential association of smoking with at least one of the outcomes of interest following THA or TKA. A study was excluded if it (1) was based on a cross-sectional questionnaire, pilot study, case report, case series report, or brief report; (2) was published only as an abstract; or (3) involved revision surgery, hemiarthroplasty, unicompartment knee arthroplasty, or arthroscopic surgery.
All retrieved studies were imported into Endnote × 7 (Thomson Scientific, Stamford, Connecticut, United States). The same two authors who had searched the databases independently excluded irrelevant studies based on titles and abstracts. These two authors then read the full text of the remaining articles to produce a final list of studies. Any discrepancies between the two authors were resolved through discussion with a third author.
The same two authors independently extracted the following data from each eligible study: first author's name, country, publication year, surgery type, sample size, study type, and follow-up time. Surgical complications included wound complications (any wound problems such as superficial/deep infection, exudation, or hematoma), prosthetic joint infection, aseptic loosening, dislocation, and revision. Systemic complications included circulatory complications, respiratory complications, urinary complications, venous thromboembolism, and sepsis. Patient-reported outcomes were evaluated based on various scales such as Harris Hip Scores and Oxford Hip/Knee Score.
The same two authors who searched the databases also independently evaluated the methodological quality of each study based on the Newcastle-Ottawa Scale (NOS), which is a widely used quality evaluation tool for observational studies (28). The maximum total NOS score is 9, and only studies scoring at least 5 were included in the meta-analysis. The quality of evidence for each outcome was evaluated according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (29, 30).
Data on patient-reported outcomes were synthesized qualitatively because the evaluation methods varied substantially across studies. Other outcomes were meta-analyzed using random-effects model and displayed as forest plots using STATA Release 14 (Stata Corp, College Station, Texas, United States). All variables in this study were dichotomous, and pooled risk estimates were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs). A 2-tailed P value < 0.05 was defined as statistically significant.
Heterogeneity across studies was assessed using the I2 test and was considered substantial if I2 > 50% (31). In meta-analyses involving at least 10 studies and substantial heterogeneity, meta-regression analyses were conducted based on publication year, country, follow-up time, surgery type, or NOS score in order to identify potential sources of heterogeneity (32). Subgroup analysis based on the study type (cohort vs. case-control) was also conducted to detect any influence on outcomes.
We planned to perform some or all of the following sensitivity analyses for each outcome: (1) exclude studies involving fewer than 500 or 1,000 patients, (2) exclude studies involving more than 100,000 or 1,000,000 patients, (3) exclude studies involving fewer than 100 events, (4) exclude studies scoring no more than 6 on the NOS, and (5) exclude studies flagged as causing heterogeneity after running the “hetred” command in STATA.
A total of 7,410 records were identified and no additional records were found through manual searching of references. An initial screening removed 4,021 duplicate records, and another 3,131 records were excluded as irrelevant after reading titles and abstracts. A further 218 records were removed because they failed to satisfy selection criteria. In the end, 40 studies involving 3,037,683 cases were included for meta-analysis (10–16, 18–24, 33–58). The details of study identification, inclusion, and exclusion are shown in Figure 1. The included 40 studies contained 20 cohort studies (10, 11, 14, 15, 19, 20, 23, 40, 41, 43, 45–48, 51–53, 55–57) and 20 case-control studies (12, 13, 16, 18, 21, 22, 24 33–39, 42, 44, 49, 50, 54, 58),. Nearly all studies had been published since 2005, including 35 since 2010 and 20 since 2017. Table 1 provides a detailed description of the study characteristics.
Total complications were investigated in eight cohort studies involving 552,553 patients (10, 14, 15, 19, 20, 45, 53, 55). Meta-analysis showed that smoking patients were at higher risk of total complications after THA and TKA than non-smoking patients (OR 1.41, 95% CI 1.01–1.98; I2 = 99%; Table 2).
Wound complications were assessed in 13 cohort studies (10, 14, 15, 19, 20, 41, 45–48, 55–57) and seven case-control studies (16, 24, 34, 38, 39, 42, 58) involving 706,107 patients. Meta-analysis showed that smoking increased the risk of wound complications after THA and TKA (OR 1.77, 95% CI 1.50–2.10; I2 = 85%; Table 2).
Data on prosthetic joint infection were extracted from eight cohort studies (10, 20, 41, 46–48, 56, 57) and six case-control studies (24, 34, 38, 39, 42, 58) involving 234,937 patients. Meta-analysis showed that patients who smoked were at higher risk of prosthetic joint infection than patients who did not (OR 1.84, 95% CI 1.52–2.24; I2 = 63%; Table 2).
Aseptic loosening was assessed in six cohort studies (23, 45, 46, 48, 50, 57) and one case-control study (18), involving a combined total of 112,637 patients. Meta-analysis showed that smoking increased the risk of aseptic loosening after THA/TKA (OR 1.62, 95% CI 1.12–2.34; I2 = 34%; Table 2).
Three cohort studies (48, 52, 57) and one case-control study (50) involving 113,130 patients reported the incidence of dislocation after THA. Meta-analysis showed that there was no significant difference in the risk of dislocation between smoking or non-smoking patients (OR 1.23, 95% CI 1.00–1.50; I2 = 0%; Table 2).
Seven cohort studies (11, 45–48, 56, 57) and three case-control studies (35, 49, 50) involving 171,261 patients reported the incidence of revision. Meta-analysis showed that risk of revision was significantly higher among smoking patients (OR 2.12, 95% CI 1.46–3.08; I2 = 90%; Table 2).
Myocardial infarction was reported in seven cohort studies involving 2,350,799 patients (10, 14, 15, 19, 20, 51, 55). Meta-analysis showed that the risk of myocardial infarction after THA/TKA was greater in smoking patients than non-smoking patients, although the difference was not significant (OR 2.14, 95% CI 0.89–5.17; I2 = 99%; Table 2).
Data on cardiac arrest were extracted from five cohort studies involving 2,200,439 patients (10, 14, 19, 20, 51). Meta-analysis showed that smoking was associated with significantly higher risk of cardiac arrest after THA or TKA (OR 4.90, 95% CI 2.26–10.60; I2 = 93%; Table 2).
A total of six cohort studies involving 2,349,988 patients assessed the risk of cerebrovascular accident after THA/TKA (10, 15, 19, 20, 51, 55). Meta-analysis showed that smoking was associated with significantly greater risk of cerebrovascular accident (OR 2.22, 95% CI 1.01–4.85; I2 = 98%; Table 2).
Pneumonia was assessed in seven cohort studies (10, 15, 19, 20, 45, 51, 55) and one case-control study (36) involving 2,521,518 patients. Meta-analysis showed that smoking significantly increased risk of pneumonia after THA or TKA (OR 2.35, 95% CI 1.17–4.74; I2 = 100%; Table 2).
Seven cohort studies (10, 14, 19, 20, 46, 51, 55) and three case-control studies (21, 22, 33) involving 2,235,826 patients reported the incidence of acute renal insufficiency after THA or TKA. Meta-analysis showed smoking to be associated with significantly higher risk of acute renal insufficiency (OR 2.01, 95% CI 1.48–2.73; I2 = 97%; Table 2).
Data on urinary tract infection was extracted from eight cohort studies involving 2,351,420 patients (10, 14, 15, 19, 20, 46, 51, 55). Meta-analysis showed that risk of urinary tract infection was not significantly different between patients who smoked or did not (OR 1.40, 95% CI 0.94–2.08; I2 = 99%; Table 2).
