
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Surg. , 12 September 2022
Sec. Cardiovascular Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.966410
Post-operative pericardial adhesions remain a serious complication after cardiac surgery that can lead to increased morbidity and mortality. Fibrous adhesions can destroy tissue planes leading to injury of surrounding vasculature, lengthening of operation time, and increased healthcare costs. While animal models are necessary for studying the formation and prevention of post-operative pericardial adhesions, a standardized animal model for inducing post-operative pericardial adhesions has not yet been established. In order to address this barrier to progress, an analysis of the literature on animal models for post-operative pericardial adhesions was performed. The animal model, method used to induce adhesions, and the time to allow development of adhesions were analyzed. Our analysis found that introduction of autologous blood into the pericardial cavity in addition to physical abrasion of the epicardium caused more severe adhesion formation in comparison to abrasion alone or abrasion with desiccation (vs. abrasion alone p = 0.0002; vs. abrasion and desiccation p = 0.0184). The most common time frame allowed for adhesion formation was 2 weeks, with the shortest time being 10 days and the longest being 12 months. Finally, we found that the difference in adhesion severity in all animal species was similar, suggesting the major determinants for the choice of model are animal size, animal cost, and the availability of research tools in the particular model. This survey of the literature provides a rational guide for researchers to select the appropriate adhesion induction modality, animal model, and time allowed for the development of adhesions.
Post-operative pericardial adhesions remain an unsolved problem in cardiac surgery. Clinically, the most important aspects of post-operative pericardial adhesion formation are significantly increased morbidity and mortality during re-operative cardiac surgery. Re-operative cardiac surgery is particularly common in congenital cardiac surgery. An analysis of 2,555 cardiac re-operations at the Mayo Clinic revealed that iatrogenic injuries occurred in 9% of cases (1). Whereas the hospital mortality rate was 6.5% among patients without injury, this increased to 18.5% among those with injury; and the mortality rate was 25% when injury occurred during sternal division (1). The mechanism by which post-operative pericardial adhesions cause this dramatically increased mortality is obliteration of tissue planes. This puts vital structures such as the aorta, coronary arteries, right ventricle and right atrium at risk for injury during sternal re-entry and dissection. Apart from affecting patient outcomes, post-operative pericardial adhesions also have important implications for healthcare economics by significantly increasing operative time and healthcare costs.
For these reasons there is an urgent clinical need for improved strategies for prevention and treatment of post-operative pericardial adhesions. This is the focus of an intensive research effort underpinned by a wide variety of animal models. However, while animal models to study post-operative pericardial adhesions are legion, an analysis of the available animal models has not yet been performed. This is a critical barrier to progress in the field because the animal models employed are highly heterogeneous and results cannot be easily compared. Here we address this barrier to progress by analyzing the literature on animal models for post-operative pericardial adhesions.
In order to determine a standardized animal model for studying pericardial adhesions, studies were evaluated to determine the most effective method to induce adhesion formation. A literature search for relevant articles was conducted using PubMed and Google Scholar from the inception of the respective databases (Figure 1). Search strategies were “pericardial adhesions”, “intrapericardial adhesions”, “adhesions” and “cardiac surgery”, “adhesions” and “heart surgery”, “pericardial barrier”, and “pericardial membrane”. Inclusion criteria for the search were animal studies published prior to 2021 that evaluated post-operative pericardial adhesions that included a negative control group in which no intervention was used to prevent adhesions. Exclusion criteria were studies that were published in a language other than English and studies that did not provide numerical data nor an adhesion score based on a finite scale.
The appropriate studies were selected, and the negative control groups of each study were compared to determine which models produced the most severe post-operative pericardial adhesions. The variables analyzed included animal model, number of animals in the control group, method used to induce pericardial adhesions, length of time allowed for adhesion formation, and grading of the adhesions. In order to account for the various methods to grade adhesions used by each study, all reported adhesion scores were converted to a standardized severity score out of 10 which allowed for comparison of adhesion severity between all studies (Equation 1). A mean severity score and standard deviation were calculated for each study. A single pooled mean and standard deviation was calculated for studies that used a similar animal model or method (Equations 2, 3) (2). Statistical analyses and graphing were performed in Prism v5 and Python with the Matplotlib and NumPy libraries.
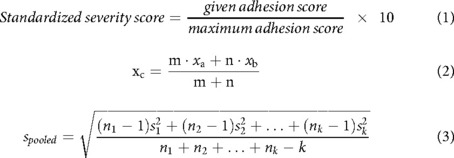
Equations 1–3. Formulas used to calculate standardized adhesion score, pooled mean, and standard deviation.
A total of 41 studies involving animal models for post-operative pericardial adhesions were identified. One study included both a pig and rabbit model which were evaluated separately (3).
The animal species used were mouse (2% of studies, n = 1 study with n = 4 total animals) (4), rat (7%, n = 3 studies with n = 28 total animals) (5–7), hamster (2%, n = 1 study with n = 11 total animals) (8), rabbit (44%, n = 18 studies with n = 235 total animals) (3, 9–25), canine (19%, n = 8 studies with n = 66 total animals) (26–33), pig (19%, n = 8 studies with n = 53 total animals) (3, 34–40), and sheep (7%, n = 3 studies with n = 15 total animals) (41–43) (Table 1).
