- 1Department of General Surgery, Hillingdon Hospital, The Hillingdon Hospitals NHS Foundation Trust, London, United Kingdom
- 2Department of Thoracic Surgery, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
- 3Department of Thoracic Surgery, University College London Hospitals, London, United Kingdom
- 4Department of Thoracic Surgery, Cantonal Hospital Lucerne, Lucerne, Switzerland
- 5Department of Thoracic Surgery, Imperial College Healthcare NHS Trust, London, United Kingdom
Background: Video-Assisted and Robotic-Assisted techniques become constantly more prominent practice in thoracic surgery for lung cancer. Furthermore, the increased frequency in detection of small lung cancers makes the intra-operative identification of these cancers even more challenging. Indocyanine Green (ICG) is one of the most commonly used dyes that assists surgeons identify small lung cancers intra-operatively. Our study aimed to evaluate the effectiveness and safety of ICG in lung cancer detection.
Methods: We performed a systematic review of the literature by screening the databases of MEDLINE, EMBASE, CENTRAL and Scopus until 30th April 2022 and the first 300 articles of Google Scholar for any suitable grey literature. We included any study that investigated the effectiveness of ICG in lung cancer detection. We excluded studies that explored the use of ICG only in identification of intersegmental planes, lymph node mapping, case reports and non-English articles. We aimed to perform a meta-analysis on test accuracy studies using hierarchical summary receiver operating characteristic (HSROC) and the bivariate random-effects models. In cases where the data for a localization technique was not sufficient for that analysis, it was presented with tables with narrative purposes. Each study was assessed for Risk of Bias (RoB) and Applicability using the QUADAS-2 tool.
Results: We found 30 eligible studies that included a total of 1,776 patients who underwent ICG localization of pulmonary nodules. We identified three ICG localization techniques: CT-guided, endobronchial and intravenous. From the 30 studies, 13 investigated CT-guided localization, 12 explored an endobronchial method while 8 studies administered ICG intravenously the median reported success rate was 94.3% (IQR: 91.4%–100%) and 98.3% (IQR: 94%–100%) for the first two techniques respectively. Intravenous ICG lung cancer localization showed Sensitivity of 88% (95% CI: 59%–0.97%) and Specificity of 25% (95% CI: 0.04%–0.74%). There were 15.2% (150/989) patients who experienced complications from CT guided ICG localization. No ICG-related complications were reported in endobronchial or intravenous techniques.
Conclusion: Our study provides a comprehensive review of the literature on ICG localization techniques for lung cancer. Current evidence suggests that ICG is boh effective and safe. Further prospective research with standardized protocols across multiple thoracic units is required in order to accurately validate these findings.
Introduction
Lung cancer remains one of the leading causes of mortality globally, accounting for almost a quarter of all cancer related deaths, even in developed counties (1, 2). Computer Tomography (CT) scan is considered the gold standard imaging modality for detection of even sub-centimeter lung nodules (3). Recent evidence demonstrates lower lung cancer mortality among high risk population who undergo volume-based, low-dose computed tomographic (CT) lung cancer screening program (4). Furthermore, the screening program detected higher rate of early stage lung cancers, compared to control, which subsequently offers treatment sooner to smaller pulmonary nodules with surgery being a key component (4).
The latest evidence from VIOLET trial supports that minimally invasive techniques offer more favorable results compared to the open approach (5). Furthermore, arising evidence suggests that anatomically sub-lobar resections, such as segmentectomy, provides better outcome compared to lobectomy in early stage, peripheral lung cancer (6). This evidence drives a clear trend in thoracic surgery over the last decade in resecting constantly smaller lung nodules (7–9). Despite the high quality cameras available in Video-Assisted Thoracoscopic Surgery (VATS) and Robotic-Assisted Thoracoscopic Surgery (RATS), the pre-operatively identified sub-centimeter ground glass opacities with small percentage of solid component that are located at a distance from the lung surface make their intra-operative identification a rising challenge (7–9). For that reason, multiple techniques for lung cancer localization have been developed in attempt to address this rising challenge (9, 10).
Indocyanine Green (ICG) is one of the most common localization techniques for early stage lung cancer. ICG is a near-infrared (NIR) fluorescent contrast agent with fluorescence absorption of 820 nm which is visible with appropriate cameras (11). NIR light provides high tissue penetration with low autofluorescence which makes it useful for contrast (11).
Despite a number of narrative reviews highlighting the usefulness of indocyanine green (ICG) in thoracic surgery, its true value remains equivocal (12–14). For that reason, we conducted a systematic review of the literature that would investigate the effectiveness and safety of ICG in detection of malignant lung nodules.
Materials and methods
Team set up and study selection criteria
To complete this project according to the guidance for systematic review and meta-analysis we set up a research team. After discussions amongst team members, we designed our research protocol which was published in PROSPERO (CRD42022338004). The results of the study were reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PRISMA Checklist is provided in the Appendix).
We concluded on the inclusion and exclusion criteria below:
Inclusion Criteria:
• Patients undergoing resection of pulmonary nodules following the use of indocyanine green (ICG) with near-infrared fluorescence imaging (NIR).
• All surgical approaches (open, video-assisted or robotic-assisted thoracoscopic surgery).
Exclusion Criteria:
• Overlapping patient cohorts (inclusion of the latest study only to avoid duplication of data).
• Studies that investigate the use of ICG only for identification of intersegmental planes.
• Studies that investigate sentinel lymph node mapping.
• Articles that contribute no data for analysis (reviews, commentaries, editorials, etc.).
• Case reports.
• Language other than English.
• Full text unavailable.
Search strategy
To achieve a broad and inclusive review of the literature we used only keywords relevant to the disease of lung cancer, the surgical approach and ICG.
This systematic review is comprised of studies that investigate the effectiveness of peri-operative indocyanine green (ICG) administration in the detection of malignant pulmonary nodules. There was no limitation with regards to the year of publication, method of ICG administration or surgical approach for lung resection.
We included randomized clinical trials and observational studies (case-control, case-series, retrospective and prospective cohorts). Systematic, narrative reviews and meta-analysis were used to identify further eligible primary studies through screening of their included studies.
We concluded on the following detailed search strategy:
#1: (“Lung Neoplasms”[Mesh] OR (lung cancer) OR (lung nodule) OR (lung mass) OR (lung carcinoma))
#2: (“Thoracic Surgery”[Mesh] OR “Thoracotomy”[Mesh] OR “Thoracic Surgical Procedures"[Mesh] OR “Thoracic Surgery, Video-Assisted”[Mesh] OR “Robotic assisted thoracic surgery” OR “RATS” OR “VATS”)
#3: (“Indocyanine Green”[Mesh] OR (ICG) OR (Indocyanine Green) OR (near infrared range) OR (NIR)).
#1 AND #2 AND #3
A two-stage screening process was performed until 30th April 2022 against our inclusion and exclusion criteria from the following electronic bibliographic databases: MEDLINE, EMBASE, CENTRAL and Scopus. We also assessed for eligibility the first 300 articles from Google Scholar in order to identify suitable grey literature (15). The databases for MEDLINE and EMBASE were screened together using OVID. Initially, records were screened by title and abstract and then duplicate studies were identified and removed across the different electronical platforms (Ovid, CENTRAL, Scopus and Google Scholar) using EndNote X9. For the second stage of screening we performed full text review of all eligible studies from the title and abstract screening. Both stages were performed by two authors (AG, SL) and any disagreements were resolved through discussion. If a consensus could not be reached between the two authors, then the other members of the team were consulted.
Data collection
Data extraction forms were designed, reviewed and approved by all authors involved in the project following guidance of Cochrane Handbook for systematic reviews (sample of the form attached at the end of Appendix) (16). The collected data from the eligible studies included:
(1) General Information about the study and its methods: authors, year of publication, name of publishing journal, country of origin of where the study was conducted, type of study, study population inclusion criteria, methods used for analysis of outcome.
(2) Demographics and baseline characteristics of study participants: total number of patients who underwent ICG localization of lung nodules, approach of ICG localization, concentration and volume of administered ICG, technology of NIR intra-operative identification, the patients age, gender, number of nodules, size of nodules, distance of the nodule from the lung surface, final nodule histology.
(3) Procedural Characteristics: Surgical approach used, type of lung excision performed, duration of localization, duration of the lung resection procedure.
(4) Outcome of the study: Intra-operative identification success rate of fluorescent substance, reasons for localization failure, complications from localization, complications after the end of the procedure.
Data were extracted in duplicate by two authors (AG, SL), with disagreements again resolved through discussion and further consultation with the team if required.
Quality assessment
The quality assessment for risk of bias and applicability of the test was performed according to Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS 2) (17). Two authors (AG, SL) completed independently their assessment for every eligible study across the four key domains of the QUADAS 2 tool for risk of bias (Low/High/Unclear) and the three key domains of the same tool on applicability. The index test was the localization technique with ICG while the reference standard was the identification of each localized nodule on histology. Any disagreements on the overall score between the two assessors were resolved through discussions with the rest of the team.
Data and statistical analysis
Details from the included studies are presented with summary tables for study characteristics and quality assessment. Continuous variables are expressed as means and SD or as median and IQR depending on data distribution. Categorical variables are expressed as proportions and percentages.
