
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 06 January 2023
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.968897
 Guangbin Chen1,2,3,†
Guangbin Chen1,2,3,† Jie Yin1,2,†
Jie Yin1,2,† Qun Chen1,2
Qun Chen1,2 Jishu Wei1,2
Jishu Wei1,2 Kai Zhang1,2
Kai Zhang1,2 Lingdong Meng1,2
Lingdong Meng1,2 Yichao Lu1,2
Yichao Lu1,2 Pengfei Wu1,2
Pengfei Wu1,2 Baobao Cai1,2
Baobao Cai1,2 Zipeng Lu1,2*
Zipeng Lu1,2* Yi Miao1,2,4
Yi Miao1,2,4 Kuirong Jiang1,2*
Kuirong Jiang1,2*
Background: Despite the advancements in surgical techniques, postoperative pancreatic fistula (POPF) remains a potentially life-threatening complication of pancreaticoduodenectomy (PD). Pancreatic duct occlusion (PDO) without anastomosis has also been proposed to alleviate the clinical consequences of POPF in selected patients after PD.
Objectives: To assess the safety and effectiveness of PDO with mechanical closure after PD in patients with an atrophic pancreatic body-tail and a small pancreatic duct.
Methods: We retrospectively identified two female and two male patients from April 2019 to October 2020 through preoperative computed tomography of the abdomen. Among them, three patients underwent PDO with mechanical closure after PD, and one underwent PDO after pylorus-preserving PD. In addition, patients' medical records and medium-and long-term follow-up data were analyzed.
Results: Postoperative histological examination revealed a solid pseudopapillary tumor in two patients, pancreatic ductal adenocarcinoma in one patient, and chronic pancreatitis with pancreatic duct stones in one patient. However, none of the patients developed biochemical or clinically relevant POPF, with no postpancreatectomy hemorrhage, biliary leakage, delayed gastric emptying, intra-abdominal abscess, or chyle leakage. Among the four patients, three developed new-onset diabetes mellitus, and one had impaired glucose tolerance. Furthermore, three patients received pancreatic enzyme supplementation at a dose of 90,000 Ph. Eur. units/d, and one was prescribed a higher dose of 120,000 Ph. Eur. units/d.
Conclusions: PDO with mechanical closure is an alternative approach for patients with an atrophic pancreatic body-tail and a small pancreatic duct after PD. Therefore, further evidence should evaluate the potential benefits of selective PDO in these patients.
Pancreaticoduodenectomy (PD) is one of the most complex and challenging procedures in abdominal surgery. Postoperative pancreatic fistula (POPF) is a major severe complication of PD, with a reported incidence of 10%–15% (1–3). POPF is a lethal complication that can significantly prolong hospital stays and increase healthcare costs and postoperative mortality (4, 5). The design and surgical technique for pancreatic-enteric anastomosis are significant determinants of POPF; however, no preferred re-establishing procedure has been demonstrated to be superior to others (5–7).
Pancreatic duct occlusion (PDO) without pancreatic anastomosis has been explored as an alternative to mitigate pancreatic fistulas following PD. The rationale behind this surgical design is that when a pure pancreatic fistula forms, it is not triggered by biliary and/or enteric juices, thereby reducing the risk of serious pancreatic fistula consequences. Occluding the pancreatic duct has been reportedly associated with a high rate of biochemical leakage, which is self-limiting, with no deviation from the clinical pathway (8, 9). Postoperative pancreatic exocrine and endocrine functions are similarly preserved in patients who have undergone PDO compared with those who have undergone pancreatic anastomosis (10). During pancreatic anastomosis in patients with a small pancreatic duct in PD, identifying the duct and securing the anastomosis remain technical challenges. Therefore, we hypothesized that PDO is a good alternative to pancreaticojejunostomy in this clinical scenario. Since 2019, we have selectively used PDO with a linear stapler in PD for patients with a small duct and atrophic parenchyma in the distal pancreas. This study aimed to evaluate the safety and efficacy of this technique in a selected patient cohort.
We retrospectively identified four patients (cases 1–4) with pancreatic atrophy and a small pancreatic duct who underwent PDO by the same team of surgeons after PD at our center, which is a high-volume institution for pancreatic surgery in China, from April 2019 to October 2020 (11, 12).