Seven cohort studies involving 2,350,609 patients (10, 15, 19, 20, 46, 51, 55) and six cohort studies involving 2,317,273 patients (10, 15, 19, 20, 46, 51) separately assessed the incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE). Meta-analysis showed no significant difference between patients who smoked or did not in the case of either DVT (OR 1.54, 95% CI 0.83–2.86; I2 = 99%; Table 2) or PE (OR 1.29, 95% CI 0.60–2.79; I2 = 99%; Table 2).
Sepsis was assessed in four cohort studies (10, 19, 20, 51) and one case-control study (37) involving 2,317,563 patients. Meta-analysis showed that smoking was associated with significantly higher risk of sepsis after THA or TKA (OR 4.35, 95% CI 1.35–14.00; I2 = 99%; Table 2).
Data were separately extracted from two (19, 51) and four cohort studies (14, 19, 20, 55) on inpatient mortality (2,118,935 patients) and 30-day mortality (114,840 patients). Meta-analysis showed that smoking was associated with significantly higher inpatient mortality (OR 12.37, 95% CI 4.46–34.28; I2 = 95%; Table 2), but it did not significantly increase risk of 30-day mortality (OR 0.88, 95% CI 0.68–1.13; I2 = 0%; Table 2).
Two studies involving 5,409 patients reported that smoking patients were at elevated risk of 30-day readmission (20), but there was no difference in readmission at 90 days after surgery (54).
Two cohort studies (15, 40) and two case-control studies (13, 44) involving 183,792 patients reported postoperative opioid consumption. One study showed that smokers consumed significantly more opioids than non-smokers immediately after THA as well as 90 days after the surgery (40). Meta-analysis of another three studies showed that smoking was associated with elevated incidence of persistent opioid consumption within 1 year after THA or TKA (OR 1.64, 95% CI 1.39–1.92; I2 = 93%; Table 2).
A total of four cohort studies (15, 43, 46, 47) and one case-control study (12) involving 1,235,726 patients reported data on patient-reported outcomes based on different scales. In one study, smokers achieved significantly smaller improvements on the WOMAC and SF-12 PCS than non-smokers within 1 year after THA or TKA (43). Another study found that smokers had lower HHS than non-smokers at six months (47). A study of THA patients found that smoking was associated with two trajectories of OHS-assessed functional recovery within the first postoperative year: “slow start”, characterized by no initial improvement, followed later by improvement; and “late dip”, characterized by initial improvement but subsequent deterioration (12). Another study found that smokers had lower OHS/OKS after THA/TKA at 6-month follow-up (15) but not at follow-up longer than 1 year (46, 47).
Subgroup analyses based on study type revealed no significant differences between cohort or case-control studies in incidences of wound complications, prosthetic joint infection, aseptic loosening, dislocation, revision, or persistent opioid consumption. Case-control studies were associated with significantly lower incidences of pneumonia, acute renal insufficiency and sepsis (Figures 2–10). Meta-regression analyses exploring the effects of potential sources of heterogeneity were conducted for the outcomes of wound complications, prosthetic joint infection, revision, and acute renal insufficiency, and significant subgroup effects were not found.
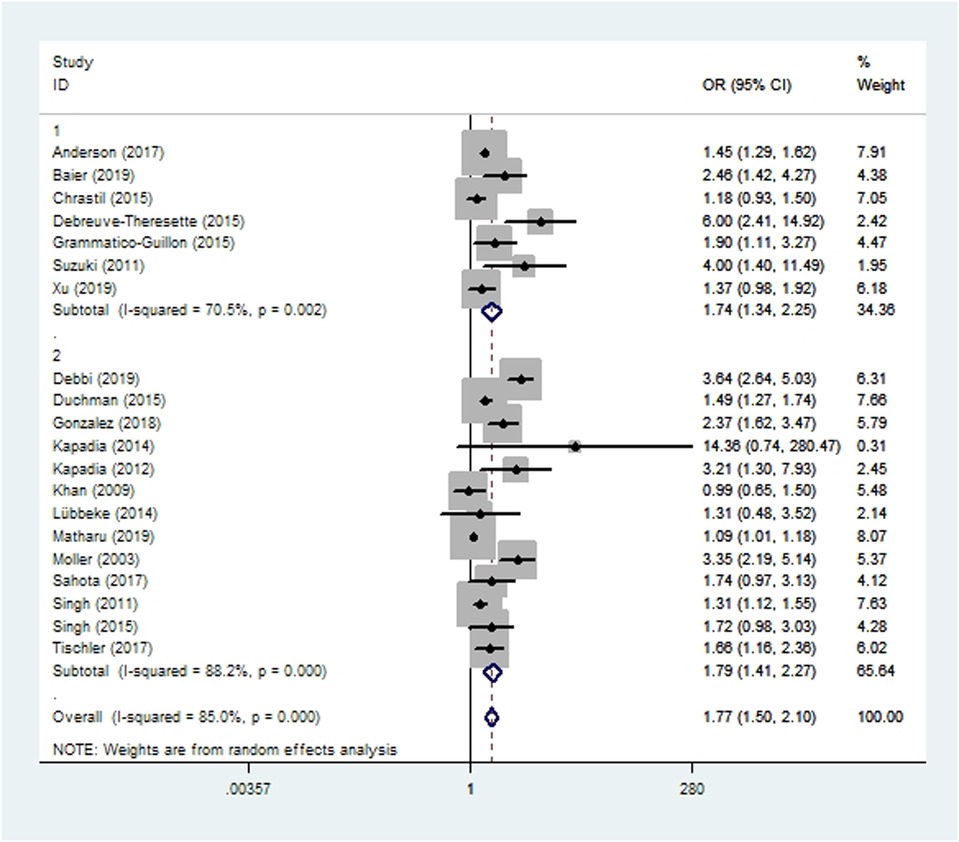
Figure 2. Forest plot of subgroup analysis for wound complications based on study type (1: case-control study; 2: cohort study).
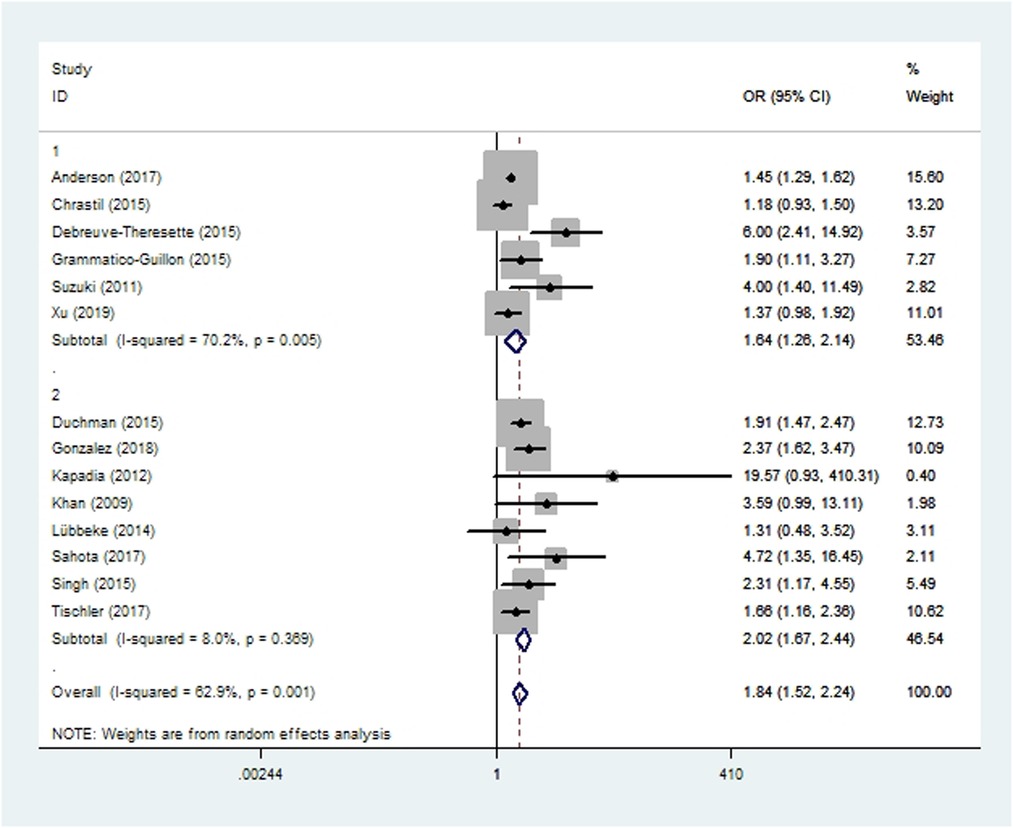
Figure 3. Forest plot of subgroup analysis for prosthetic joint infection based on study type (1: case-control study; 2: cohort study).