Prior to data collection, we aimed to perform a meta-analysis on test accuracy studies using hierarchical summary receiver operating characteristic (HSROC) and the bivariate random-effects models (18). Our goal was to plot the summary receiver operating characteristic (SROC) curve which would include the prediction region, the summary point and its confidence region. In cases where the data for one of the localization techniques was not sufficient for that analysis, that was presented with tables with narrative purposes.
For the meta-analysis of the sensitivity and specificity of each localization method we adhered to the following assessments: For CT-guided and endobronchial ICG localization of pulmonary nodules, the administration of ICG was used as a marking dye to guide surgical excision and not as an identification for malignancy. Therefore, true positive (TP) was considered a lesion which was fluorescent after ICG localization and was found completely resected on histopathology. False positive (FP) was considered a lesion which was fluorescent after ICG localization and was either absent or not completely resected on histopathology. True negative (TN) was considered a resected lesion with no identified nodules on histopathology that was not intra-operatively fluorescent because it was not found for localization with ICG. False negative (FN) was considered a lesion which was not fluorescent after ICG localization either due to localization failure or because the fluorescent dye was not identifiable intra-operatively and if resected, there was a nodule present on histopathology. The lesions that were not resected despite pre-operative planning due to wrong localization were considered localization failures and therefore were considered false negative.
Those assessments were different for the intravenous ICG administration because that technique aimed at identifying malignant lung nodules in the lung parenchyma as it relied on previous evidence suggesting that ICG could remain in tumors 24 h after intravenous injection by enhanced permeability and retention (EPR) effect (19). For that reason, we considered true positive (TP) lesions those which were fluorescent intra-operatively while also found to be malignant on histopathology. False positive (FP) was considered a lesion which was fluorescent but was not malignant on histopathology. True negative (TN) was considered a resected lesion that was not fluorescent and was not malignant on histopathology. False negative (FN) was considered a resected lesion which was not fluorescent but was malignant on histopathology and all localization failures that resulted in malignant lesions to remain unresected.
The accuracy of each technique was calculated by the model: (TN + TP)/(TN + TP + FN + FP). Data analysis and synthesis was performed using STATA 17 (StataCorp. 2015. Stata Statistical Software: Release 17. College Station, TX: StataCorp LP).
The results are presented according to PRISMA guidelines.
Results
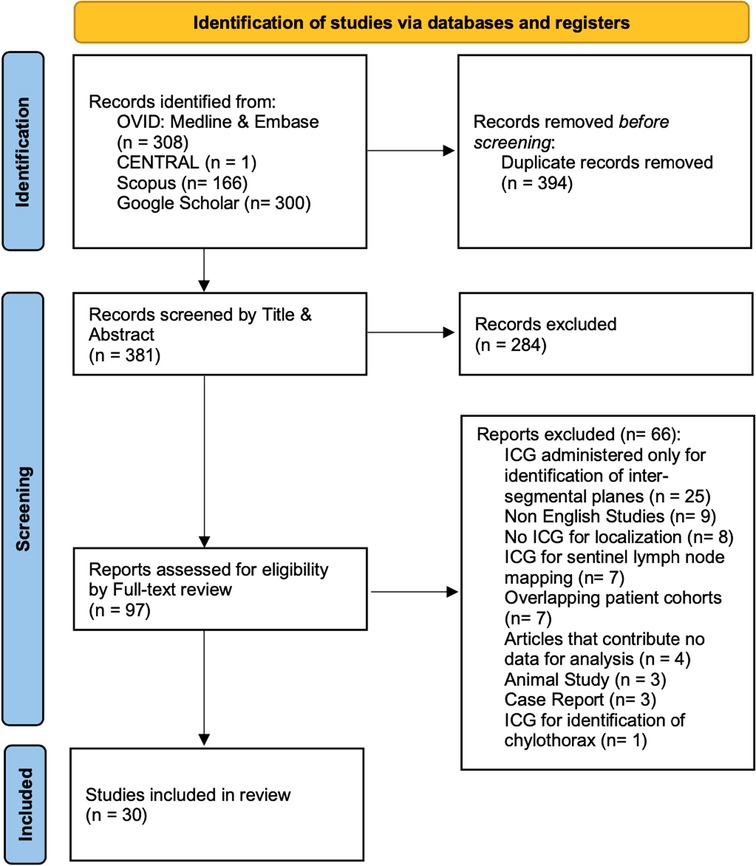
Our initial search identified 775 studies across the 5 electronic databases. After removal of 394 duplicates, we screened 381 studies by title and abstract. Of the 381 articles, 97 full-text articles were assessed. The second stage of the screening process resulted in 30 studies to be included for further analysis (Figure 1) (19–48).
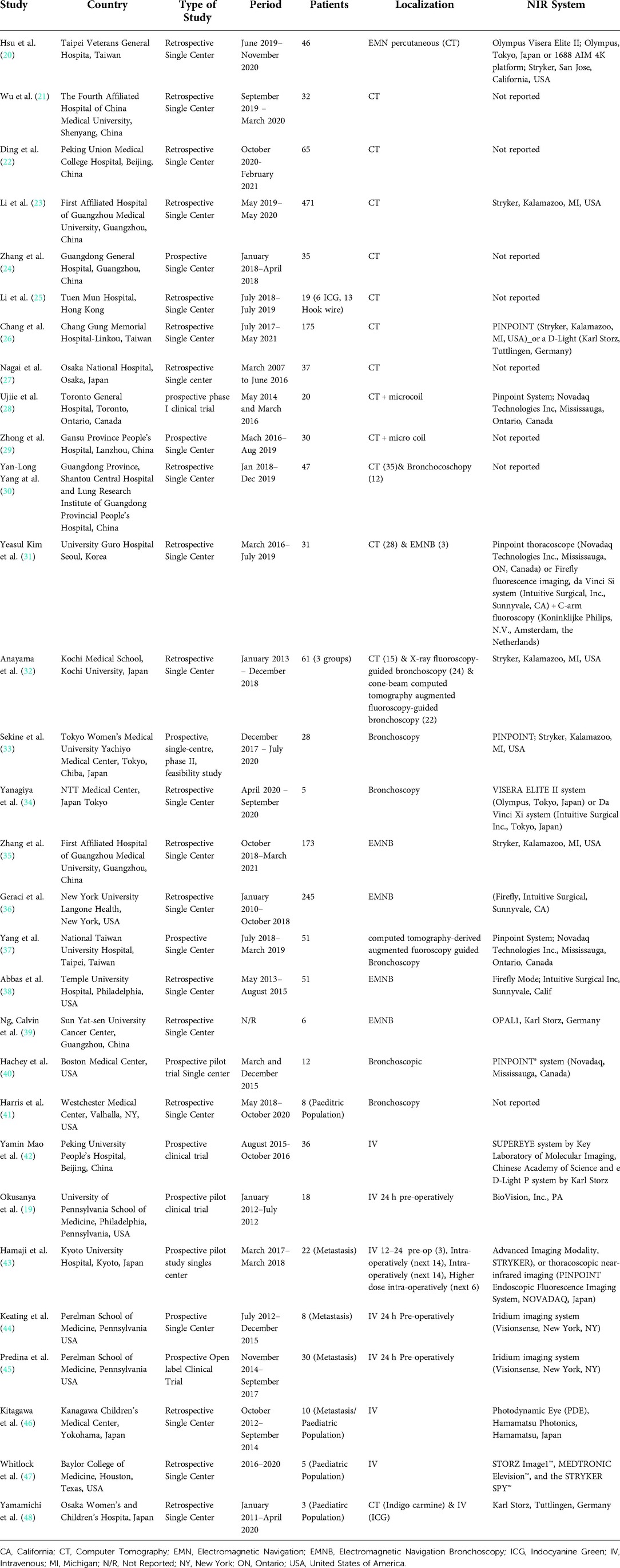
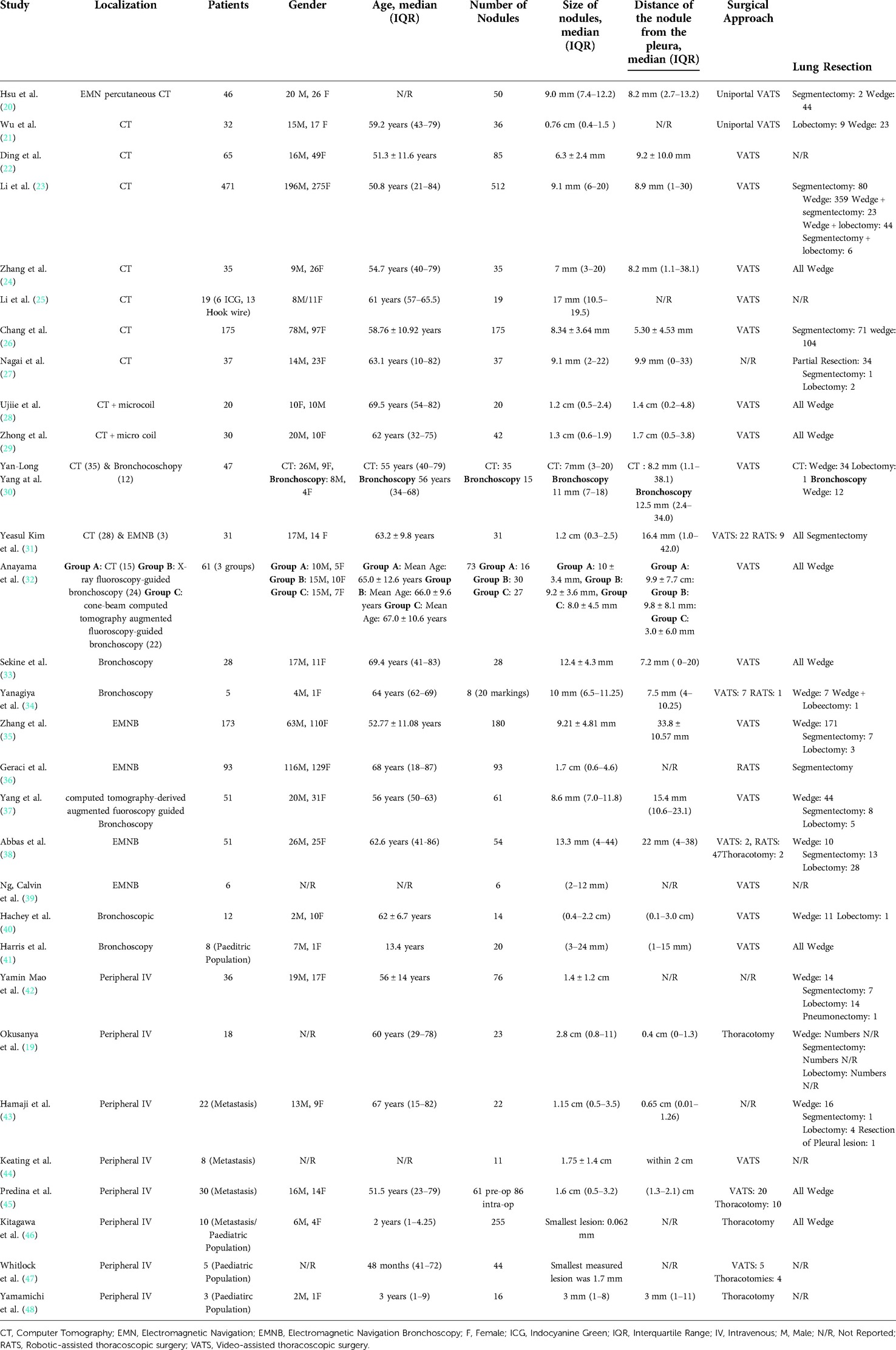
