
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 30 August 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.969995
This article is part of the Research Topic Clinical and Basic Research on Endovascular Repair and Branch Reconstruction for the Treatment of Lesions involving the Aortic Arch View all 12 articles
 Xichun Qin1,2,†
Xichun Qin1,2,† Yaxuan Gao1,2,†
Yaxuan Gao1,2,† Yi Jiang1,3
Yi Jiang1,3 Feng Zhu1,2
Feng Zhu1,2 Wei Xie1,2
Wei Xie1,2 Xinlong Tang1,2
Xinlong Tang1,2 Yunxing Xue1,2
Yunxing Xue1,2 Dongjin Wang1,2*
Dongjin Wang1,2* Hailong Cao1,2*
Hailong Cao1,2*
Background: Acute Stanford-A aortic dissection (AAAD) is a devastating cardiovascular condition with high mortality, therefore identifying risk prognosis factors is vital for the risk stratification of patients with AAAD. Here, we investigated peripheral blood eosinophil (EOS) counts in patients with AAAD and their possible biological implications.
Methods: We performed a single center retrospective cohort study. From 2011 to 2021, a total of 1,190 patients underwent AAAD surgery. Patients were categorized first by death and then admission EOS counts (0.00 × 109/L or >0.00 × 109/L). Demographics, laboratory data, and outcomes were analyzed using standard statistical analyses. Ascending aorta specimens were used for western blotting and histological assessments.
Results: Death group patients had lower EOS counts than the non-death group (P = 0.008). When patients were stratified using mean blood EOS counts: 681 patients had low (0.00 × 109/L) and 499 had high (>0.00 × 109/L) counts. Patients with low EOS counts at admission were more likely to have a higher mortality risk (P = 0.017) and longer treatment in the intensive care unit (ICU) days (P = 0.033) than patients with normal EOS counts. Also, the five blood coagulation items between both groups showed significantly different (P < 0.001). Hematoxylin & eosin-stained cross-sections of the ascending aorta false lumen showed that EOSs were readily observed in thrombi in the false lumen of the aorta.
Conclusions: Peripheral blood EOS counts may be involved in thrombosis and could be an effective and efficient indicator for the diagnosis, evaluation, and prognosis monitoring of patients with AAAD.
Acute aortic dissection (AAD) is a serious life-threatening disease, with a gradually increasing incidence rate in recent years (1). Depending on the rupture site, AAD is classified as Stanford-A when it involves the thoracic segment of the ascending aorta and/or the aortic arch, and Stanford-B when it involves the descending aorta and/or the thoracoabdominal aorta (2). Acute Stanford-A aortic dissection (AAAD) is the most common type, accounting for 75% of all cases. If treatment is not timely, many patients with AAAD will die suddenly due to aortic wall rupture. The mortality rate is as high as 90% (3). With the establishment of regional referral centers, more patients with AAAD can now receive timely surgical intervention, but all-cause mortality remains up to 10%–30% (4, 5).
Patients with AAAD usually undergo vital sign monitoring and blood tests upon admission in the emergency department, therefore, more clinicians are now heeding circulating biomarkers, such as routine bloods, in an attempt to analyze or predict the risk of death in these patients (6, 7). Laboratory test results are easily generated and do not impose additional risk and financial burden on patients. Many clinical and basic research studies have reported that inflammation has important roles in cardiovascular disease, with several inflammatory factors predicting cardiovascular disease (CVD) progression and prognosis (8, 9). These findings are also applicable to AAAD (10). White blood cells (WBC) are generally elevated in patients with AAAD and associated with a more severe prognosis and higher mortality (11). Eosinophils (EOS) are a type of WBC, and are implicated in several CVDs. For example, high blood EOS counts are positively associated with major cardiovascular risk factors and CVD prevalence (12), low EOS counts are negatively associated with peripheral arterial disease (13), and higher blood EOS counts are recorded in patients with abdominal aortic aneurysm when compared with normal controls, and can act as independent risk factors for the condition (14). However, we found that in the majority of patients with AAAD, EOS counts were <0.02 × 109/L, and even dropped below the lower limit of detection (denoted as 0.00 × 109/L). Compared to the change in the WBC counts, the EOS counts which were “0.00” seemed to be more striking.