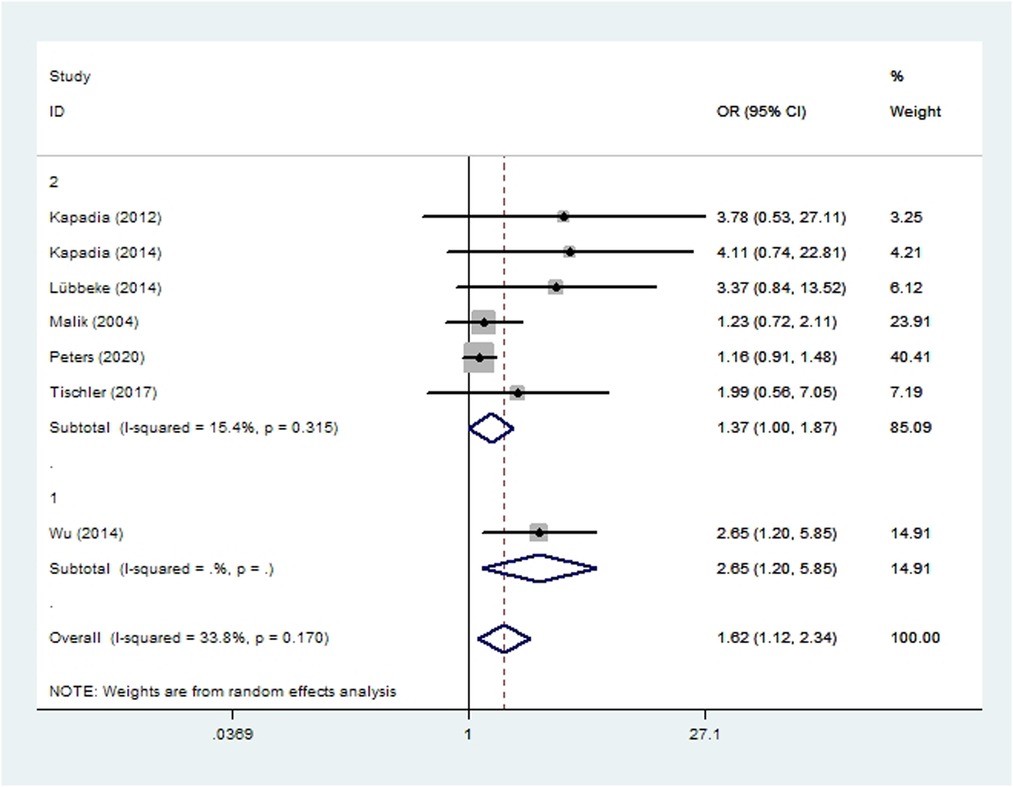
Figure 4. Forest plot of subgroup analysis for aseptic loosening based on study type (1: case-control study; 2: cohort study).
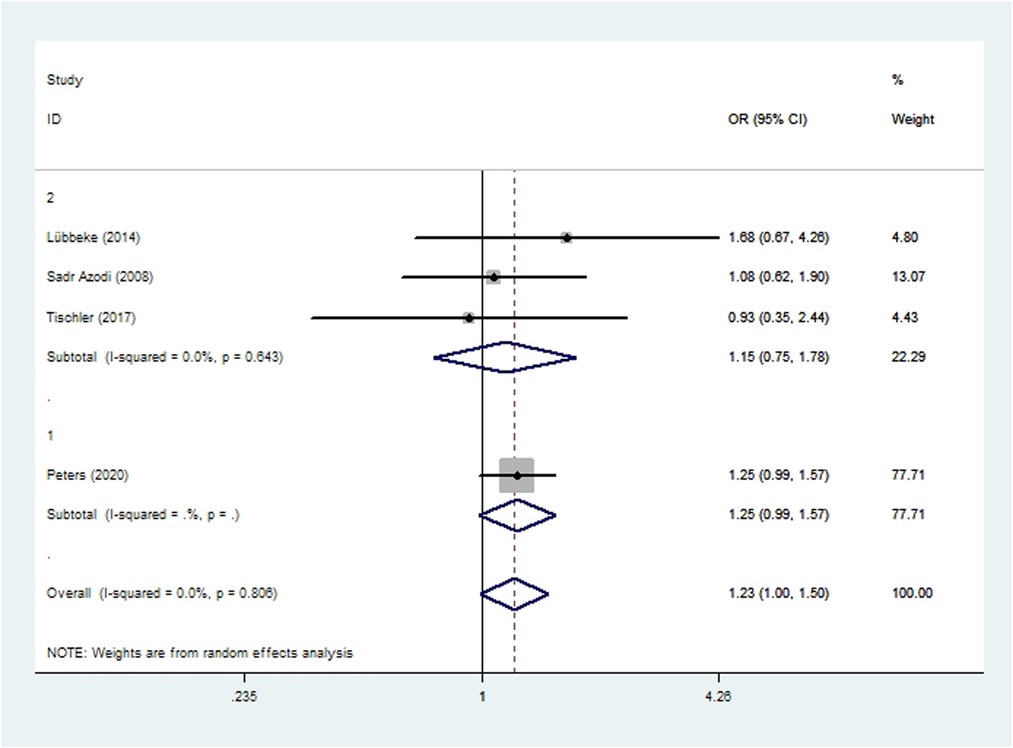
Figure 5. Forest plot of subgroup analysis for dislocation based on study type (1: case-control study; 2: cohort study).