Overall, there were 1,776 patients who underwent ICG localization of pulmonary nodules in the 30 eligible studies. These studies were subsequently categorized on three subgroups according to the administration technique of ICG for localization. The three techniques which were identified from this systematic review of the literature were CT guided, under bronchoscopy and through peripheral intravenous administration (Table 1). Those included 13, 12 and 8 studies subsequently to each group. In three of the studies, the investigators performed both CT guided and endobronchial localization of the pulmonary nodules (30–32). There was one study that performed both CT guided localization with indigo carmine in all of their patients (n = 12) but 3 patients (16 nodules) received ICG IV 24 h pre-operatively and was thus included in our IV group (48). All the studies were conducted in single centers and 63% of them had retrospective study design (19/30). There were four studies that investigated the effectiveness of ICG localization in pediatric population (41, 46–48) while five studies assessed the performance of ICG localization in patients with potential pulmonary metastases (43–47).
Apart from three pediatric studies, that performed lung resections via thoracotomy, the vast majority of papers showed a preference towards VATS or RATS. There was only one adult trial in which the operating team performed their lung resections via thoracotomy (19). The vast majority of lung resections was undertaken with sub-lobar excisions in 96% of the operations (1442/1500). Further details of each study's population are illustrated on Table 2.
Quality assessment
The quality assessment of the studies demonstrated not only low risk of bias (RoB) on reference standard and flow and timing but also low concern regarding applicability in more than 80% of the included studies (26/30, 28/30 and 28/30 respectively). The index test assessment showed high risk of bias in 36.7% (11/30) which also reflected on the high level of concern regarding applicability in 30% of the studies (9/30). This was the result of studies administering a mixture of ICG with other localization techniques such as micro-coils and other dyes (Iopamidol, Methylene Blue, Indigo Carmine) which introduced significant bias on the assessment of the effectiveness of ICG as independent marker (25, 28, 29, 34, 38, 39, 41). Furthermore, two studies that investigated the IV administration of ICG, had significant variation on ICG dose and time interval between ICG administration and the operation among the participants of each study (43, 47).
Finally, the RoB was low in 53% of the studies (16/30) on patient selection. This was predominantly the result of poor population description with regards to co-morbidities and peri-operative data which can be attributed to restrictions which are usually encountered in retrospective studies. The summary of QUADAS-2 assessment is illustrated in Figure 2 and the review of each study can be found in the Appendix.
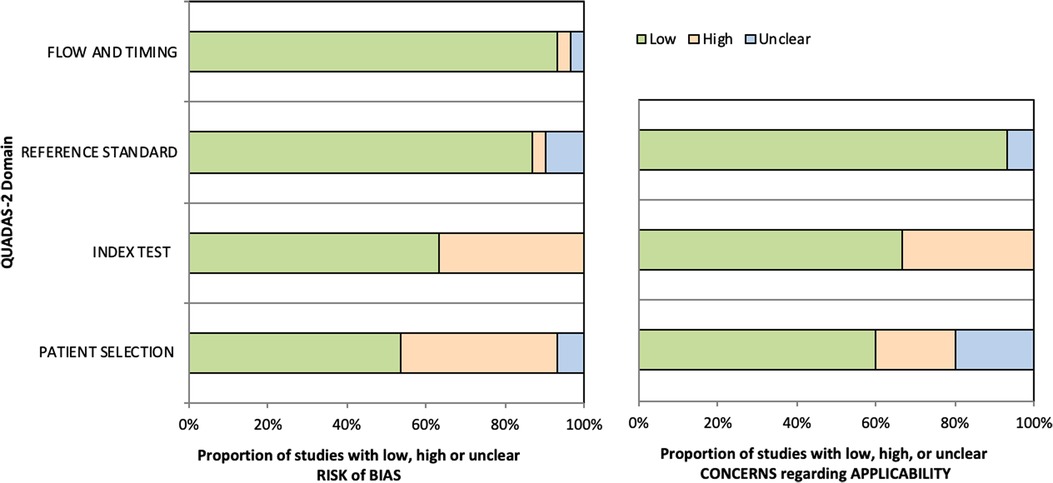
Figure 2. QUADAS-2 tool assessment on Risk of Bias (Left bar chart) and Applicability (Right bar chart).
Outcome assessment
The primary outcome of this study was to evaluate the success rate for ICG localization. The studies that evaluated the CT-guided and endobronchial techniques did not report any true negative or false positive lesions based on our predefined criteria. Subsequently, this resulted in inability to use the bivariate model or HSROC. We therefore, summarize the findings of our systematic review for these two localization techniques on Tables 3, 4.
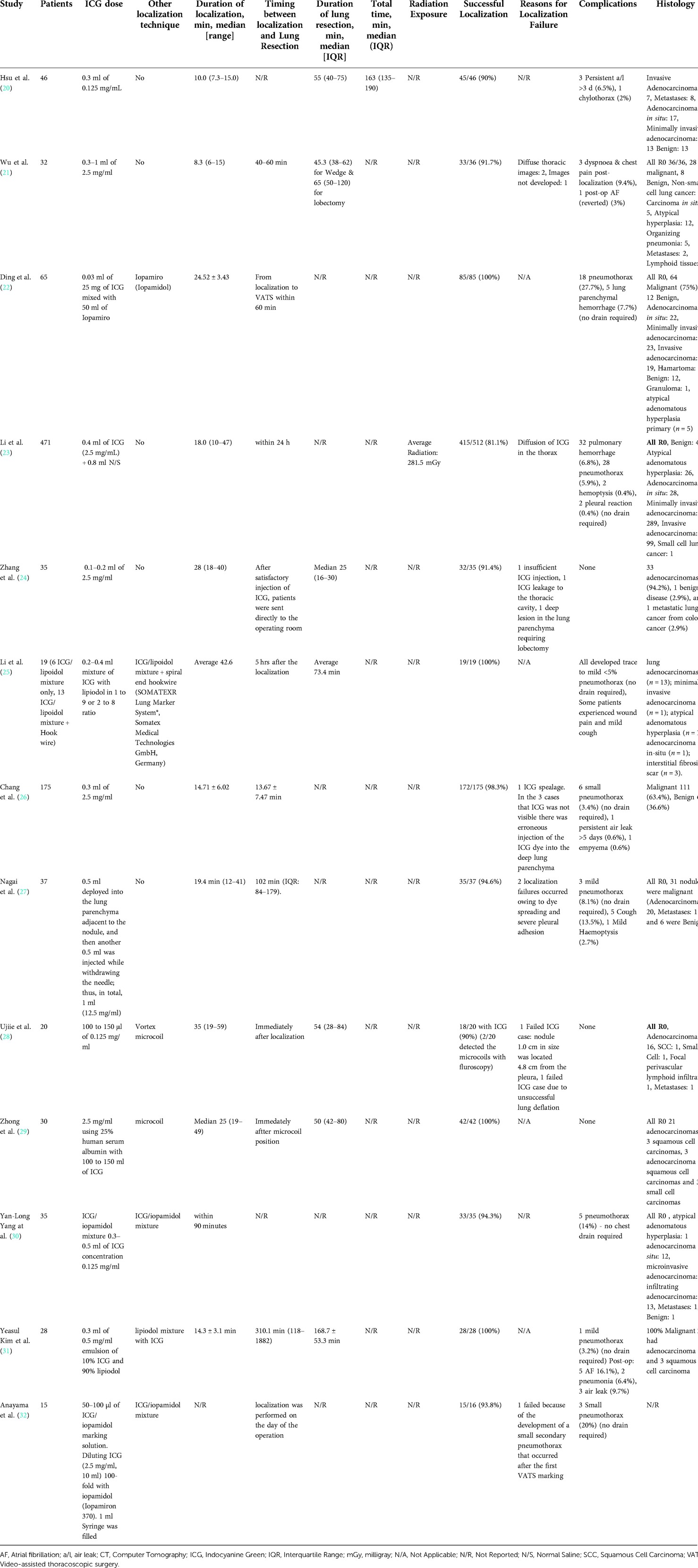
Table 3. Operative details and outcomes from studies that used CT-guided Indocyanine Green localization.