While research on the pathogenesis of AAAD has progressed, EOS alterations during the condition and their possible biological ramifications are rarely considered. In this study, we investigated this phenomenon, characterized EOS changes of AAAD patients, and examined their roles.
Materials and extension methods are described in Supplementary Materials.
The principal study was a single-center, retrospective study. That study was approved by the Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School.
From January 2011 to December 2021, 1,190 AAAD surgeries were performed in Nanjing Drum Tower Hospital. An AAAD diagnosis was confirmed by aorta angiography using multi-detector computed tomographic scanning. Stanford type A (DeBakey I and II) dissection involved the ascending aorta and/or the aortic arch according to previously published criteria. All patients underwent serological testing in the emergency department preoperatively, and the results were considered as admission laboratory data. Patients were excluded if they died preoperatively or had no laboratory data (routine blood examinations including EOS counts) or had taken medications such as aspirin, antibiotics, and glucocorticoids affecting blood counts. The study was reviewed by the hospital Ethics Committee. Laboratory analytes measured at admission included routine blood examinations, biochemical analysis, D-dimer, cTNT, and five blood coagulation items.
Patient demographic data, including age, gender, body mass index (BMI), disease onset, and previous medical history, including hypertension, diabetes, atrial fibrillation, Marfan syndrome, chronic obstructive pulmonary disease, coronary artery bypass grafting history, and smoking and drinking status. Other clinical characteristics included symptoms and signs at admission. The rationale for surgery and the surgical strategy were both determined by attending surgeons.
The primary study outcome was inpatient mortality which was defined as all-cause death. Secondary outcomes included the time in the intensive care unit (ICU), post- tracheostomy, post-stroke, and post-intracranial hemorrhage after surgeries during the index hospitalization.
Continuous variables were expressed as the mean ± standard deviation and compared using t tests if they were normally distributed. Skewed continuous variables were analyzed as the median and inter quartile range (IQR), and comparisons were made using the Mann-Whitney U test, with significance accepted at P < 0.05. Binary and categorical variables were expressed as counts and percentages, and compared using the χ2 or Fisher’s exact test. A two-sided α < 0.05 was considered statistically significant. Statistical analyses were performed in SPSS version 26.0 (SPSS, Inc., Chicago, IL, United States).
In total, 1,190 patients with AAAD were investigated. We excluded patients with no laboratory data and who had taken medications affecting blood counts. Finally, 1,180 patients with AAAD were analyzed, of whom 1,009 survived and 171 died. Next, we divided AAAD patients into death and non-death groups. Demographics were similar between groups, except for age (P < 0.001) and hypertension history (P = 0.005). Moreover, patients in the death group were more likely to have hypotension (22 [12.2%] vs. 66 [6.5%], P < 0.01) and preoperative limb ischemia before surgery (44 [24.4%] vs. 135 [13.4%], P = 0.001) (Table 1).
In terms of laboratory data, normal EOS counts were between 0.02–0.52 × 109/L, however, over 80% of EOS counts below 0.02 × 109/L, and 57.7% of AAAD patients dropped below the lower limit of detection (0.00 × 109/L). Moreover, when EOS counts were considered as a continuous variable, we identified significant differences between death and non-death patients (P = 0.008), and EOS percentages lower in the death group (P = 0.002). When EOS counts were considered a categorical variable, we identified differences between groups (P = 0.038/0.017). Higher WBC and neutrophil counts were identified in the death group. In contrast, lymphocyte and monocyte counts in both groups were not significantly different (Table 2). These data suggested that reduced EOS counts were almost a universal feature in patients with AAAD.
We selected tissue from patients with AAAD (EOS count = 0.00 × 109/L) and normal human ascending aorta tissue (EOS count = 0.02–0.52 × 109/L), and investigated Siglec-8 expression (EOS marker protein). Interestingly, in ascending aortic tissue from patients with AAAD, we observed no abnormal Siglec-8 expression (Supplementary Figures S1A,B). Additionally, we observed no EOS infiltration or enrichment in AAAD patient’s aorta tissue using H&E staining (Supplementary Figure S1C).