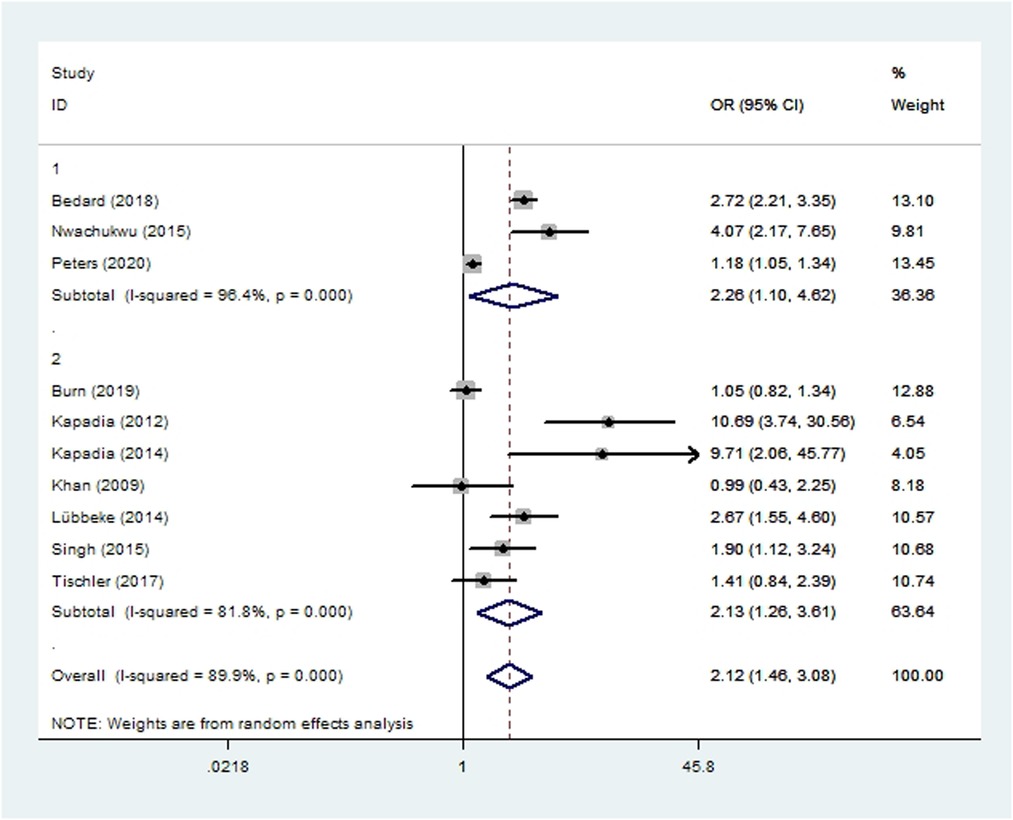
Figure 6. Forest plot of subgroup analysis for revision based on study type (1: case-control study; 2: cohort study).
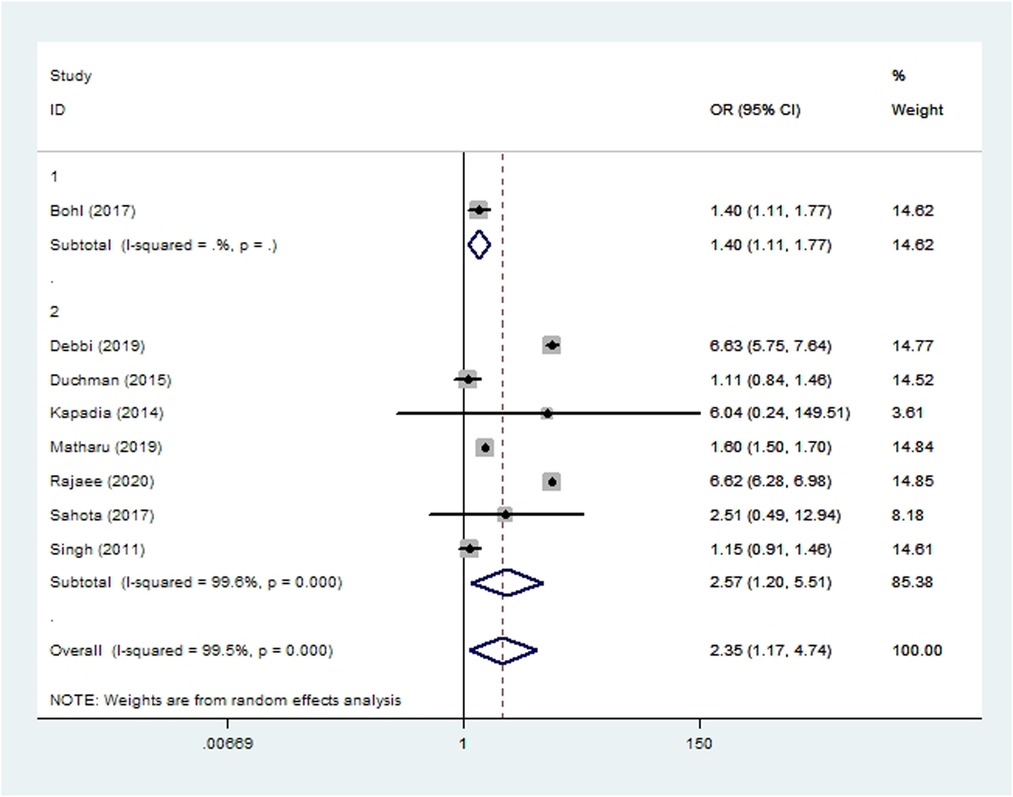
Figure 7. Forest plot of subgroup analysis for pneumonia based on study type (1: case-control study; 2: cohort study).
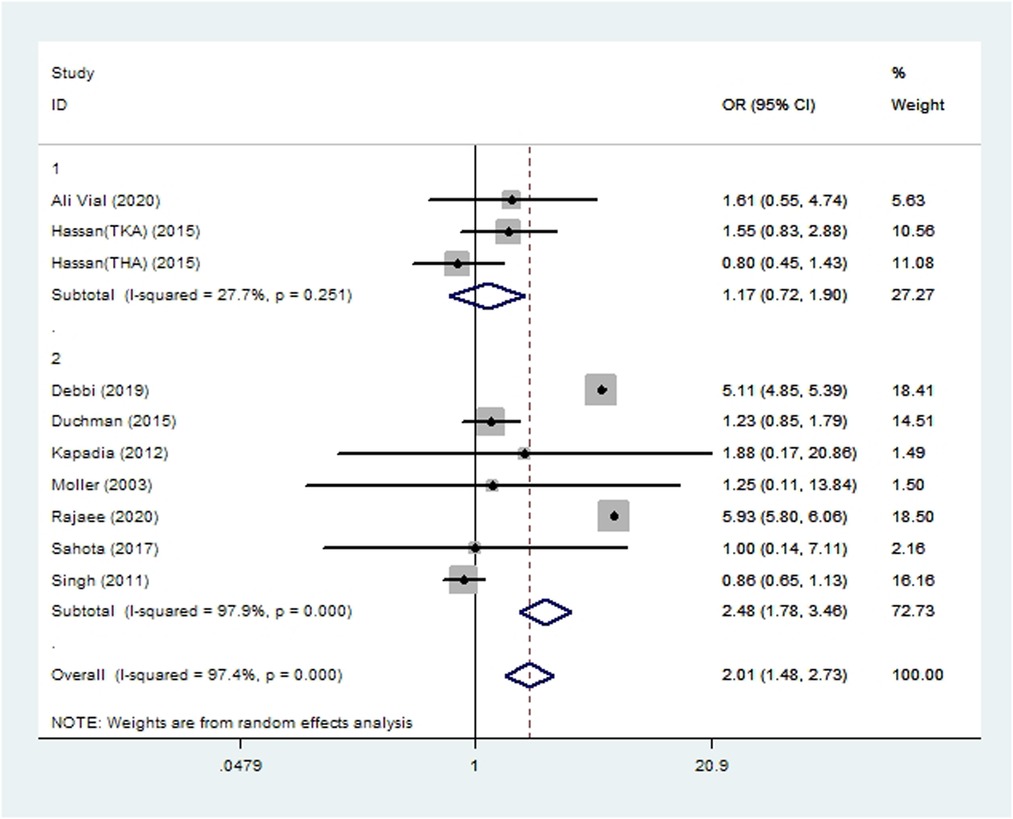
Figure 8. Forest plot of subgroup analysis for acute renal insufficiency based on study type (1: case-control study; 2: cohort study).