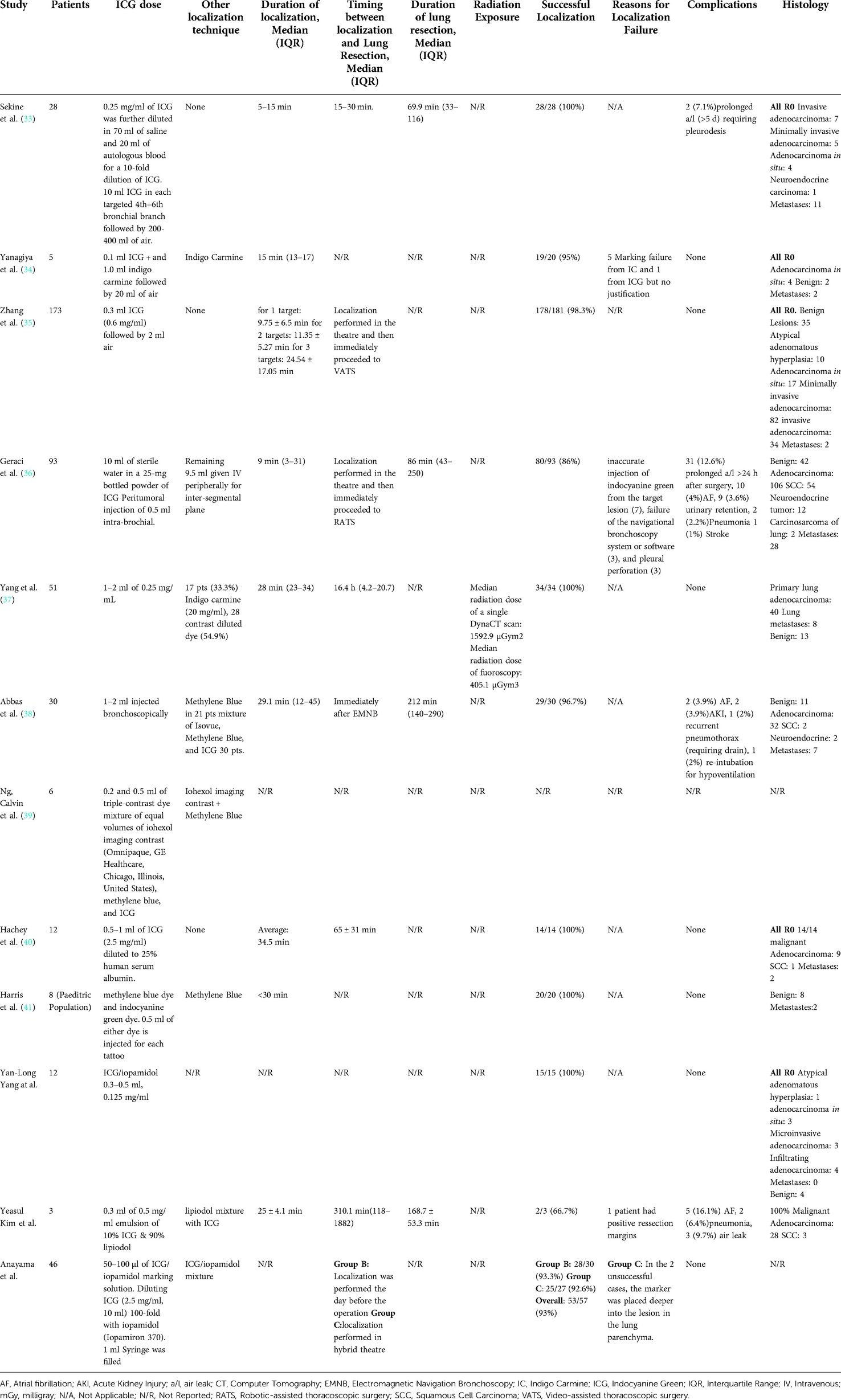
Table 4. Operative details and outcomes from studies that used bronchoscopy Indocyanine Green localization.
The ICG localization of pulmonary nodules under CT guidance had an overall success rate of 97.6%, with median success rate 94.3% (IQR: 91.4%–100%). The accuracy of the technique was estimated at 97.3%. There were only 2.4% (26/1089) of false negative ICG markings which resulted from localization failure, most commonly due to ICG spillage and subsequent diffused dye in the chest cavity. There were 15.2% (150/989) of patients who developed complications during their hospitalization. In 131 of these patients their complication was related to CT guided localization. However, in 120 of them the complications were small traces of pneumothorax and intra-parenchymal hemorrhage which was only visible on CT during guidance. None of these patients required a chest drain or any other intervention for these complications. The other 11 patients experienced either dyspnea, chest pain or mild hemoptysis after localization. There were no reported allergic reactions in any of the studies. The duration of localization time was reported by 12 studies and ranged from 6 to 59 min while the lung resection time was reported by 7 studies with a range from 16 to 221 min. From the 11 studies that reported the timing between localization and lung resection, the vast majority of them (90.9%, 10/11) proceeded with lung resection within a few hours.
From the 12 studies that ICG localization of the pulmonary nodules was performed under bronchoscopy had an overall success of 95.4%, median 98.3% (IQR: 94%–100%). The accuracy of bronchoscopy-guided ICG localization was calculated at 95.5%. The overall false negative ICG markings were 4.4% (20/458). Only in 1 study, the patients underwent bronchoscopic localization of their nodules the day before their scheduled lung resection (32). That was only offered to 24 patients of that study during an interval in which the department was setting up a hybrid thoracic theatre. There were no reported complications following ICG localization. The overall duration of endobronchial localization was reported by 75% (9/12) studies and had a reported range from 3 to 45 min. One study showed that localization time increased significantly when multiple nodules were localized and that was estimated at 9.75 ± 6.5 min, 11.35 ± 5.27 min and 24.54 ± 17.05 min for 1, 2 and 3 targets respectively (35).
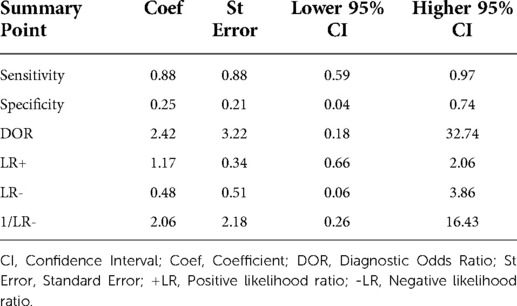
The 8 studies that investigated the effectiveness of ICG localization after peripheral IV administration reported enough data that allowed for bivariate model and HSROC to function (Tables 5, 6). The point estimates for Sensitivity and Specificity were 88% (95% CI: 59%–0.97%) and 25% (95% CI: 0.04%–0.74%) respectively (Figure 3). These results are illustrated in detail on the SROC curve and Table 6. The accuracy of IV ICG administration for malignant lung tumor localization was estimated at 83.5%. There were no ICG localization related complications in any of the patients. The time interval between ICG administration and the lung resection ranged from 12 h to 96 h but the dose was mostly unanimously administered at 5 mg/kg in 87.5% of the studies (7/8). The only studies that administered a smaller dose was a pediatric study and an adult study that later raised the dose to 5 mg/kg after poor localization success (43, 48).
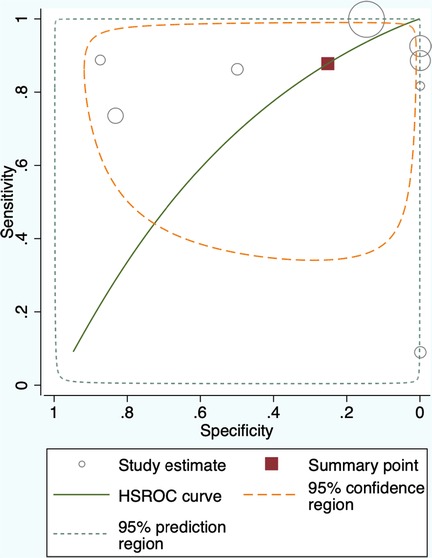
Figure 3. Summary Receiver Operating Characteristic (SROC) curve for Intravenous administration of Indocyanine Green for lung cancer localization. HSROC: hierarchical summary receiver operating characteristic.