To further investigate the role of EOS counts in AAAD, we divided patients into two groups based on circulating EOS counts at admission; a lower EOS group (0.00 × 109/L) and a higher EOS group (>0.00 × 109/L). No significant clinical differences were identified between groups. Similarly, no differences in age (P = 0.628), BMI (P = 0.226), and the median interval from onset to hospitalization (P = 0.196) were identified.
The proportion of patients in the lower EOS group, who developed pericardial effusion (65.8% vs. 72.3%, P = 0.017) was significantly lower than the higher EOS group. Moreover, patients in the lower EOS group who developed cerebral ischemia attack (10.0% vs. 5.8%, P = 0.010) were higher than the higher EOS group (Table 3).
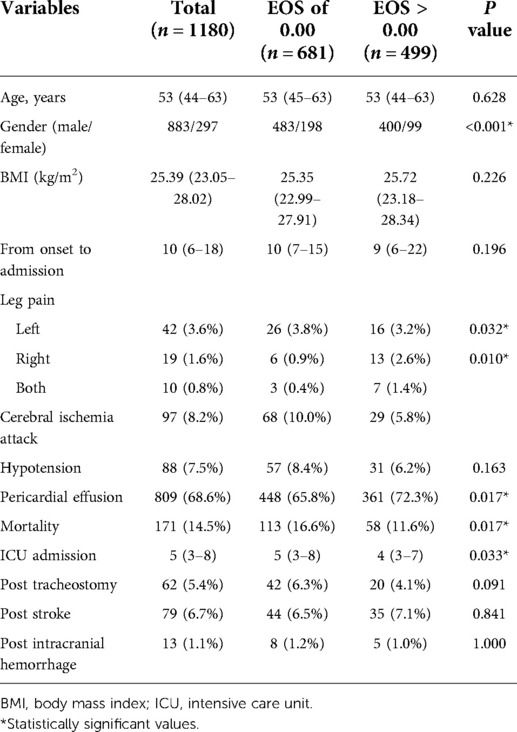
Table 3. The effects of different eosinophil levels on the clinical characteristics, primary and secondary outcomes of patients with AAD.
By examining primary and secondary outcomes in both EOS groups, patients with an admission EOS count of 0.00 × 109/L had higher mortality rates (113 [16.6%] vs. 58 [11.6%], P = 0.017) and increased ICU treatment days [5.0 days, (IQR 3.0–8.0) vs. 4.0 days, (IQR 3.0–7.0), P = 0.033]. No significant differences in tracheotomy (42 [6.3%] vs. 20 [4.1%], P = 0.091) and intracranial hemorrhage rates (8 [1.2%] vs. 5 [1.0%], P = 1.000) were identified (Table 3).
The results of univariate regression analysis of predictors of mortality were shown in Table 4. Admission EOS count was associated with mortality both as a continuous variable (OR = 0.004, 95% CI 0.000–0.241, P = 0.006) and as a cutoff value of >0.00 × 109/L (OR = 0.661, 95% CI 0.470–0.929, P = 0.017). Other risk factors associated with all-cause mortality included age, hypertension and WBC count. Add risk factors which P < 0.05 into multivariable logistic regression models. Multivariable-adjusted ORs for mortality according to per 1.0 × 109 cells/L increase, the cutoff value of 0.00 × 109/L, were presented in Table 5. Admission EOS count was an independent predictor of death when considered as a continuous variable (OR = 0.010, 95% CI 0.000–0.650, P = 0.031) or as a categorical variable (OR = 1.46, 95% CI 1.033–2.070, P = 0.032) using the cutoff value of 0.00 × 109/L after adjustment for age, hypertension and WBC count.
Aortic false lumen is a typical feature of AAAD (15). We noticed changes in coagulation function in AAAD patients, and some studies suggested that EOS might be involved in thrombosis (16, 17). Comparisons between EOS groups were significant for the five blood coagulation items. Patients with 0.00 × 109/L EOS counts had higher PT [(12.8 s, IQR 11.8–14.35 s) vs. (12.4 s, IQR 11.5–13.9 s), P < 0.001], APTT [(30.4 s, IQR 26.9–39.3 s) vs. (29.3 s, IQR 26.8–34.55 s), P = 0.004], INR [(1.12 s, IQR 1.03–1.25 s) vs. (1.09 s, IQR 1.00–1.22 s), P < 0.001], TT [(19.3 s, IQR 17.5–22.1 s) vs. (18.6 s, IQR 16.9–20.9 s), P < 0.001], and lower fibrinogen levels [(2.1 g/L, IQR 1.6–2.7 g/L) vs. (2.4 g/L, IQR 1.7–3.3 g/L), P < 0.001] (Table 6). Furthermore, EOSs were observed in thrombi in the false lumen of the aorta (Supplementary Figure S2). Therefore, decreased peripheral blood EOS counts may be due to the involvement of EOSs in aortic false lumen thrombosis.