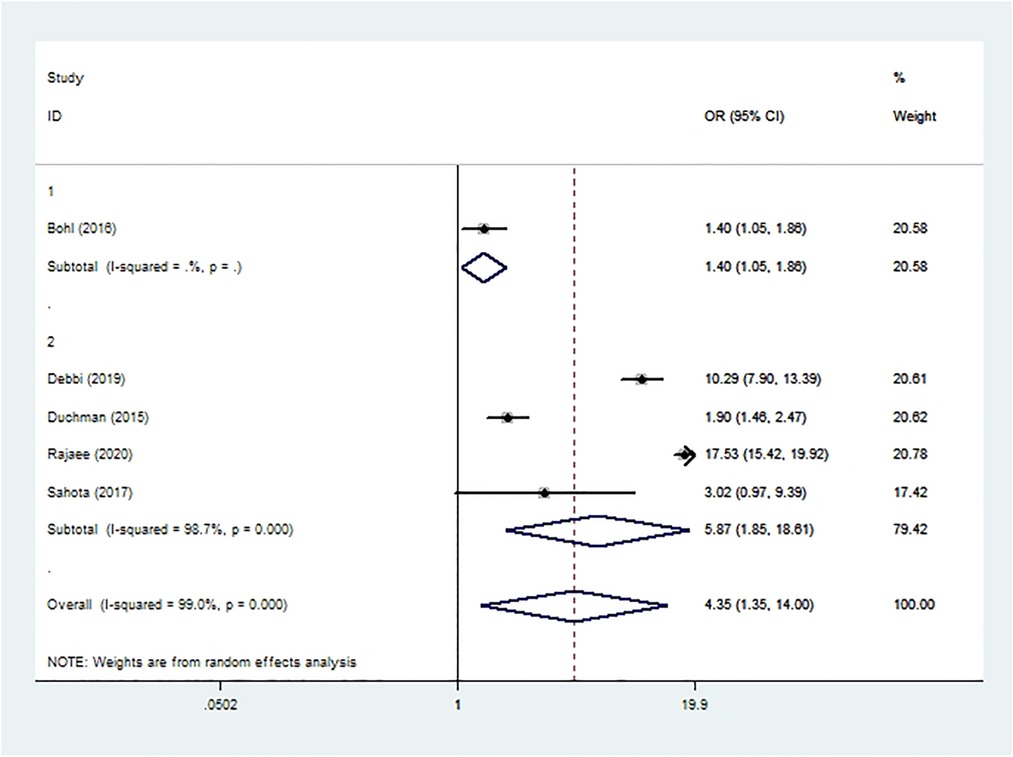
Figure 9. Forest plot of subgroup analysis for sepsis based on study type (1: case-control study; 2: cohort study).
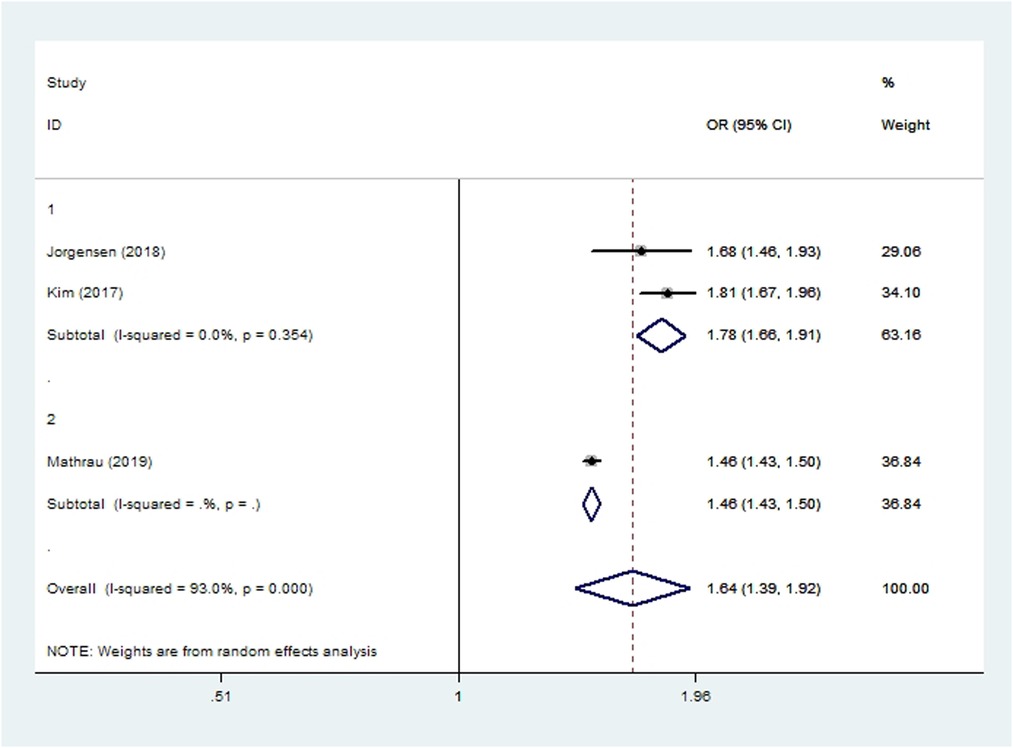
Figure 10. Forest plot of subgroup analysis for persistent opioid consumption based on study type (1: case-control study; 2: cohort study).
Sensitivity analyses gave similar results as overall meta-analysis in the case of wound complications, prosthetic joint infection, dislocation, revision, pneumonia, urinary tract infection, DVT, PE, sepsis, 30-day mortality, and opioid consumption (Table 3). This suggests that these meta-analyses are likely to be robust and to represent true associations. In contrast, sensitivity analyses gave different results from the overall meta-analysis in the case of total complications, aseptic loosening, myocardial infarction, cardiac arrest, cerebrovascular accident, and acute renal insufficiency. These meta-analyses may therefore not be so robust.
The quality of evidence according to the GRADE system was low or very low for all outcomes (Supplementary Table S1).
A systematic review of studies published up to 2010 reported an association between smoking and composite risk of any postoperative complication or death (25), but numerous studies published since then have suggested that smoking exerts more complex effects on outcomes after THA or TKA. Therefore, we conducted the present review and meta-analysis to gain a comprehensive understanding based on all available evidence.
Smoking interferes with all phases of wound healing, including hemostasis, wound contraction, proliferation and remodeling (59), and this has been observed following many types of surgery (7, 59, 60). In addition, recent reports have described a possible correlation between smoking and prosthetic joint infection. The results of the present meta-analysis show that smoking is associated with higher incidences of wound complications and prosthetic joint infection, and the similar results obtained in all sensitivity analyses suggests that our findings are robust.