Patients underwent either open PD or pylorus-preserving PD (PPPD) for any disease with ductal occlusions of the pancreatic duct of the distal remnant (without re-establishing pancreaticojejunal anastomosis [PJA]). In all cases, the pancreatic stump was closed using a linear stapler device (Echelon Flex EC60A with a 2.5 mm staple load; Ethicon-Endo Surgery™). Three abdominal silicon drainages with gravity suction were placed (two proximal to the pancreatic remnant and one posterior to the hepaticojejunostomy) to ensure effective surveillance and drainage of any possible POPF. Passive drainage with gravity was applied after surgery, and amylase level in the drainage fluid was routinely measured on postoperative days 1, 3, and 5 and on additional days when needed. Finally, drains were usually removed on postoperative day 7 when a fistula was not observed.
Pancreatic atrophy was defined as a pancreatic body width of < 10 mm on preoperative computed tomography (CT) (13). Classification of the pancreatic duct size and texture, which predicts POPF, was based on the consensus of the International Study Group of Pancreatic Surgery (ISGPS) (14). POPF, postpancreatectomy hemorrhage (PPH), and delayed gastric emptying (DGE) were defined and graded distinctively according to ISGPS criteria (15–18). In addition, the diagnosis of biliary leakage (BL) and diabetes mellitus (DM) was performed following the International Study Group of Liver Surgery recommendations (19) and the American Diabetes Association recommendations (20), respectively.
Patient's medical records, including clinical history, preoperative investigations, intraoperative data (texture of the pancreatic remnant and main pancreatic duct size), postoperative complications, postoperative hospital stays, and postoperative pancreatic endocrine and exocrine functions, were prospectively collected and retrospectively analyzed. Morbidity and mortality were evaluated within 30 days of surgery or during hospitalization. Medium-and long-term follow-up data were acquired from the same medical database at the pancreatic center. General routine examinations, including hematologic and biochemical examinations, as well as abdominal CT, were performed postoperatively in all patients. Follow-up evaluations were conducted via telephone interviews or outpatient service visits. The endocrine pancreatic function was indirectly evaluated by measuring the postoperative fasting blood glucose (FBG) level and performing an oral glucose tolerance test (OGTT). Conversely, the exocrine pancreatic function was evaluated based on symptoms of steatorrhea and the dosage of pancreatic enzyme supplementation.
The study protocol was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Jiangsu Provincial People's Hospital). All patients provided written informed consent before participation in this study.
This study included two female and two male patients with atrophic glands and small ducts in the left pancreas who underwent PDO without pancreaticojejunostomy during PD. These patients (age range, 40–63 years) were diagnosed with a pancreatic head lesion with atrophy in the distal part preoperatively on an abdominal contrast-enhanced CT, and the width of their pancreatic body ranged from 7 to 9 mm (Table 1; Figure 1). The physical status of all patients was categorized as American Society of Anesthesiologists grade II. None of the patients had a history of DM, and preoperative FBG levels were between 5.39 and 5.99 mmol/L (Table 1). In addition, none of the patients complained of symptoms of exocrine pancreatic insufficiency, such as steatorrhea or oral pancreatic enzyme supplements before surgery.
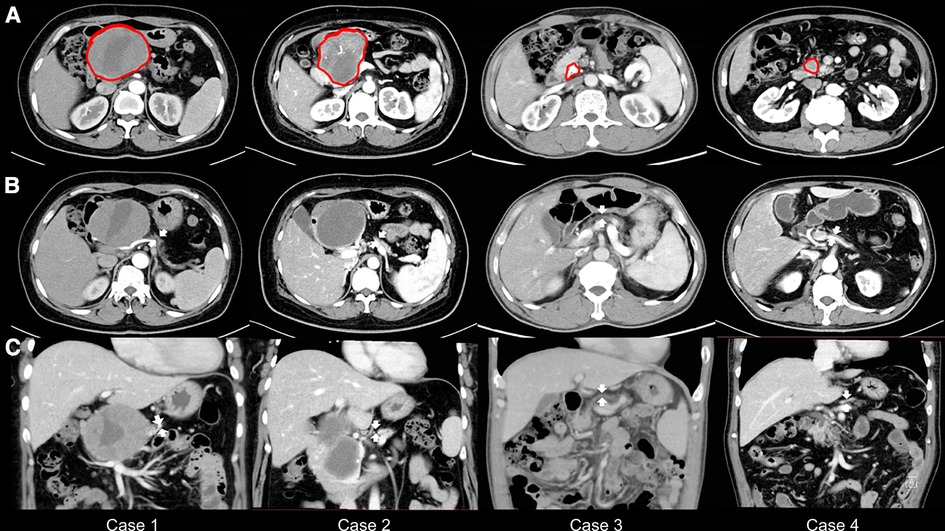
Figure 1. Preoperative abdominal computed tomography scans. (A) The tumor or stone is marked in red. (B) The width of the pancreatic bodies is < 10 mm, with arrows highlighting the atrophy of the pancreatic bodies. (C) The thickness of the pancreatic bodies is < 10 mm in the image, with arrows indicating the atrophy of the pancreatic bodies.