Our study found that EOS counts in the peripheral blood of patients with AAAD were significantly lower; up to 81.1% patients EOS counts below 0.02 × 109/L and 57.7% of patients had undetectable EOS counts (0.00 × 109/L), concomitant with higher mortality and longer ICU treatment days. When we compared Siglec-8 expression in the control group with AAAD patients (preoperative EOS counts = “0.00”), and also histological examinations, we observed no evidence that EOSs accumulated in the aortic interstitial spaces of patients with AAAD. From statistical analyses of coagulation functions (APTT, TT, INR, PT, and Fibrinogen), D-dimer levels, and platelet counts in patients with AAAD, lower EOS counts tended to represent worse coagulation functions, higher D-dimer levels, and lower platelet counts. Furthermore, infiltrating EOSs were observed from the thrombus in the false lumen. Therefore, EOSs may be recruited from the peripheral blood into the false lumen and be associated with thrombus formation.
Aortic dissection is characterized by damage and remodeling of the aortic media leading to secondary thrombosis and inflammation, with systemic signs of inflammatory activation and local inflammatory cell infiltration (10, 18). Inflammatory cells, such as neutrophils (11), lymphocytes (19), and macrophages (20) have been investigated during AAAD pathogenesis, but EOS studies are lacking. EOSs are not only inflammatory effector cells, but they exert several immune regulatory functions. EOS counts in a large CVD patient cohort and experimental rat data on aortic aneurysms support the conclusion that EOS exert major protective roles in CVD (12). EOS deficiency increased abdominal aortic aneurysm growth, inflammatory cell lesion levels, angiogenesis, elastic rupture of the arterial wall, lesion cell apoptosis, SMC loss, and M1 macrophage marker expression (14). False lumen formation and subsequent thrombosis are important features in AAAD acute phases. Reports of thrombotic events in patients with EOS-related disease confirmed EOS involvement in thrombosis, i.e., promoting thrombosis through eosinophilic extracellular traps, thereby enhancing platelet activation, leading to atherosclerosis and stable thrombosis (16). Additionally, EOS may be involved in coronary thrombosis in acute myocardial infarction via inflammatory mechanisms (21). Activated PLT is associated with EOS pathology in several diseases, including asthma and hyper-eosinophilic syndrome (22). PLT is activated by EOS particles, major basic protein, and EOS peroxidase (23). Our data also suggested that EOS may be involved in false lumen thrombus formation in patients with AAAD.
Whether EOSs promote vascular injury, induce pro-inflammatory effects, or are simply recruited to tissue injury sites remain unclear. The main goal of AAAD surgery is to prevent fatal complications, and the more severe the preoperative vascular injury, the more likely it is to cause rupture, cardiac tamponade, and poor perfusion. Additionally, damaged vessels also make graft anastomosis more difficult during surgical treatment, resulting in postoperative complications such as bleeding and infection. Decreased EOS percentages may indicate severe vascular injury and more extensive thrombosis, therefore, EOS counts may be useful in assessing the extent of preoperative aortic injury in patients with AAAD.
Our research had some limitations. Most AAAD patients were first diagnosed in local hospitals, therefore it was difficult to obtain accurate information on false lumen thrombosis. Similarly, information on the effects of false lumen thrombosis on postoperative in-hospital survival rates are lacking. Partial thrombosis of the false cavity is an important independent predictor of mortality in patients with type B dissection, but it does not affect the long-term survival rate of AAAD survivors after discharge. Blood flow and thrombus may coexist, and most patients had EOS counts below the lower limit of detection, therefore it was difficult to quantify associations between false lumen thrombosis and the degree of EOS reduction through limited specimen. Additionally, in considering intraoperative bleeding, blood transfusion, and postoperative anti-infection treatment, we did not continuously monitor EOS counts during hospitalization. Therefore, when EOSs leave the peripheral blood system, are they deposited in a thrombus or elsewhere, or are they destroyed or degraded? Furthermore, peripheral blood eosinophil counts have a circadian rhythm (peaked during nighttime) (24), the time of drawing blood may affect results. These questions require further investigation.