Smoking also has deleterious effects on bone metabolism (61, 62). It exerts toxic effects directly on bone cells and indirectly by affecting hormones, vitamin D, and oxygenation; it inhibits bone formation and accelerates bone absorption (61–63). Since smoking causes bone loss, it may be associated with periprosthetic osteolysis and subsequent aseptic loosening. After total joint arthroplasy, smokers show significantly lower serum levels of osteogenic markers than non-smokers, suggesting that smoking affects bone formation (61); whether the same is true for aseptic loosening is unclear (18, 23, 64, 65). The present meta-analysis supports the idea that smoking increases the risk of aseptic loosening after THA and TKA, but the different results obtained from sensitivity analyses suggest the need to verify these findings in future work. Since prosthetic joint infection and aseptic loosening are common causes for revision after THA and TKA (66, 67), the ability of smoking to increase the risk of these two complications may explain why it increased the risk of revision in our meta-analysis.
Our study highlights associations between smoking and significantly elevated risk of pneumonia and sepsis after THA and TKA. Smoking can increase the incidence of community-acquired pneumonia by impairing mucociliary clearance and increasing bacterial adherence (68, 69); as well as by causing changes in cellular and humoral immune system function (70). Smoking patients may therefore be at higher risk of postoperative pneumonia due to structural mechanisms and systemic immune dysfunction, which may help explain the results of our meta-analysis. Sepsis following total joint arthroplasty can prove devastating: it significantly increases risk of mortality as well as healthcare costs (37). Surgical site infection and pneumonia are the common sources of sepsis after THA and TKA (37), and the ability of smoking to increase the risk of both types of infection may explain its association with elevated risk of sepsis in our meta-analysis.
Smoking contributes significantly to nearly all cardio-cerebrovascular morbidity and mortality, ranging from chronic diseases of hypertension to acute clinical events such as myocardial infarction and cardiac arrest (71). The primary cause of cardio-cerebrovascular dysfunction appears to be oxidative stress caused by smoke exposure (72). Consistent with this literature, our meta-analysis shows that smoking is associated with significantly higher risk of postoperative cardiac arrest and cerebrovascular accident. In addition, we found that patients who smoke are also more likely to experience myocardial infarction after THA and TKA, although the difference in risk is not significant.
Acute renal insufficiency and urinary tract infection are the most common urinary complications after THA and TKA. In smokers, the genitourinary system is directly exposed to tobacco toxins that are excreted in urine, and the system may also be affected by systemic immune dysfunction caused by smoking (3). Our meta-analysis suggests that smoking is associated with elevated risk of acute renal insufficiency, but not of urinary tract infection, after THA or TKA.
In addition to complications, patient management after total joint arthroplasty aims to optimize the subjective feelings of patients, so patient-reported outcomes are increasingly used in clinical settings (43). Although we were able to conduct only qualitative synthesis, the outcomes of included studies are consistent: smoking is associated with worse patient-reported outcomes during the first year after THA and TKA. The association between smoking and elevated postoperative opioid consumption after these surgeries is an interesting finding. Nicotine in tobacco modulates pain perception and the body's natural neuroendocrine opioid system: it partly counteracts the analgesic effects of opioid medications and increases pain sensation, leading to greater opioid consumption and even dependence (73).
The strengths of this meta-analysis include the extensive literature searching, inclusion of a large amount of updated literature, and comprehensive investigation of the association between smoking and various postoperative complications and clinical outcomes. Moreover, we conducted subgroup analyses, meta-regression, sensitivity analyses, and GRADE evaluation of the evidence. On the other hand, our study also has several limitations. First, as the definitions of smoking status in most included studies were unclear or ambiguous, we could not evaluate possible differences in outcomes between current or previous smokers, between patients with long or short smoking histories, or between patients who smoke fewer or more cigarettes per day. Second, due to the lack of available data, we were unable to assess whether smoking shows a dose-response association with complications. Third, the unexplained heterogeneity and the generally low level of quality of evidence for outcomes made the conclusions less robust.
Despite these limitations, this systematic review and meta-analysis provide an up-to-date overview of the impact of smoking on clinical outcomes after TKA or THA. We find that smoking is associated with increased risks of numerous complications, inpatient mortality, persistent opioid consumption, and worse 1-year patient-reported outcomes. These findings suggest clinicians to make every effort to persuade THA/TKA patients to quit smoking before surgery, and pre-surgical protocols and perioperative managements should give special consideration to smoking patients undergoing THA or TKA.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
XZ and C Yue conceived and designed the study, and wrote the paper. GC, MM, YT, HL and YL completed the searching, and data extraction and analysis. All authors contributed to the article and approved the submitted version.
This work is supported in China by National Natural Science Foundation of China (82004396); Project of science and technology of the Henan province (212102311089/222102310368); Heluo Youth Talent Promotion Project (2022HLTJ15).
We thank A. Chapin Rodríguez PhD for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.970537/full#supplementary-material.
1. Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol. (2017) 14(3):156–70. doi: 10.1038/nrcardio.2016.179
2. Kim SY, Sim S, Choi HG. Active, passive, and electronic cigarette smoking is associated with asthma in adolescents. Sci Rep. 2017 7(1):17789. doi: 10.1038/s41598-017-17958-y
3. Samet JM. Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin. (2013) 23(2):103–12. doi: 10.1016/j.thorsurg.2013.01.009
4. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. (2004) 45(Suppl 2):S3–9. doi: 10.1016/j.lungcan.2004.07.998
5. Shields M, Wilkins K. Smoking, smoking cessation and heart disease risk: a 16-year follow-up study. Health Rep. (2013) 24(2):12–22.24257906
6. Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. (2012) 380(9842):668–79. doi: 10.1016/S0140-6736(12)61085-X
7. Grønkjær M, Eliasen M, Skov-Ettrup LS, Tolstrup JS, Christiansen AH, Mikkelsen SS, et al. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg. (2014) 259(1):52–71. doi: 10.1097/SLA.0b013e3182911913
8. Tønnesen H, Nielsen PR, Lauritzen JB, Møller AM. Smoking and alcohol intervention before surgery: evidence for best practice. Br J Anaesth. (2009) 102(3):297–306. doi: 10.1093/bja/aen401
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. (2007) 89(4):780–5. doi: 10.2106/00004623-200704000-00012
10. Duchman KR, Gao Y, Pugely AJ, Martin CT, Noiseux NO, Callaghan JJ. The effect of smoking on short-term complications following total hip and knee arthroplasty. J Bone Joint Surg Am. (2015) 97(13):1049–58. doi: 10.2106/JBJS.N.01016
11. Burn E, Edwards CJ, Murray DW, Silman A, Cooper C, Arden NK, et al. The impact of BMI and smoking on risk of revision following knee and hip replacement surgery: evidence from routinely collected data. Osteoarth Cart. (2019) 27(9):1294–300. doi: 10.1016/j.joca.2019.05.012
12. Hesseling B, Mathijssen NMC, van Steenbergen LN, Melles M, Vehmeijer SBW, Porsius JT. Fast starters, slow starters, and late dippers: trajectories of patient-reported outcomes after total hip arthroplasty: results from a Dutch nationwide database. J Bone Joint Surg Am. (2019) 101(24):2175–86. doi: 10.2106/JBJS.19.00234