In total, one and three patients underwent PPPD and PD, respectively. The operative duration and intraoperative blood loss ranged from 252 to 400 min and 200 to 400 ml, respectively. Based on the ISGPS POPF risk stratification, all patients were classified as type B with a non-soft pancreatic texture and a small duct (Table 2). In addition, the postoperative histological examination revealed a solid pseudopapillary tumor in two patients, pancreatic ductal adenocarcinoma in one patient, and chronic pancreatitis with pancreatic duct stones in one patient (Table 2).
However, none of the patients showed biochemical leakage or clinically relevant POPF (grades B and C). No cases of PPH, DGE, BL, intra-abdominal abscess, intra-abdominal fluid collection, or wound complications were observed (Table 3). None of the patients required an interventional procedure or had a 90-day readmission, and the 90-day mortality was not recorded. Furthermore, the mean postoperative hospital stay was 11 days (range, 9–16 days) (Table 3).
All four patients survived over an average follow-up period of 13 months (range, 11–26 months. The patients underwent abdominal CT during the follow-up assessments (Figure 2), where imaging showed that the distal pancreatic remnant was well preserved because of sufficient blood supply, with no indications of inflammation or further atrophy (Figure 2). Patients' median short-term postoperative FBG level was 6.69 mmol/L (range, 6.32–7.5 mmol/L) (Table 4). Therefore, to evaluate the median-term change in glucose metabolic status after the procedure, all four patients underwent a 75 g oral OGTT at a median time of 13 months postoperatively (range, 11–26 months) (Table 4). Three patients developed new-onset DM (3/4), whereas one (case 2) had impaired glucose tolerance (IGT). Insulin and C-peptide levels were also measured to further evaluate the pancreatic endocrine function (Figures 3A–C). The insulin levels ranged from 21.4 to 149.6 mIU/L and 271.8 to 1058 mIU/L for the base and peak values, respectively; the base and peak values for C-peptide levels ranged from 286.4 to 707.1 pmol/L and 1234 to 4568 pmol/L, respectively. In addition, the peak times were 30 to 120 min. Because of the patient's diabetic status, cases 1, 3, and 4 had flat release curves. However, case 2 showed a release curve with a delayed peak (Table 4; Figures 3B–C). All patients received oral pancreatic enzyme supplementation at a dosage of 90,000 Ph. Eur. units/d after discharge. During the follow-up, only one patient (case 3) reported steatorrhea after consuming fatty food, which was alleviated after adjusting the pancreatic enzyme dosage to 120,000 Ph. Eur. units/d (Table 4).
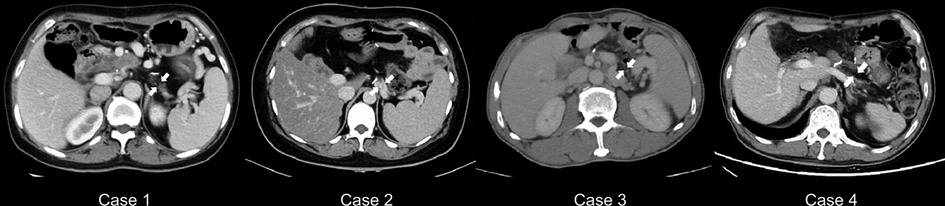
Figure 2. Abdominal CT scans during the follow-up studies. Abdominal CT reveals that the distal pancreas is intact in all cases (indicated by arrows). CT = computed tomography.

Figure 3. Results of pancreatic endocrine function, including the 75 g OGTT during the follow-up studies. (A) OGTT curve; (B) OGTT insulin-release curve; and (C) OGTT C-peptide release curve. OGTT = oral glucose tolerance test.