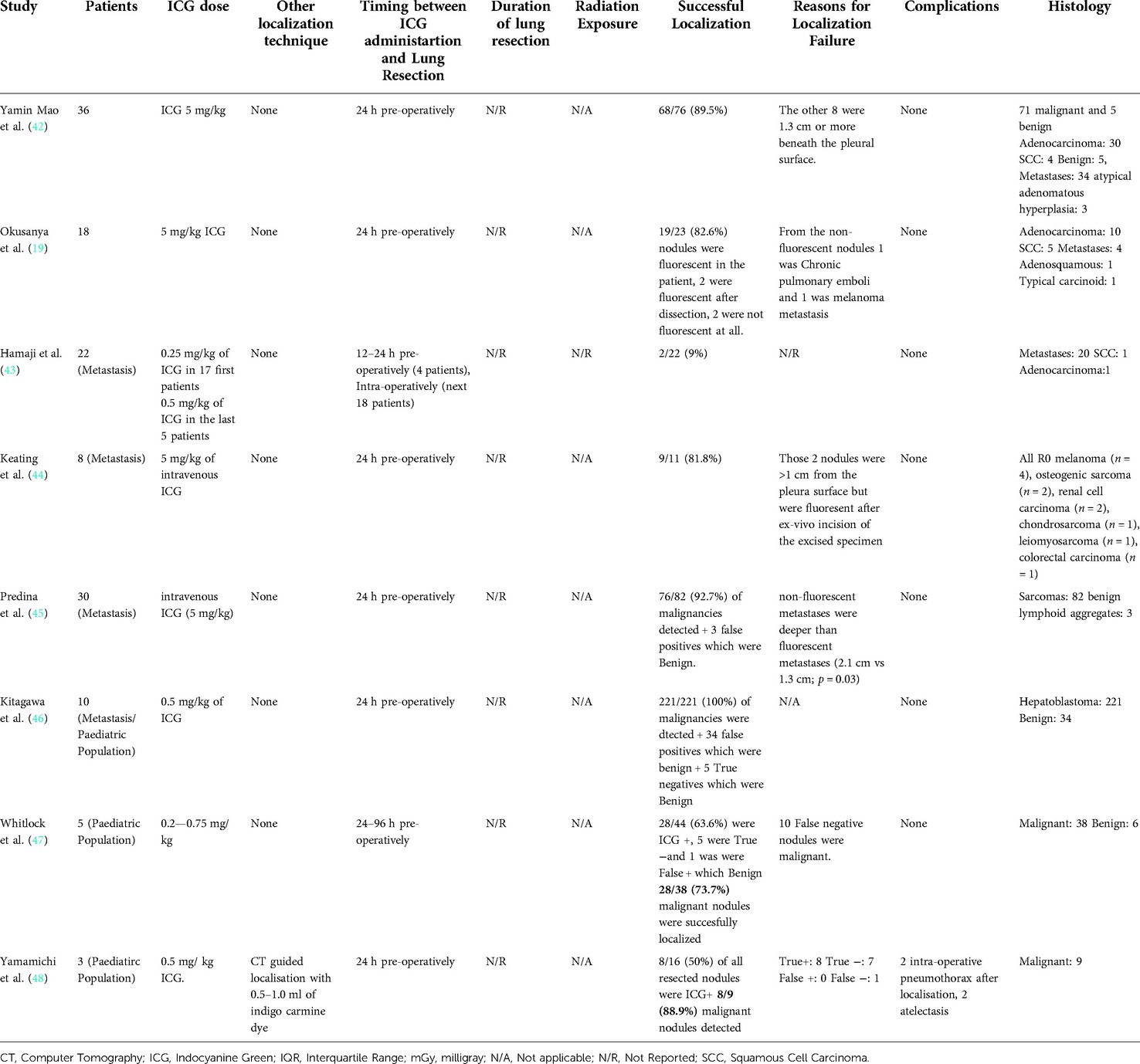
Table 5. Operative details and outcomes from studies that used intravenous Indocyanine Green localization.
Discussion
Following the 10-year follow-up results from the NELSON trial, the low-dose CT lung cancer screening showed reduced lung-cancer mortality among high-risk population and higher detection of earlier stage lung cancer (4). This finding in combination with the recent publications of the VIOLET trial and the study by the Japan Clinical Oncology Group/West Japan Oncology Group (JCOG/WJOG4607L) show a clear trend towards minimally-invasive techniques and anatomical sublobar resections for smaller lung cancers (5, 6). Therefore, localization techniques that will guide the operating surgeon to safely identify intra-operatively constantly smaller lung cancers, show an increased scientific interest over the last decade. In this study, we performed a thorough systematic review of the literature and meta-analysis on ICG localization techniques for detection of lung malignancies.
There have been several localization techniques already described in the literature for lung cancer. Hook-wire localization was one of the first described and most commonly used techniques which has also been widely performed in surgical oncology for other organs such as breast (49, 50). However, hook-wire insertion for lung cancer carries a relatively high rate of complications such as pneumothorax and wire migration or dislodgement (51). Furthermore, some studies have reported cases of massive air embolism following hook-wire insertion which is life-threatening complication (52, 53).
Micro-coil localization is another reported approach with documented high success rate (96.9%–100%) (54, 55). Their use for lung nodule localization in thoracoscopic surgery has been performed since 1994 (56). Micro-coils overcome some of the hook-wire complications as they are being deployed in the lung parenchyma without parts of wire being left outside the chest. Migration of the micro-coil has been described and can lead to procedure failure albeit less frequently compared to hook-wire (57). One of the main disadvantages in this technique is that it requires hybrid operating theatre as the lung resection has to be performed under fluoroscopy guidance in order to safely resect the targeted nodule. This demands adequately trained theatre staff and the team needs to wear lead-protecting equipment, to counteract the radiation exposure from fluoroscopy, which adds weight on the back of operating surgeons and could restrict dexterity and range of movement.
In attempt to resolve migration of metallic markers, researchers have focused on investigating multiple dyes and contrast agents that can identify lung nodules. These are Methylene Blue (MB), Indigo Carmine (IC), Barium, Lipiodol and Iopamidol (57). The latter three dyes, despite their successful intra-operative localization rate (93.3%–100%) require fluoroscopic guidance during lung resection. Furthermore, Barium localization induces some acute inflammatory response and edema on the surrounding lung parenchyma which can impair the histopathological diagnosis (55). MB, is a widely used dye for localization of peripheral lung nodules both under CT guidance and under bronchoscopy with good success rates (93.3%–100%) (57, 58). Even though it has shown satisfactory outcome, a significant restriction in its use, is the rapid diffusion and poor identification in severely anthracotic lungs (58). Furthermore, MB dyes the resected specimen which can create difficulties on histopathology assessment. IC is another dye which has been administered both percutaneously under CT-guidance and fluoroscopy but also under bronchoscopy with reported successful identification rate of 93.2% (95% CI: 90.8–95.1) (59, 60). Similarly to MB, the localization with IC is also difficult to be identified in the background of lung parenchyma with significant carbon particle deposition (55).
NIR imaging utilizes fluorescent dyes that emit in the near-infrared spectrum (700–900 nm) and offer high tissue penetration and low autofluorescence (11). Those two key features allow for sufficient contrast between the localized and non-localized area with ICG being a widely used representative of these dyes in clinical practice and medical research. ICG has increased tissue penetration at 820 nm and a greater “brightness” compared to its other NIR counterpart, MB, which emits at 700 nm and is subject to higher autofluorescence background (61). Another advantage from the use of ICG, compared to MB, is that it does not interfere with the surgical field without the use of NIR imaging system because it is otherwise non-visible to the human eye. Furthermore, ICG localized areas in patients with lung anthracosis are better visualized compared to IC or MB (55, 62). NIR imaging systems have been developed in order to detect the fluorescent dyes and aid thoracic surgeons intra-operatively in accurately detecting the localized lung nodules (63). This is perceived as a drawback for ICG because it requires specialized NIR equipment to identify the localized area. However, the availability of NIR thoracoscope cameras in thoracic departments could also be used for other parts of the procedure like the identification of intersegmental planes in anatomic sublobar lung resections (36). A second disadvantage is its poor detection of nodules that are located deep in the lung parenchyma. Even though ICG has shown better tissue penetration than the 0.5 mm of MB, its fluorescence diminshes in deep-seated nodule localization (62). Furthermore, ICG can diffuse easily in the surrounding lung parenchyma which could reduce its accuracy as a localization method. In attempt to address the two previously mentioned limitations, researchers have shown that a mixed solution of ICG with oil-based radiopaque agents, like Lipiodol can offer some benefits. That combination allows for improved localization of deep pulmonary nodules through the use of fluoroscopy and also reduces ICG diffusion (62). However, for the purposes of our study the papers that did not investigate solely the administration of ICG for localization were considered high risk of bias. This is because it was impossible to assess the true effect of ICG as a localization dye when also other techniques were in use in the same patients.