13. Kim SC, Choudhry N, Franklin JM, Bykov K, Eikermann M, Lii J, et al. Patterns and predictors of persistent opioid use following hip or knee arthroplasty. Osteoarthritis Cartilage. (2017) 25(9):1399–406. doi: 10.1016/j.joca.2017.04.002
14. Møller AM, Pedersen T, Villebro N, Munksgaard A. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. (2003) 85(2):178–81. doi: 10.1302/0301-620X.85B2.13717
15. Matharu GS, Mouchti S, Twigg S, Delmestri A, Murray DW, Judge A, et al. The effect of smoking on outcomes following primary total hip and knee arthroplasty: a population-based cohort study of 117,024 patients. Acta Orthop. (2019) 90(6):559–67. doi: 10.1080/17453674.2019.1649510
16. Baier C, Adelmund S, Schwab F, Lassahn C, Chaberny IF, Gossé F, et al. Incidence and risk factors of surgical site infection after total knee arthroplasty: results of a retrospective cohort study. Am J Infect Control. (2019) 47(10):1270–2. doi: 10.1016/j.ajic.2019.04.010
17. Crowe B, Payne A, Evangelista PJ, Stachel A, Phillips MS, Slover JD, et al. Risk factors for infection following total knee arthroplasty: a series of 3836 cases from one institution. J Arthropl. (2015) 30(12):2275–8. doi: 10.1016/j.arth.2015.06.058
18. Wu C, Qu X, Mao Y, Li H, Liu F, Zhu Z. Developmental dysplasia of the hip, age, BMI, place of residence and tobacco abuse increase the odds of aseptic loosening in Chinese patients. PLoS One. (2014) 9(1):e85562. doi: 10.1371/journal.pone.0085562
19. Debbi EM, Rajaee SS, Spitzer AI, Paiement GD. Smoking and total hip arthroplasty: increased inpatient complications, costs, and length of stay. J Arthropl. (2019) 34(8):1736–9. doi: 10.1016/j.arth.2019.03.059
20. Sahota S, Lovecchio F, Harold RE, Beal MD, Manning DW. The effect of smoking on thirty-day postoperative complications after total joint arthroplasty: a propensity score-matched analysis. J Arthropl. (2018) 33(1):30–5. doi: 10.1016/j.arth.2017.07.037
21. Hassan B, Sahlström A, Dessau R. Risk factors for renal dysfunction after total knee joint replacement. Acta Orthop Belg. (2015) 81(4):647–53.26790786
22. Hassan BK, Sahlström A, Dessau RB. Risk factors for renal dysfunction after total hip joint replacement; a retrospective cohort study. J Orthop Surg Res. (2015) 10:158. doi: 10.1186/s13018-015-0299-0
23. Malik MH, Gray J, Kay PR. Early aseptic loosening of cemented total hip arthroplasty: the influence of non-steroidal anti-inflammatory drugs and smoking. Int Orthop. (2004) 28(4):211–3. doi: 10.1007/s00264-004-0556-z
24. Suzuki G, Saito S, Ishii T, Motojima S, Tokuhashi Y, Ryu J. Previous fracture surgery is a major risk factor of infection after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. (2011) 19(12):2040–4. doi: 10.1007/s00167-011-1525-x
25. Singh JA. Smoking and outcomes after knee and hip arthroplasty: a systematic review. J Rheumatol. (2011) 38(9):1824–34. doi: 10.3899/jrheum.101221
26. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. Jama. (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
27. Mueller M, D'Addario M, Egger M, Cevallos M, Dekkers O, Mugglin C, et al. Methods to systematically review and meta-analyse observational studies: a systematic scoping review of recommendations. BMC Med Res Methodol. (2018) 18(1):44. doi: 10.1186/s12874-018-0495-9
28. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses: Ottawa Hospital Research Institute; 2019. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
29. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE Guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026
30. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE Guidelines: a new series of articles in the journal of clinical epidemiology. J Clin Epidemiol. (2011) 64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011
31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
32. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019): Cochrane; 2019. Available at: www.training.cochrane.org/handbook
33. Vial IA A, Babar T, Boutros I. Incidence and risk factors of acute kidney injury after total joint arthroplasty; a retrospective cohort study. J Clin Orthop Trauma. (2020) 11(Suppl 2):S255–s9. doi: 10.1016/j.jcot.2019.10.012
34. Anderson MB, Curtin K, Wong J, Pelt CE, Peters CL, Gililland JM. Familial clustering identified in periprosthetic joint infection following primary total joint arthroplasty: a population-based cohort study. J Bone Joint Surg Am. (2017) 99(11):905–13. doi: 10.2106/JBJS.16.00514
35. Bedard NA, DeMik DE, Dowdle SB, Owens JM, Liu SS, Callaghan JJ. Preoperative opioid use and its association with early revision of total knee arthroplasty. J Arthropl. (2018) 33(11):3520–3. doi: 10.1016/j.arth.2018.06.005
36. Bohl DD, Saltzman BM, Sershon RA, Darrith B, Okroj KT, Della Valle CJ. Incidence, risk factors, and clinical implications of pneumonia following total hip and knee arthroplasty. J Arthropl. (2017) 32(6):1991–5. doi: 10.1016/j.arth.2017.01.004
37. Bohl DD, Sershon RA, Fillingham YA, Della Valle CJ. Incidence, risk factors, and sources of sepsis following total joint arthroplasty. J Arthropl. (2016) 31(12):2875–9. doi: 10.1016/j.arth.2016.05.031
38. Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL, Pelt CE. Is hemoglobin A1c or perioperative hyperglycemia predictive of periprosthetic joint infection or death following primary total joint arthroplasty? J Arthropl. (2015) 30(7):1197–202. doi: 10.1016/j.arth.2015.01.040
39. Debreuve-Theresette A, Diallo S, Siboni R, Ohl X, Dehoux E, Bajolet O. Infections in total hip and total knee arthroplasty: development of a score to assess endogenous risk of surgical site infections. Surg Infect (Larchmt). (2015) 16(6):794–8. doi: 10.1089/sur.2014.155
40. Etcheson JI, Gwam CU, George NE, Walia N, Jerjian C, Han GR, et al. Opiate pain medication consumption in cigarette smokers following total hip arthroplasty. Joints. (2018) 6(3):157–60. doi: 10.1055/s-0038-1673405
41. Gonzalez AI, Luime JJ, Uçkay I, Hannouche D, Hoffmeyer P, Lübbeke A. Is there an association between smoking Status and prosthetic joint infection after primary total joint arthroplasty? J Arthropl. (2018) 33(7):2218–24. doi: 10.1016/j.arth.2018.02.069
42. Grammatico-Guillon L, Baron S, Rosset P, Gaborit C, Bernard L, Rusch E, et al. Surgical site infection after primary hip and knee arthroplasty: a cohort study using a hospital database. Infect Control Hosp Epidemiol. (2015) 36(10):1198–207. doi: 10.1017/ice.2015.148
43. Halawi MJ, Allen DA, Baron S, Savoy L, Williams VJ, Cote MP. Tobacco smoking independently predicts lower patient-reported outcomes: new insights on a forgotten epidemic. J Arthropl. (2019) 34(7s):S144–s7. doi: 10.1016/j.arth.2018.10.036
44. Jørgensen CC, Petersen M, Kehlet H, Aasvang EK. Analgesic consumption trajectories in 8975 patients 1 year after fast-track total hip or knee arthroplasty. Eur J Pain. (2018) 22(8):1428–38. doi: 10.1002/ejp.1232
45. Kapadia BH, Issa K, Pivec R, Bonutti PM, Mont MA. Tobacco use may be associated with increased revision and complication rates following total hip arthroplasty. J Arthropl. (2014) 29(4):777–80. doi: 10.1016/j.arth.2013.08.023
46. Kapadia BH, Johnson AJ, Naziri Q, Mont MA, Delanois RE, Bonutti PM. Increased revision rates after total knee arthroplasty in patients who smoke. J Arthropl. (2012) 27(9):1690–5. doi: 10.1016/j.arth.2012.03.057
47. Khan LA, Cowie JG, Ballantyne JA, Brenkel IJ. The complication rate and medium-term functional outcome after total hip replacement in smokers. Hip Int. (2009) 19(1):47–51. doi: 10.1177/112070000901900109
48. Lübbeke A, Rothman KJ, Garavaglia G, Barea C, Christofilopoulos P, Stern R, et al. Strong association between smoking and the risk of revision in a cohort study of patients with metal-on-metal total hip arthroplasty. J Orthop Res. (2014) 32(6):762–8. doi: 10.1002/jor.22603
49. Nwachukwu BU, Gurary EB, Lerner V, Collins JE, Thornhill TS, Losina E, et al. Effect of smoking and soft tissue release on risk of revision after total knee arthroplasty: a case- control study. BMC Musculoskelet Disord. (2015) 16:245. doi: 10.1186/s12891-015-0694-z
50. Peters RM, van Steenbergen LN, Stewart RE, Stevens M, Rijk PC, Bulstra SK, et al. Patient characteristics influence revision rate of total hip arthroplasty: american society of anesthesiologists score and body mass Index were the strongest predictors for short-term revision after primary total hip arthroplasty. J Arthropl. (2020) 35(1):188–92. doi: 10.1016/j.arth.2019.08.024
51. Rajaee SS, Debbi EM. Increased Prevalence, Complications, and Costs of Smokers Undergoing Total Knee Arthroplasty. 2020.