With the recent advances in medical technology and perioperative management, the mortality rate after PD has decreased to < 5% (21–23); however, the morbidity rate remains high at 30%–50% (24–26). A pancreatic fistula is the most common cause of morbidity and mortality after pancreatic anastomosis (27, 28). Furthermore, the activation of pancreatic enzymes from the distal pancreas by intestinal and/or biliary juices is the major cause of complications related to POPF, leading to erosion of the anastomosis, leakage of the biliary-enteric anastomosis, intra-abdominal abscesses, severe sepsis, hemorrhage, and DGE (1, 9, 27). However, the consensus risk factors associated with POPF include a small pancreatic duct (≤ 3 mm) and soft pancreatic texture (3, 4, 29, 30), with a soft pancreatic texture reflecting active exocrine function and a small-sized pancreatic duct requiring a more precise anastomosis of the pancreatic duct (5, 9, 14). Therefore, various strategies, including preoperative, intraoperative, and postoperative interventions and management, have been attempted to prevent the development of POPF, particularly surgical procedures (5, 27, 31–35).
According to the 2017 ISGPS statement (7), no universal standard modality is currently available for reconstruction after pancreatic surgery to avoid clinically relevant POPF. Nonetheless, surgeons dedicated to the pancreas have attempted to overcome these difficulties and make individualized decisions based on the clinical characteristics of patients and the pancreas (e.g., metabolic comorbidities, pancreatic duct diameter, pancreatic fibrosis, and the degree of tissue inflammation).
PDO/closure without pancreatic-enteric anastomosis after PD has been proposed to mitigate clinically relevant POPF, with the pancreatic remnant injected with chemical substances, stapled, or sutured (8–10, 32). This approach is technically simple, relatively safe, and less labor-intensive than pancreatic anastomosis. Moreover, occluding the pancreatic duct can prevent pancreatic enzyme activation by enteric and/or biliary juices in the case of POPF. A prospective, non-randomized clinical study in 2019 revealed that the early postoperative outcome of selected patients at high risk of POPF undergoing PDO with glue injection is equivalent to that of patients at low risk of POPF undergoing PJA (32). Although Mauriello et al. (8) used a linear stapler and Alfieri et al. (9) injected glue, they both reported that the occlusion of the pancreatic duct was related to a high rate of grade A fistulas (redefined as “biochemical leak” in 2017 [15]), which were considered self-limiting with no deviation from the conventional postoperative clinical pathway. Hemorrhage is another major complication of PD for artery skeletonization after curative lymph node dissection, exposing activated pancreatic enzymes and subsequent eroding (9). Regarding hemorrhage, pancreatic anastomosis leakage after PDO could be less threatening than PJA. Furthermore, endocrine and exocrine pancreatic insufficiency are other concerns for surgeons after surgery. Postoperative pancreatic exocrine insufficiency is common in patients with pancreatic cancer, and 74% of those undergoing PD with PJA require enzyme supplementation (36). Alfieri et al. (10) conducted long-term follow-ups objectively and subjectively after PD and reported no significant difference in postoperative pancreatic exocrine and endocrine functionality between PDO and PJA. Despite a higher frequency of objective exocrine insufficiency in the PDO group, the need for postoperative substitutive enzymes did not increase (10).
To date, several reports have focused on the differences in POPF and pancreatic functional outcomes between PDO and PJA after PD, with few studies on patients with pancreatic atrophy and a small pancreatic duct undergoing PD. Therefore, it is difficult to effectively perform pancreaticojejunostomy in a patient with a narrow pancreatic duct, which may lead to the risk of severe POPF. This study's innovative characteristics depend on surgical decision-making, which considers the type of anastomotic reconstruction and fibrosis/atrophy of the pancreatic remnant with a small pancreatic duct, as well as the recommendations for selected patients undergoing PD who are eligible for PDO without anastomosis. Since a pancreatic head tumor or pancreatic duct stone obstruction of the main duct induces atrophy of the body and tail of the pancreas (37–39), PDO can be practically considered a typical model of exocrine pancreatic insufficiency due to atrophy of the pancreatic remnant (10). In this study, four selected patients with an atrophic pancreatic body tail and a small pancreatic duct underwent PDO without anastomosis after PD/PPPD. Three patients developed new-onset DM, and one developed IGT. All four patients received pancreatic enzyme supplementation postoperatively. Furthermore, pancreatic insufficiency after surgery is expected, considering fibrosis and/or atrophy of the distal pancreas and loss of partial function preoperatively.