There has been a limited number of published studies that compared dye localization with other techniques. A study by Kleedehn et al. from 2016 that compared the use of hookwire with MB, showed no statistically significant difference on successful localization between the two methods (64). However, the hookwire group had more severe and frequent complications. Similarly to these findings, a more recent study by Ding et al. indicated that ICG localization also had lower complication rate compared to hookwire localization (22). Furthermore, even though the success rate was higher in the ICG group, it was also not statistically significant (22). A randomized trial from China which aims to compare ICG localization under electromagnetic navigation bronchoscopy (ENB) with percutaneous hookwire insertion could provide more evidence on the effectiveness and safety between the two techniques (NCT04182152).
To our knowledge, there are no studies available in the literature that have investigated a direct comparison between different localization dyes for lung cancer (55). For that reason, a meaningful comparison between ICG and other dyes was not possible and thus we focused our study's aim on assessing the evidence behind the effectiveness and safety amongst the different ICG localization techniques for lung cancer.
Through our systematic review, we identified three main techniques of ICG administration for lung cancer detection: percutaneous CT-guided, Intrabronchial and through peripheral Intravenous access.
CT-guided percutaneous localization of lung nodules has been widely used and is considered a well-established technique. Specifically, after using ICG as a marker, our study has shown a high reported success rate on tumor localization (96.7%) with false negative rate of merely 2.4% (26/1089). Our study also verifies the experiences from previous researchers which have shown a high incidence of complications post-localization with markers other than ICG. We found a complication rate of 15.2% (150/989) amongst patients who underwent percutaneous CT-guided localization with ICG. However, it is important to highlight that none of the cases that developed a small pneumothorax or small intra-parenchymal haematoma required any further intervention while only 11/989 patients experienced symptoms such as dyspnea, chest pain or mild hemoptysis after localization. Furthermore, the additional radiation exposure is another theoretical drawback form this procedure for which we could not collect significant evidence from our systematic review due to lack of available data.
Emerging evidence suggests that the electromagnetic navigation bronchoscopy-guided dye marking could counteract some of the problems encountered in CT-guided localization (65, 66). Our study also confirmed a high success rate of 95.4% for pre-operative localization of pulmonary nodules with ICG under bronchoscopy. We did not observe any complications related to ICG localization through this method in the literature. We assume that this can be attributed to the “single-stage” approach between localization and lung resection because the patient remains anaesthetized following localization and thus their symptoms of pain, cough or discomfort from the latter may overlap with those that the patient experiences post-operatively. Furthermore, endobronchial localization offers better access to tumors which may be located to a more central position or close to great vessels. However, effective endobronchial localization demands skillful operators who would be able to perform this procedure and train their more junior colleagues.
Finally, we reviewed the evidence behind IV administration of ICG for detection of lung malignancy. ICG is not a lung cancer specific marker but it tends to accumulate in certain tumors due to the enhanced permeability and retention (EPR) effect (67, 68). This occurs due to high permeability of the porous tumor blood vessels which results in accumulation of ICG. The retention effect is caused by the dysfunctional lymphatic system of the tumor which subsequently leads ICG to remain around the tumor's micro-environment for long enough period to make it detectable intra-operatively.
Our meta-analysis showed that IV administration of ICG has Sensitivity of 88% (95% CI: 59%–0.97%) and Specificity of 25% (95% CI: 0.04%–0.74%) in detecting lung malignancy. The wide confidence interval for Specificity reflects the small number of true negative incidences reported in the studies. Similarly, to the bronchoscopy technique, we did not find any localization-related complications among the studies that investigated IV ICG. The theoretical advantage in IV administration of ICG compared to the other two techniques is that it can identify lung nodules which may not even be detectable pre-opratively by current imaging modalities. There has been an increasing interest over the last 5 years to create NIR substances that are specific to lung cancer cells and could therefore provide more accurate tumor localization. The most widely researched are the two folate analogs EC17 and OTL38 and the oral photosensitizer 5-ALA (69).
Overall, our study shows that ICG has demonstrated high success rates as a localization dye for lung cancer. In order to accurately interpret that finding, it is important to acknowledge that some tumor-specific characteristics were incorporated in the eligibility criteria of studies that investigated different ICG localization techniques (19–48). Those frequently included the size of the nodules and their distance from the visceral pleura. In more detail, several CT-guided ICG localization studies enrolled only patients with small (<3 cm) nodules, located peripherally in the lung parenchyma at a distance of less than 3 cm from the visceral pleura (21, 23, 24, 31, 32). On the other hand, in bronchoscopy-guided ICG studies, patients were recruited when they had smaller nodules (<1 cm) on their pre-operative imaging and those were located deeper in the lung parenchyma (>1 cm from the visceral pleura) (35–38). Finally, none of the studies that investigated the IV ICG administration restricted their eligibility criteria based on tumor size or distance from the visceral pleura (19, 42–48). This discrepancy, compared to the other two localization techniques, reflects the aim of these studies, which was to identify intra-operatively even malignant nodules which were not visible on pre-operative imaging. Therefore, despite lack of general consensus, there appears to be a trend among researchers to localize lesions which are located deeper in the lung parenchyma via bronchoscopy whereas more superficial nodules were localized via CT-guidance.
The timing between administration of ICG and lung resection is essential, as the evidence suggests that it can affect the level of fluorescence in NIR imaging (62, 70). For CT- and bronchoscopy-guided ICG localization, we identified great discrepancy on time protocols between the eligible studies. Some studies reported the average time (26), others the median time (27, 31) while others only described that they proceeded with lung resection immediately after localization without providing any numerical data (24, 28, 29). The lack of homogeneity among time reporting values and the absence of analysis of the time interval between ICG localization and lung resection as a parameter that can affect successful NIR identification of ICG among the published literature does not allow for a meaningful data synthesis. For that reason, we could not accurately conclude on a specific time-interval as the ideal period before proceeding with lung resection after localization. However, it appears to be a consensus among the researchers, that lung resection is performed in less than 24 h after ICG localization. In more detail, 6/13 studies performed lung resection either immediately after CT-guided ICG localization or within 60 min (21, 22, 24, 26, 28, 29) and 5/13 within the same day (23, 25, 27, 31, 32). Similarly, from the articles that investigated bronchoscopy-guided ICG localization, in 6/12 the surgeons proceeded with lung resection immediately after localization or within 60 min (32, 33, 35, 36, 38, 40) while in 3/12 the operation was performed within 24 h (31, 32, 37). From the 8 studies that investigated the IV ICG localization, only one study administered the ICG earlier than 24 h before lung resection (43) and only one study after 24 h (47). These two studies showed the lowest successful localization estimated at 9% (43) and 73.7% respectively (47). Therefore, it appears that the consensus among most researchers (75%, 6/8) is that ICG should be administered intravenously 24 h pre-operatively (19, 42, 44, 45, 46, 48).
There are some limitations in our study's findings which derive from the design of the included studies in our review. Due to the predominantly small sample sized, single centered, retrospective, observational studies, our results should be interpreted with caution. Also, there is inevitably significant bias introduced in our results because of variations on treatment and operating protocols among the different units. Given that there are no available data in the literature from meaningful comparison between different ICG localization techniques or different dyes, we were not able to perform any analysis on this topic. Furthermore, is important to mention that the absence of allergic reactions from our findings could have been influenced by the exclusion of patients with iodine allergies from the included studies.
Nevertheless, our study offers an updated and thorough review on how effective is ICG in detecting pulmonary malignancies. Our findings will complement further research projects that will advance evidence-based medicine in thoracic surgery. Large prospective multicenter studies are required in order to safely validate our results. Presumably, a 2-arm, multicentre, randomised, surgeon-blinded, parallel design clinical trial that will compare CT- with bronchoscopy-guided ICG localization of lung nodules will provide meaningful data. The two groups should be stratified according to nodule size and their distance from the visceral pleura which could allow for further sensitivity analysis of their effect on successful ICG localization.
The design of standardized localization protocols will be a key component in future research projects in thoracic surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
FM and AG contributed to conception and design of the study. AG and SL organized the database. AG performed the statistical analysis. AG wrote the first draft of the manuscript. SL and FM wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.967897/full#supplementary-material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Canadian Cancer Society. Lung cancer statistics. Available from: http://www.cancer.ca/en/cancer-information/cancer-type/lung/statistics/?region=on (Accessed June 4, 2022).