52. Sadr Azodi O, Adami J, Lindström D, Eriksson KO, Wladis A, Bellocco R. High body mass index is associated with increased risk of implant dislocation following primary total hip replacement: 2,106 patients followed for up to 8 years. Acta Orthop. (2008) 79(1):141–7. doi: 10.1080/17453670710014897
53. Sadr Azodi O, Bellocco R, Eriksson K, Adami J. The impact of tobacco use and body mass index on the length of stay in hospital and the risk of post-operative complications among patients undergoing total hip replacement. J Bone Joint Surg Br. (2006) 88(10):1316–20. doi: 10.1302/0301-620X.88B10.17957
54. Sikora-Klak J, Zarling B, Bergum C, Flynn JC, Markel DC. The effect of comorbidities on discharge disposition and readmission for total joint arthroplasty patients. J Arthropl. (2017) 32(5):1414–7. doi: 10.1016/j.arth.2016.11.035
55. Singh JA, Houston TK, Ponce BA, Maddox G, Bishop MJ, Richman J, et al. Smoking as a risk factor for short-term outcomes following primary total hip and total knee replacement in veterans. Arthritis Care Res (Hoboken. (2011) 63(10):1365–74. doi: 10.1002/acr.20555
56. Singh JA, Schleck C, Harmsen WS, Jacob AK, Warner DO, Lewallen DG. Current tobacco use is associated with higher rates of implant revision and deep infection after total hip or knee arthroplasty: a prospective cohort study. BMC Med. (2015) 13:283. doi: 10.1186/s12916-015-0523-0
57. Tischler EH, Matsen Ko L, Chen AF, Maltenfort MG, Schroeder J, Austin MS. Smoking increases the rate of reoperation for infection within 90 days after primary total joint arthroplasty. J Bone Joint Surg Am. (2017) 99(4):295–304. doi: 10.2106/JBJS.16.00311
58. Xu C, Guo H, Wang Q, Qu P, Bell K, Chen J. Interaction of obesity with smoking and inflammatory arthropathies increases the risk of periprosthetic joint infection: a propensity score matched study in a Chinese han population. J Hosp Infect. (2019) 101(2):222–8. doi: 10.1016/j.jhin.2018.06.017
59. Sørensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg. (2012) 255(6):1069–79. doi: 10.1097/SLA.0b013e31824f632d
60. Sørensen LT, Jørgensen S, Petersen LJ, Hemmingsen U, Bülow J, Loft S, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. (2009) 152(2):224–30. doi: 10.1016/j.jss.2008.02.066
61. Ehnert S, Aspera-Werz RH, Ihle C, Trost M, Zirn B, Flesch I, et al. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. J Clin Med. (2019) 8(3). doi: 10.3390/jcm8030406
62. Barbosa AP, Lourenço JD, Junqueira JJM, Larissa Emidio de França S, Martins JS, Oliveira Junior MC, et al. The deleterious effects of smoking in bone mineralization and fibrillar matrix composition. Life Sci. (2020) 241:117132. doi: 10.1016/j.lfs.2019.117132
63. Kim BS, Kim SJ, Kim HJ, Lee SJ, Park YJ, Lee J, et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. (2012) 90(3-4):109–15. doi: 10.1016/j.lfs.2011.10.019
64. Cherian JJ, Jauregui JJ, Banerjee S, Pierce T, Mont MA. What host factors affect aseptic loosening after THA and TKA? Clin Orthop Relat Res. (2015) 473(8):2700–9. doi: 10.1007/s11999-015-4220-2
65. Teng S, Yi C, Krettek C, Jagodzinski M. Smoking and risk of prosthesis-related complications after total hip arthroplasty: a meta-analysis of cohort studies. PLoS One. (2015) 10(4):e0125294. doi: 10.1371/journal.pone.0125294
66. Postler A, Lützner C, Beyer F, Tille E, Lützner J. Analysis of total knee arthroplasty revision causes. BMC Musculoskelet Disord. (2018) 19(1):55. doi: 10.1186/s12891-018-1977-y
67. Kahlenberg CA, Swarup I, Krell EC, Heinz N, Figgie MP. Causes of revision in young patients undergoing total hip arthroplasty. J Arthroplasty. (2019) 34(7):1435–40. doi: 10.1016/j.arth.2019.03.014
68. Piatti G, Gazzola T, Allegra L. Bacterial adherence in smokers and non-smokers. Pharmacol Res. (1997) 36(6):481–4. doi: 10.1006/phrs.1997.0255
69. Baskaran V, Murray RL, Hunter A, Lim WS, McKeever TM. Effect of tobacco smoking on the risk of developing community acquired pneumonia: a systematic review and meta-analysis. PLoS One. (2019) 14(7):e0220204. doi: 10.1371/journal.pone.0220204
70. Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. (2004) 164(20):2206–16. doi: 10.1001/archinte.164.20.2206
71. Banks E, Joshy G, Korda RJ, Stavreski B, Soga K, Egger S, et al. Tobacco smoking and risk of 36 cardiovascular disease subtypes: fatal and non-fatal outcomes in a large prospective Australian study. BMC Med. (2019) 17(1):128. doi: 10.1186/s12916-019-1351-4
72. Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. (2004) 43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047
Keywords: smoking, clinical outcomes, THA, TKA, meta-analysis
Citation: Yue C, Cui G, Ma M, Tang Y, Li H, Liu Y and Zhang X (2022) Associations between smoking and clinical outcomes after total hip and knee arthroplasty: A systematic review and meta-analysis. Front. Surg. 9:970537. doi: 10.3389/fsurg.2022.970537
Received: 16 June 2022; Accepted: 11 October 2022;
Published: 2 November 2022.
Edited by:
Miguel A Ortega, University of Alcalá, SpainReviewed by:
Jian-ke Pan, Guangdong Provincial Hospital of Chinese Medicine, China© 2022 Yue, Cui, Ma, Tang, Li, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Zhang MTU4NTYwNDAxOTRAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.