This study was limited due to its retrospective, non-randomized nature and involved a few patients with an atrophic pancreatic body tail and a small pancreatic duct. Nevertheless, based on this study and an extensive literature review, PDO is an alternative procedure for patients with an atrophic pancreatic body-tail and a small pancreatic duct in PD. Furthermore, PDO after PD may be considered in selected “high-risk” patients, particularly those with a soft pancreas and small pancreatic duct, to prevent and reduce POPF-related complications (8, 9, 32). In addition, PDO remains an option to manage the pancreatic remnant and prevent the completion of pancreatectomy in “difficult circumstances,” such as severe POPF requiring relaparotomy (40, 41).
Although a larger sample size is required to confirm our results, this study suggests that PDO with mechanical closure is a safe, easy-to-perform preferred procedure to manage the pancreatic stump during PD in patients with an atrophic pancreatic body tail and a small-sized duct. However, the long-term function of the remnant pancreas after this procedure warrants further evaluation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Jiangsu Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
GC, JY, ZL, KJ: conception and design. YM, KJ: administrative support. JY, JW, KZ, PW, BC: provision of study materials or patients. GC, JY, QC, LM, YL: collection and assembly of data. GC, JY, ZL: data analysis and interpretation. GC, JY, ZL: manuscript writing. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (numbers (nos.): 81871980 and 82072706), National Science Foundation for Distinguished Young Scholars of China (no.: 82103280), Jiangsu Key Medical Discipline (General Surgery, no.: ZDXKA2016005), and Project “333” of Jiangsu Province (no.: BRA2019096).
We thank all of our colleagues at the Pancreas Center for their invaluable discussions and help with our work. We would also like to thank Editage for English language editing assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mirrielees JA, Weber SM, Abbott DE, Greenberg CC, Minter RM, Scarborough JE. Pancreatic fistula and delayed gastric emptying are the highest-impact complications after whipple. J Surg Res. (2020) 250:80–7. doi: 10.1016/j.jss.2019.12.041
2. Vining CC, Kuchta K, Schuitevoerder D, Paterakos P, Berger Y, Roggin KK, et al. Risk factors for complications in patients undergoing pancreaticoduodenectomy: a NSQIP analysis with propensity score matching. J Surg Oncol. (2020) 122:183–94. doi: 10.1002/jso.25942
3. Eshmuminov D, Schneider MA, Tschuor C, Raptis DA, Kambakamba P, Muller X, et al. Systematic review and meta-analysis of postoperative pancreatic fistula rates using the updated 2016 international study group pancreatic Fistula definition in patients undergoing pancreatic resection with soft and hard pancreatic texture. HPB (Oxford). (2018) 20:992–1003. doi: 10.1016/j.hpb.2018.04.003
4. Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. (2016) 22:7797–805. doi: 10.3748/wjg.v22.i34.7797
5. Kawaida H, Kono H, Hosomura N, Amemiya H, Itakura J, Fujii H, et al. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol. (2019) 25:3722–37. doi: 10.3748/wjg.v25.i28.3722
6. Wang W, Zhang Z, Gu C, Liu Q, Liang Z, He W, et al. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: a network meta-analysis of randomized control trials. Int J Surg. (2018) 57:111–6. doi: 10.1016/j.ijsu.2018.04.005
7. Shrikhande SV, Sivasanker M, Vollmer CM, Friess H, Besselink MG, Fingerhut A, et al. Pancreatic anastomosis after pancreatoduodenectomy: a position statement by the international study group of pancreatic surgery (ISGPS). Surg. (2017) 161:1221–34. doi: 10.1016/j.surg.2016.11.021
8. Mauriello C, Polistena A, Gambardella C, Tartaglia E, Orditura M, De Vita F, et al. Pancreatic stump closure after pancreatoduodenectomy in elderly patients: a retrospective clinical study. Aging Clin Exp Res. (2017) 29:35–40. doi: 10.1007/s40520-016-0657-8