3. Diederich S, Thomas M, Semik M, Lenzen H, Roos N, Weber A, et al. Screening for early lung cancer with low-dose spiral computed tomography: results of annual follow-up examinations in asymptomatic smokers. Eur Radiol. (2004) 14(4):691–702. doi: 10.1007/s00330-003-2200-5
4. de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. (2020) 382(6):503–13. doi: 10.1056/NEJMoa1911793
5. Lim E, Batchelor Tim JP, Dunning J, Shackcloth M, Anikin V, Naidu B, et al. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. NEJM Evid. (2022) 1(3): EVIDoa2100016. doi: 10.1056/EVIDoa2100016
6. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy vs. lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. (2022) 399(10335):1607–17. doi: 10.1016/S0140-6736(21)02333-3
7. Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. (1999) 115(2):563–8. doi: 10.1378/chest.115.2.563
8. Saito H, Minamiya Y, Matsuzaki I, Tozawa K, Taguchi K, Nakagawa T, et al. Indication for preoperative localization of small peripheral pulmonary nodules in thoracoscopic surgery. J Thorac Cardiovasc Surg. (2002) 124(6):1198–202. doi: 10.1067/mtc.2002.127331
9. Powell TI, Jangra D, Clifton JC, Lara-Guerra H, Church N, English J, et al. Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg. (2004) 240(3):481–8; discussion 488–9. doi: 10.1097/01.sla.0000137132.01881.57
10. Zaman M, Bilal H, Woo CY, Tang A. In patients undergoing video-assisted thoracoscopic surgery excision, what is the best way to locate a subcentimetre solitary pulmonary nodule in order to achieve successful excision? Interact Cardiovasc Thorac Surg. (2012) 15(2):266–72. doi: 10.1093/icvts/ivs068
11. Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. (2011) 104(3):323–32. doi: 10.1002/jso.21943
12. Ferrari-Light D, Geraci TC, Sasankan P, Cerfolio RJ. The utility of near-infrared fluorescence and indocyanine green during robotic pulmonary resection. Front Surg. (2019) 6(47). doi: 10.3389/fsurg.2019.00047.
13. Newton AD, Predina JD, Nie S, Low PS, Singhal S. Intraoperative fluorescence imaging in thoracic surgery. J Surg Oncol. (2018) 118(2):344–55. doi: 10.1002/jso.25149
14. Newton AD, Kennedy GT, Predina JD, Low PS, Singhal S. Intraoperative molecular imaging to identify lung adenocarcinomas. J Thorac Dis. (2016) 8(Suppl 9):S697–704. doi: 10.21037/jtd.2016.09.50
15. Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of google scholar in evidence reviews and its applicability to grey literature searching. PLoS One. (2015) 10(9):e0138237. doi: 10.1371/journal.pone.0138237
16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019.
17. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
18. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol. (2015) 16(6):1188–96. doi: 10.3348/kjr.2015.16.6.1188
19. Okusanya OT, Holt D, Heitjan D, Deshpande C, Venegas O, Jiang J, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg. (2014) 98(4):1223–30. doi: 10.1016/j.athoracsur.2014.05.026
20. Hsu PK, Lee YY, Chuang LC, Ting CK, Tsou MY. Nonintubated versus intubated “one-stage” preoperative localization and thoracoscopic lung resection. JTCVS Tech. (2021) 10:517–25. doi: 10.1016/j.xjtc.2021.09.032
21. Wu Z, Zhang L, Zhao X, Zhou D, Yang XY. Localization of subcentimeter pulmonary nodules using an indocyanine green near-infrared imaging system during uniportal video-assisted thoracoscopic surgery. J Cardiothorac Surg. (2021) 16(1):224. doi: 10.1186/s13019-021-01603-x
22. Ding N, Wang K, Cao J, Hu G, Wang Z, Jin Z. Targeted near-infrared fluorescence imaging with iodized indocyanine green in preoperative pulmonary localization: comparative efficacy, safety, patient perception with hook-wire localization. Front Oncol. (2021) 11:707425. doi: 10.3389/fonc.2021.707425.
23. Li X, Xu K, Cen R, Deng J, Hao Z, Liu J, et al. Preoperative computer tomography-guided indocyanine green injection is associated with successful localization of small pulmonary nodules. Transl Lung Cancer Res. (2021) 10(5):2229–36. doi: 10.21037/tlcr-21-425
24. Zhang C, Lin H, Fu R, Zhang T, Nie Q, Dong S, et al. Application of indocyanine green fluorescence for precision sublobar resection. Thorac Cancer. (2019) 10(4):624–30. doi: 10.1111/1759-7714.12972
25. Li A, Chan S, Thung KH. Pre-operative CT localization for patients with subsolid opacities expecting video-assisted thoracoscopic surgery-single center experience of fluorescent iodized emulsion and hook-wire localization technique. Br J Radiol. (2020) 93(1109):20190938. doi: 10.1259/bjr.20190938
26. Chang CJ, Lu CH, Gao X, Fang HY, Chao YK. Safety and efficacy of cone-beam computed tomography-guided lung tumor localization with a near-infrared marker: a retrospective study of 175 patients. Life (Basel. (2022) 12(4):494. doi: 10.3390/life12040494
27. Nagai K, Kuriyama K, Inoue A, Yoshida Y, Takami K. Computed tomography-guided preoperative localization of small lung nodules with indocyanine green. Acta Radiol. (2018) 59(7):830–5. doi: 10.1177/0284185117733507
28. Ujiie H, Kato T, Hu HP, Patel P, Wada H, Fujino K, et al. A novel minimally invasive near-infrared thoracoscopic localization technique of small pulmonary nodules: a phase I feasibility trial. J Thorac Cardiovasc Surg. (2017) 154(2):702–11. doi: 10.1016/j.jtcvs.2017.03.140
29. Zhong L, Hu W, Li S, Wei Z, Zhu Z, Jin G, et al. Clinical study of video-assisted thoracoscopic surgery wedge resection in early-stage lung cancer by tumor mapping with indocyanine green. Wideochir Inne Tech Maloinwazyjne. (2019) 14(4):545–50. doi: 10.5114/wiitm.2019.89986
30. Yang YL, Li ZZ, Huang WC, Zhuang J, Lin DY, Zhong WZ, et al. Electromagnetic navigation bronchoscopic localization versus percutaneous CT-guided localization for thoracoscopic resection of small pulmonary nodules. Thorac Cancer. (2021) 12(4):468–74. doi: 10.1111/1759-7714.13775
31. Kim Y, Rho J, Quan YH, Choi BH, Han KN, Kim HK, et al. Simultaneous visualization of pulmonary nodules and intersegmental planes on fluorescent images in pulmonary segmentectomy. Eur J Cardiothorac Surg. (2020) 58(Suppl_1):i77–84. doi: 10.1093/ejcts/ezaa064
32. Anayama T, Yamamoto M, Hirohashi K, Miyazaki R, Okada H, Doi A, et al. The accuracy of cone-beam computed tomography and augmented fluoroscopy-guided bronchoscopic marking of multiple small-sized pulmonary nodules in a hybrid operating room: a retrospective cohort study. Quant Imaging Med Surg. (2021) 11(2):725–36. doi: 10.21037/qims-20-781
33. Sekine Y, Koh E, Hoshino H. The efficacy of transbronchial indocyanine green instillation for fluorescent-guided wedge resection. Interact Cardiovasc Thorac Surg. (2021) 33(1):51–9. doi: 10.1093/icvts/ivab054
34. Yanagiya M, Amano Y, Hiyama N, Matsumoto J. Initial experience of virtual-assisted lung mapping utilizing both indocyanine green and indigo carmine. Gen Thorac Cardiovasc Surg. (2021) 69(6):1035–9. doi: 10.1007/s11748-020-01565-2
35. Zhang J, He J, Chen J, Zhong Y, He J, Li S. Application of indocyanine green injection guided by electromagnetic navigation bronchoscopy in localization of pulmonary nodules. Transl Lung Cancer Res. (2021) 10(12):4414–22. doi: 10.21037/tlcr-21-699
36. Geraci TC, Ferrari-Light D, Kent A, Michaud G, Zervos M, Pass HI, et al. Technique, outcomes with navigational bronchoscopy using indocyanine green for robotic segmentectomy. Ann Thorac Surg. (2019) 108(2):363–9. doi: 10.1016/j.athoracsur.2019.03.032
37. Yang SM, Yu KL, Lin KH, Liu YL, Sun SE, Meng LH, et al. Real-time augmented fluoroscopy-guided lung marking for thoracoscopic resection of small pulmonary nodules. Surg Endosc. (2020) 34(1):477–84. doi: 10.1007/s00464-019-06972-y
38. Abbas A, Kadakia S, Ambur V, Muro K, Kaiser L. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg. (2017) 153(6):1581–90. doi: 10.1016/j.jtcvs.2016.12.044
39. Ng CSH, Zhao Z, Long H, Lau RWH. Electromagnetic navigation bronchoscopy triple contrast dye marking for lung nodule localization. Thorac Cardiovasc Surg. (2020) 68(3):253–5. doi: 10.1055/s-0038-1676964