Peripheral blood EOS counts may be valid indicators for preoperative risk assessment in patients with AAAD. Circulating EOS levels below detection limits may not only indicate thrombosis, but may be significant in predicting AAAD severity and prognosis in patients with AAAD. Therefore, a rapid, simple, and low-cost peripheral blood EOS count test can be used to effectively assess preoperative risk in patients with AAAD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HLC and DJW designed the project. YJ, FZ, WX, XLT, YXX participated in the collection of data. XCQ and YXG performed the experiments, analyzed data and wrote the manuscript. All authors participated in writing and provided feedback. All authors contributed to the article and approved the submitted version.
This study was supported by funds from Nanjing Medical Science and Technology Development Key Project (ZKX20026), the National Natural Science Foundation of China (81970401).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.969995/full#supplementary-material.
1. Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. (2021) 18(5):331–48. doi: 10.1038/s41569-020-00472-6
2. Malaisrie SC, Szeto WY, Halas M, Girardi LN, Coselli JS, Sundt TM 3rd, et al., The American association for thoracic surgery expert consensus document: surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg. (2021) 162(3):735–58.e2. doi: 10.1016/j.jtcvs.2021.04.053
3. Cifani N, Proietta M, Tritapepe L, Di Gioia C, Ferri L, Taurino M, et al. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: a review. Ann Med. (2015) 47(6):441–6. doi: 10.3109/07853890.2015.1073346
4. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. (2018) 137(17):1846–60. doi: 10.1161/CIRCULATIONAHA.117.031264
5. Clough RE, Nienaber CA. Management of acute aortic syndrome. Nat Rev Cardiol. (2015) 12(2):103–14. doi: 10.1038/nrcardio.2014.203
6. Morello F, Piler P, Novak M, Kruzliak P. Biomarkers for diagnosis and prognostic stratification of aortic dissection: challenges and perspectives. Biomark Med. (2014) 8(7):931–41. doi: 10.2217/bmm.14.38
7. Zeng T, Shi L, Ji Q, Shi Y, Huang Y, Liu Y, et al. Cytokines in aortic dissection. Clin Chim Acta. (2018) 486:177–82. doi: 10.1016/j.cca.2018.08.005
8. Haybar H, Shokuhian M, Bagheri M, Davari N, Saki N. Involvement of circulating inflammatory factors in prognosis and risk of cardiovascular disease. J Mol Cell Cardiol. (2019) 132:110–9. doi: 10.1016/j.yjmcc.2019.05.010
9. Ajala ON, Everett BM. Targeting inflammation to reduce residual cardiovascular risk. Curr Atheroscler Rep. (2020) 22(11):66. doi: 10.1007/s11883-020-00883-3
10. Luo F, Zhou XL, Li JJ, Hui RT. Inflammatory response is associated with aortic dissection. Ageing Res Rev. (2009) 8(1):31–5. doi: 10.1016/j.arr.2008.08.001
11. Wen D, Wu HY, Jiang XJ, Zhang HM, Zhou XL, Li JJ, et al. Role of plasma C-reactive protein and white blood cell count in predicting in-hospital clinical events of acute type A aortic dissection. Chin Med J. (2011) 124(17):2678–82. doi: 10.3760/cma.j.issn.0366-6999.2011.17.020
12. Verdoia M, Schaffer A, Cassetti E, Di Giovine G, Marino P, Suryapranata H, et al. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J Thromb Thrombolysis. (2015) 39(4):459–66. doi: 10.1007/s11239-014-1120-3
13. Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart. (2016) 3(2):e000477. doi: 10.1136/openhrt-2016-000477
14. Liu CL, Liu X, Zhang Y, Liu J, Yang C, Luo S, et al. Eosinophils protect mice from angiotensin-II perfusion-induced abdominal aortic aneurysm. Circ Res. (2021) 128(2):188–202. doi: 10.1161/CIRCRESAHA.120.318182