9. Alfieri S, Quero G, Rosa F, Di Miceli D, Tortorelli AP, Doglietto GB. Indications and results of pancreatic stump duct occlusion after duodenopancreatectomy. Updates Surg. (2016) 68:287–93. doi: 10.1007/s13304-016-0384-x
10. Alfieri S, Agnes A, Rosa F, Di Miceli D, Grieco DL, Scaldaferri F, et al. Long-term pancreatic exocrine and endometabolic functionality after pancreaticoduodenectomy. Comparison between pancreaticojejunostomy and pancreatic duct occlusion with fibrin glue. Eur Rev Med Pharmacol Sci. (2018) 22:4310–8. doi: 10.26355/eurrev_201807_15427
11. Radenkovic D, Farnell MB, Bassi C, Besselink M. Evolving techniques in pancreatic surgery. Gastroenterol Res Pract. (2016) 2016:1–2. doi: 10.1155/2016/4289724
12. Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surg. (1999) 125:250–6. doi: 10.1016/S0039-6060(99)70234-5
13. Masuda A, Shiomi H, Matsuda T, Takenaka M, Arisaka Y, Azuma T, et al. The relationship between pancreatic atrophy after steroid therapy and diabetes mellitus in patients with autoimmune pancreatitis. Pancreatol. (2014) 14:361–5. doi: 10.1016/j.pan.2014.07.005
14. Schuh F, Mihaljevic AL, Probst P, Trudeau MT, Muller PC, Marchegiani G, et al. A simple classification of pancreatic duct size and texture predicts postoperative pancreatic fistula: a classification of the international study group of pancreatic surgery (ISGPS). Ann Surg. (2021). doi: 10.1097/SLA.0000000000004855. [Epub ahead of print]33914473
15. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu HM, Adham M, et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surg. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014
16. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition. Surgery. (2007) 142:20–5. doi: 10.1016/j.surg.2007.02.001
17. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS). Surg. (2007) 142:761–8. doi: 10.1016/j.surg.2007.05.005
18. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surg. (2005) 138:8–13. doi: 10.1016/j.surg.2005.05.001
19. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the international study group of liver surgery. Surg. (2011) 149:680–8. doi: 10.1016/j.surg.2010.12.002
20. Association AD. Classification and diagnosis of diabetes. Diabetes Care. (2017) 40:S11–24. doi: 10.2337/dc17-S005
21. Beger HG, Mayer B. Early postoperative and late metabolic morbidity after pancreatic resections: an old and new challenge for surgeons - a review. Am J Surg. (2018) 216:131–4. doi: 10.1016/j.amjsurg.2018.02.014
22. Kirihara Y, Takahashi N, Hashimoto Y, Sclabas GM, Khan S, Moriya T, et al. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: the use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg. (2013) 257:512–9. doi: 10.1097/SLA.0b013e31827827d0
23. Marchegiani G, Andrianello S, Salvia R, Bassi C. Current definition of and controversial issues regarding postoperative pancreatic fistulas. Gut Liver. (2019) 13:149–53. doi: 10.5009/gnl18229
24. Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): a systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Med (Baltimore). (2017) 96:e6858. doi: 10.1097/MD.0000000000006858
25. Soreide K, Labori KJ. Risk factors and preventive strategies for post-operative pancreatic fistula after pancreatic surgery: a comprehensive review. Scand J Gastroenterol. (2016) 51:1147–54. doi: 10.3109/00365521.2016.1169317
26. Bassi C, Buchler MW, Fingerhut A, Sarr M. Predictive factors for postoperative pancreatic fistula. Ann Surg. (2015) 261:e99. doi: 10.1097/SLA.0000000000000577
27. Crippa S, Falconi M. Pancreatic fistula after pancreaticoduodenectomy-does surgical technique matter? Ann Transl Med. (2020) 8:669. doi: 10.21037/atm.2020.03.123
28. Crippa S, Salvia R, Falconi M, Butturini G, Landoni L, Bassi C. Anastomotic leakage in pancreatic surgery. HPB (Oxford). (2007) 9:8–15. doi: 10.1080/13651820600641357