40. Hachey KJ, Digesu CS, Armstrong KW, Gilmore DM, Khullar OV, Whang B, et al. A novel technique for tumor localization and targeted lymphatic mapping in early-stage lung cancer. J Thorac Cardiovasc Surg. (2017) 154(3):1110–8. doi: 10.1016/j.jtcvs.2016.12.058
41. Harris K, Schaefer E, Rosenblum J, Stewart FD, Arkovitz MS. Intraoperative electromagnetic navigation bronchoscopy (IENB) to localize peripheral lung lesions: a new technique in the pediatric oncology population. J Pediatr Surg. (2021) S0022-3468(21)00783-1. doi: 10.1016/j.jpedsurg.2021.11.011
42. Mao Y, Chi C, Yang F, Zhou J, He K, Li H, et al. The identification of sub-centimetre nodules by near-infrared fluorescence thoracoscopic systems in pulmonary resection surgeries. Eur J Cardiothorac Surg. (2017) 52(6):1190–6. doi: 10.1093/ejcts/ezx207
43. Hamaji M, Chen-Yoshikawa TF, Minami M, Date H. Near-infrared imaging using intravenous indocyanine green at a conventional dose to locate pulmonary metastases: a pilot study. Thorac Cardiovasc Surg. (2019) 67(8):688–91. doi: 10.1055/s-0038-1675346
44. Keating J, Newton A, Venegas O, Nims S, Zeh R, Predina J, et al. Near-infrared intraoperative molecular imaging can locate metastases to the lung. Ann Thorac Surg. (2017) 103(2):390–8. doi: 10.1016/j.athoracsur.2016.08.079
45. Predina JD, Newton AD, Corbett C, Shin M, Sulfyok LF, Okusanya OT, et al. Near-infrared intraoperative imaging for minimally invasive pulmonary metastasectomy for sarcomas. J Thorac Cardiovasc Surg. (2019) 157(5):2061–9. doi: 10.1016/j.jtcvs.2018.10.169
46. Kitagawa N, Shinkai M, Mochizuki K, Usui H, Miyagi H, Nakamura K, et al. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr Surg Int. (2015) 31(4):407–11. doi: 10.1007/s00383-015-3679-y
47. Whitlock RS, Patel KR, Yang T, Nguyen HN, Masand P, Vasudevan SA. Pathologic correlation with near infrared-indocyanine green guided surgery for pediatric liver cancer. J Pediatr Surg. (2022 Apr) 57(4):700–10. doi: 10.1016/j.jpedsurg.2021.04.019
48. Yamamichi T, Nishikawa M, Takayama K, Takase K, Kim K, Umeda S, et al. Computed tomography-guided marking using a dye-staining method for preoperative localization of tiny pulmonary lesions in children. Pediatr Surg Int. (2021) 37(9):1265–72. doi: 10.1007/s00383-021-04930-1
49. Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis. (2016) 8(Suppl 9):S749–55. doi: 10.21037/jtd.2016.09.71
50. Dimitrovska MJ, Mitreska N, Lazareska M, Jovanovska ES, Dodevski A, Stojkoski A. Hook wire localization procedure and early detection of breast cancer - our experience. Open Access Maced J Med Sci. (2015) 3(2):273–7. doi: 10.3889/oamjms.2015.055
51. Iguchi T, Hiraki T, Matsui Y, Fujiwara H, Masaoka Y, Uka M, et al. Short hookwire placement under imaging guidance before thoracic surgery: A review. Diagn Interv Imaging. (2018) 99(10):591–7. doi: 10.1016/j.diii.2018.04.001
52. Horan TA, Pinheiro PM, Araújo LM, Santiago FF, Rodrigues MR. Massive gas embolism during pulmonary nodule hook wire localization. Ann Thorac Surg. (2002) 73:1647–9. doi: 10.1016/S0003-4975(01)03371-9
53. Sakiyama S, Kondo K, Matsuoka H, Yoshida M, Miyoshi T, Yoshida S, et al. Fatal air embolism during computed tomography-guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg. (2003) 126:1207–9. doi: 10.1016/S0022-5223(03)00821-3
54. Su TH, Fan YF, Jin L, He W, Hu LB. CT-guided localization of small pulmonary nodules using adjacent microcoil implantation prior to video-assisted thoracoscopic surgical resection. Eur Radiol. (2015) 25(9):2627–33. doi: 10.1007/s00330-015-3676-5.
55. Lee JW, Park CH, Lee SM, Jeong M, Hur J. Planting seeds into the lung: image-guided percutaneous localization to guide minimally invasive thoracic surgery. Korean J Radiol. (2019) 20(11):1498–514. doi: 10.3348/kjr.2019.0155
56. Asamura H, Kondo H, Naruke T, Tsuchiya R, Wakao F, Kaneko M, et al. Computed tomography-guided coil injection and thoracoscopic pulmonary resection under roentgenographic fluoroscopy. Ann Thorac Surg. (1994) 58:1542–4. doi: 10.1016/0003-4975(94)91957-7
57. Park CH, Han K, Hur J, Lee SM, Lee JW, Hwang SH, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. (2017) 151:316–28. doi: 10.1016/j.chest.2016.09.017
58. Lin CY, Chang CC, Huang LT, Chung TJ, Liu YS, Yen YT, et al. Computed tomography-guided methylene blue localization: single vs. multiple lung nodules. Front Med (Lausanne). (2021) 8:661956. doi: 10.3389/fmed.2021.661956
59. Hasegawa T, Kuroda H, Sato Y, Matsuo K, Sakata S, Yashiro H, et al. The utility of indigo carmine and lipiodol mixture for preoperative pulmonary nodule localization before video-assisted thoracic surgery. J Vasc Interv Radiol. (2019) 30(3):446–52. doi: 10.1016/j.jvir.2018.08.024
60. Sato M, Kobayashi M, Kojima F, Tanaka F, Yanagiya M, Kosaka S, et al. Effect of virtual-assisted lung mapping in acquisition of surgical margins in sublobar lung resection. J Thorac Cardiovasc Surg. (2018) 156(4):1691–1701.e5. doi: 10.1016/j.jtcvs.2018.05.122
61. Matsui A, Tanaka E, Choi HS, Winer JH, Kianzad V, Gioux S, et al. Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery. (2010) 148(1):87–95. doi: 10.1016/j.surg.2009.12.004
62. Rho J, Lee JW, Quan YH, Choi BH, Shin BK, Han KN, et al. Fluorescent and iodized emulsion for preoperative localization of pulmonary nodules. Ann Surg. (2021) 273(5):989–96. doi: 10.1097/SLA.0000000000003300. PMID: 30973387.
63. Mao Y, Wang K, He K, Ye J, Yang F, Zhou J, et al. Development and application of the near-infrared and white-light thoracoscope system for minimally invasive lung cancer surgery. J Biomed Opt. (2017) 22(6):66002. doi: 10.1117/1.JBO.22.6.066002
64. Kleedehn M, Kim DH, Lee FT, Lubner MG, Robbins JB, Ziemlewicz TJ, et al. Preoperative pulmonary nodule localization: a comparison of methylene blue and hookwire techniques. AJR Am J Roentgenol. (2016) 207(6):1334–9. doi: 10.2214/AJR.16.16272
65. Marino KA, Sullivan JL, Weksler B. Electromagnetic navigation bronchoscopy for identifying lung nodules for thoracoscopic resection. Ann Thorac Surg. (2016) 102:454–7. doi: 10.1016/j.athoracsur.2016.03.010
66. Awais O, Reidy MR, Mehta K, et al. Electromagnetic navigation bronchoscopy-guided dye marking for thoracoscopic resection of pulmonary nodules. Ann Thorac Surg. (2016) 102:223–9. doi: 10.1016/j.athoracsur.2016.02.040
67. Satou S, Ishizawa T, Masuda K, Kaneko J, Aoki T, Sakamoto Y, et al. Indocyanine green fluorescent imaging for detecting extrahepatic metastasis of hepatocellular carcinoma. J Gastroenterol. (2013) 48:1136–43. doi: 10.1007/s00535-012-0709-6
68. Tummers QR, Hoogstins CE, Peters AA, de Kroon CD, Trimbos JB, Cj VDV, et al. The value of intraoperative near-infrared fluorescence imaging based on enhanced permeability and retention of indocyanine green: feasibility and false-positives in ovarian cancer. PLoS One. (2015) 10(6):e0129766. doi: 10.1371/journal.pone.0129766
69. Matsuura Y, Ichinose J, Nakao M, Okumura S, Mun M. Recent fluorescence imaging technology applications of indocyanine green in general thoracic surgery. Surg Today. (2020 Nov) 50(11):1332–42. doi: 10.1007/s00595-019-01906-6
Keywords: Indocyianine green, ICG, pulmonary nodules, lung surgery, lung malignancy
Citation: Gkikas A, Lampridis S, Patrini D, Scarci M and Minervini F (2022) How effective is indocyanine green (ICG) in localization of malignant pulmonary nodules? A systematic review and meta-analysis. Front. Surg. 9:967897. doi: 10.3389/fsurg.2022.967897
Received: 13 June 2022; Accepted: 11 July 2022;
Published: 25 July 2022.
Edited by:
Luca Bertolaccini, European Institute of Oncology (IEO), ItalyReviewed by:
Wentao Fang, Shanghai Jiao Tong University, ChinaAkif Turna, Istanbul University-Cerrahpasa, Turkey
Marco Schiavon, University Hospital of Padua, Italy
© 2022 Gkikas, Lampridis, Patrini, Kestenholz, Scarci and Minervini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabrizio Minervini ZmFicml6aW9taW5lcnZpbmlAaG90bWFpbC5jb20=
†These authors share last authorship
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Andreas Gkikas
Andreas Gkikas Savvas Lampridis
Savvas Lampridis Davide Patrini3
Davide Patrini3 Marco Scarci
Marco Scarci Fabrizio Minervini
Fabrizio Minervini