15. Yuan X, Mitsis A, Semple T, Castro Verdes M, Cambronero-Cortinas E, Tang Y, et al. False lumen intervention to promote remodelling and thrombosis-the FLIRT concept in aortic dissection. Catheter Cardiovasc Interv. (2018) 92(4):732–40. doi: 10.1002/ccd.27599
16. Marx C, Novotny J, Salbeck D, Zellner KR, Nicolai L, Pekayvaz K, et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. (2019) 134(21):1859–72. doi: 10.1182/blood.2019000518
17. Mansiroglu AK, Sincer I, Cosgun M, Gunes Y. Dating thrombus organization with eosinophil counts in deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. (2021) 9(4):874–80. doi: 10.1016/j.jvsv.2020.10.012
18. Zhang R, Zhou J, Feng J, Zhao Z, Liu J, Li Z, et al. Inducing false lumen thrombosis for retrograde type A aortic dissection. J Thorac Cardiovasc Surg. (2017) 153(1):57–65. doi: 10.1016/j.jtcvs.2016.09.022
19. Erdolu B, As AK. C-Reactive protein and neutrophil to lymphocyte ratio values in predicting inhospital death in patients with Stanford type A acute aortic dissection. Heart Surg Forum. (2020) 23(4):E488–E92. doi: 10.1532/hsf.3055
20. Wang X, Zhang H, Cao L, He Y, Ma A, Guo W. The role of macrophages in aortic dissection. Front Physiol. (2020) 11:54. doi: 10.3389/fphys.2020.00054
21. Jiang P, Wang DZ, Ren YL, Cai JP, Chen BX. Significance of eosinophil accumulation in the thrombus and decrease in peripheral blood in patients with acute coronary syndrome. Coron Artery Dis. (2015) 26(2):101–6. doi: 10.1097/MCA.0000000000000186
22. Ames PR, Aloj G, Gentile F. Eosinophilia and thrombosis in parasitic diseases: an overview. Clin Appl Thromb Hemost. (2011) 17(1):33–8. doi: 10.1177/1076029609348314
23. Ulfman LH, Joosten DP, van Aalst CW, Lammers JW, van de Graaf EA, Koenderman L, et al. Platelets promote eosinophil adhesion of patients with asthma to endothelium under flow conditions. Am J Respir Cell Mol Biol. (2003) 28(4):512–9. doi: 10.1165/rcmb.4806
Keywords: acute Stanford-A aortic dissection, eosinophil, mortality risk, blood coagulation, thrombosis
Citation: Qin X, Gao Y, Jiang Y, Zhu F, Xie W, Tang X, Xue Y, Wang D and Cao H (2022) The role of peripheral blood eosinophil counts in acute Stanford type A aortic dissection patients. Front. Surg. 9:969995. doi: 10.3389/fsurg.2022.969995
Received: 15 June 2022; Accepted: 11 August 2022;
Published: 30 August 2022.
Edited by:
Emiliano Chisci, Nuovo Ospedale San Giovanni di Dio, ItalyReviewed by:
Kexiang Liu, Jilin University, China© 2022 Qin, Gao, Jiang, Zhu, Xie, Tang, Xue, Wang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailong Cao Y2FvaGFpbG9uZ0BuamdseXkuY29t Dongjin Wang d2FuZ2RvbmdqaW5AbmpnbHl5LmNvbQ==
†These authors have contributed equally to this work.
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
Abbreviations EOS, eosinophil; AAD, acute aortic dissection; ICU, intensive care unit; AAAD, acute Stanford-A aortic dissection; WBC, white blood cells; CVD, cardiovascular disease; CT, computed tomographic; BMI, body mass index; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IQR, inter quartile range; SD, standard deviation; SMC, smooth muscle cell; PLT, platelet; HTN, hypertension; DB, diabetes; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; CABG, coronary artery bypass grafting; CTNT, troponin T; PT, prothrombin time; INR, international normalized ratio; PT, prothrombin time; INR, international normalized ratio; TT, thrombin time; APTT, activated partial thromboplastin time
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.