29. Ke Z, Cui J, Hu N, Yang Z, Chen H, Hu J, et al. Risk factors for postoperative pancreatic fistula: analysis of 170 consecutive cases of pancreaticoduodenectomy based on the updated ISGPS classification and grading system. Med (Baltimore). (2018) 97:e12151. doi: 10.1097/MD.0000000000012151
30. Aoki S, Miyata H, Konno H, Gotoh M, Motoi F, Kumamaru H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci. (2017) 24:243–51. doi: 10.1002/jhbp.438
31. Kambakamba P, Mannil M, Herrera PE, Muller PC, Kuemmerli C, Linecker M, et al. The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: a proof-of-principle study. Surg. (2020) 167:448–54. doi: 10.1016/j.surg.2019.09.019
32. Mazzaferro V, Virdis M, Sposito C, Cotsoglou C, Droz DBM, Bongini M, et al. Permanent pancreatic duct occlusion with neoprene-based glue injection after pancreatoduodenectomy at high risk of pancreatic fistula: a prospective clinical study. Ann Surg. (2019) 270:791–8. doi: 10.1097/SLA.0000000000003514
33. Chen JS, Liu G, Li TR, Chen JY, Xu QM, Guo YZ, et al. Pancreatic fistula after pancreaticoduodenectomy: risk factors and preventive strategies. J Cancer Res Ther. (2019) 15:857–63. doi: 10.4103/jcrt.JCRT_364_18
34. Casciani F, Bassi C, Vollmer CM. Decision points in pancreatoduodenectomy: insights from the contemporary experts on prevention, mitigation, and management of postoperative pancreatic fistula. Surg. (2021) 170:889–909. doi: 10.1016/j.surg.2021.02.064
35. Mungroop TH, Klompmaker S, Wellner UF, Steyerberg EW, Coratti A, D’Hondt M, et al. Updated alternative fistula risk score (ua-FRS) to include minimally invasive pancreatoduodenectomy: pan-European validation. Ann Surg. (2021) 273:334–40. doi: 10.1097/SLA.0000000000003234
36. Tseng DS, Molenaar IQ, Besselink MG, van Eijck CH, Borel RI, van Santvoort HC. Pancreatic exocrine insufficiency in patients with pancreatic or periampullary cancer: a systematic review. Pancreas. (2016) 45:325–30. doi: 10.1097/MPA.0000000000000473
37. Olesen SS, Hagn-Meincke R, Drewes AM, Steinkohl E, Frokjaer JB. Pancreatic atrophy and exocrine insufficiency associate with the presence of diabetes in chronic pancreatitis patients, but additional mediators are operative. Scand J Gastroenterol. (2021) 56:321–8. doi: 10.1080/00365521.2020.1867891
38. Iglesia D, Avci B, Kiriukova M, Panic N, Bozhychko M, Sandru V, et al. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: a systematic review and meta-analysis. United European Gastroenterol J. (2020) 8:1115–25. doi: 10.1177/2050640620938987
39. Tsujie M, Wakasa T, Mizuno S, Ishikawa H, Manabe H, Koyama T, et al. Solid pseudopapillary neoplasm of the pancreas showing marked distal atrophy: a case report. Int J Surg Case Rep. (2019) 55:136–9. doi: 10.1016/j.ijscr.2019.01.030
40. Andaluz A, Ewertowska E, Moll X, Aguilar A, Garcia F, Fondevila D, et al. Endoluminal radiofrequency ablation of the main pancreatic duct is a secure and effective method to produce pancreatic atrophy and to achieve stump closure. Sci Rep. (2019) 9:5928. doi: 10.1038/s41598-019-42411-7
Keywords: pancreaticoduodenectomy, pancreatic duct occlusion, pancreatic fistula, pancreatic atrophy, pancreas
Citation: Chen G, Yin J, Chen Q, Wei J, Zhang K, Meng L, Lu Y, Wu P, Cai B, Lu Z, Miao Y and Jiang K (2023) Selective use of pancreatic duct occlusion during pancreaticoduodenectomy in patients with a small-size duct and atrophic parenchyma in the distal pancreas: A retrospective study. Front. Surg. 9:968897. doi: 10.3389/fsurg.2022.968897
Received: 14 June 2022; Accepted: 31 October 2022;
Published: 6 January 2023.
Edited by:
Antonio Gangemi, University of Illinois at Chicago, United States© 2023 Chen, Yin, Chen, Wei, Zhang, Meng, Lu, Wu, Cai, Lu, Miao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuirong Jiang amlhbmdrdWlyb25nQG5qbXUuZWR1LmNu Zipeng Lu c3VyZ2Vvbm1hcmtAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.