The tendency of severe postoperative adhesions to form in various animal models irrespective of the method of traumatization was compared (Figure 2). The pig models demonstrated more severe adhesion formation in comparison to all other animal models with the exception of hamster (vs. rabbit p < 0.0001; vs. canine p < 0.0001; vs. sheep p < 0.0001; vs. rat p < 0.0001; vs. mouse p < 0.0196). The rabbit model was most frequently used and showed greater adhesion severity compared to canine and rat (vs. canine p = 0.0045; vs. rat p < 0.0001).
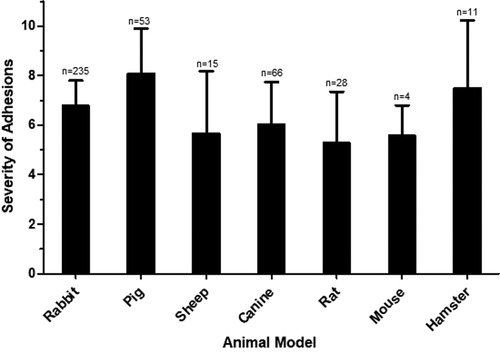
Figure 2. Severity of adhesions in various animal models. The mean severity score for each animal model is shown, with error bars representing standard deviation. N represents the number of animals used in each model.
The modalities used to induce post-operative pericardial adhesions in animal models were: pericardial excision only, physical abrasion of the epicardium, abrasion of the epicardium in addition to desiccation, abrasion of the epicardium with introduction of autologous blood into the pericardial cavity, injection of talcum, institution of extracorporeal membrane oxygenation (ECMO) and simulated cardiopulmonary bypass cannulation through various dissection and suturing techniques. Each method's effectiveness at inducing post-operative adhesion formation was analyzed by comparing the severity of pericardial adhesions formed (Figure 3). Animals that only underwent pericardial excision had significantly less severe post-operative adhesions than all of the other methods with the exception of institution of ECMO and injection of talcum (vs. abrasion p < 0.0001; vs. abrasion and desiccation p < 0.0001; vs. abrasion and blood p < 0.0001; vs. simulated bypass p < 0.0001). Introduction of autologous blood into the pericardial cavity in addition to physical abrasion caused the formation of more severe adhesions in comparison to abrasion alone or abrasion with desiccation (vs. abrasion alone p = 0.0002; vs. abrasion and desiccation p = 0.0184). Injection of talcum was less effective at inducing severe adhesions compared to all of the abrasion models and simulation of cardiopulmonary bypass and was not significantly better at inducing severe adhesions than performing pericardial excision alone (vs. abrasion p = 0.0004; vs. abrasion and desiccation p < 0.0001, vs. abrasion and blood p < 0.0001, vs. simulated bypass p < 0.0001, vs. pericardial excision only p = 0.946).

Figure 3. Effect of method of adhesion induction on severity of adhesions. The mean severity score for each method is shown, with error bars representing standard deviation. N represents the number of animals used per method.
Animal models to study post-operative pericardial adhesions are numerous, but highly heterogeneous. Our systematic analysis of the literature identified a total of 42 animal models. We analyzed these models with regards to the mechanism for adhesion induction, the animal species used, and the timing of analysis.
Pericardial adhesions are a physiologic response to mediastinal trauma that aims to seal off vascular injuries and thus ensure survival of the organism. This involves a cascade of biochemical and cellular events with an imbalance of inflammatory responses, coagulation mechanisms, angiogenesis and fibrinolysis (44). Broadly, pericardial adhesion formation is initiated by the deposition of fibrin, and the formation of fibrin bridges between mediastinal tissues. Subsequent cellular organization of these fibrin bridges by fibroblast migration and vascularization under the influence of inflammatory growth factors results in the formation of permanent adhesions (45–47). This cascade can be triggered by a wide variety of traumatic stimuli.
In the literature, seven different methods were used to induce postoperative pericardial adhesions, namely, pericardial excision only, physical abrasion of the epicardium, abrasion of the epicardium in addition to desiccation, abrasion of the epicardium with introduction of autologous blood into the pericardial cavity, and injection of talcum. In an effort to more closely replicate the conditions of cardiothoracic surgery, one study instituted ECMO (42) and others simulated cardiopulmonary bypass cannulation through various dissection and suturing techniques (31, 37, 39). Mitchel et al. hypothesized that while mild mesothelial injury alone and blood clots inside an uninjured serosal cavity are not sufficient to induce pericardial adhesions on their own, the combination of these conditions can facilitate adhesion formation (31). Our findings support this conclusion, as introduction of autologous blood into the pericardial cavity in addition to physical abrasion was found to induce more severe post-operative adhesions compared to abrasion alone or abrasion with desiccation (vs. abrasion alone p = 0.0002; vs. abrasion and desiccation p = 0.0184).
In contrast, animals that only underwent pericardial excision had significantly less severe post-operative adhesions than all of the other methods with the exception of institution of ECMO and injection of talcum, indicating that pericardial excision alone is not sufficient to induce post-operative adhesions that are representative of clinical conditions.
While administration of talcum into the pericardial space allows for a simpler way to induce adhesions without performing a thoracotomy, we found injection of talcum was less effective at inducing severe adhesions compared to all of the abrasion models and simulation of cardiopulmonary bypass, and was not significantly better at inducing severe adhesions than performing pericardial excision alone (vs. abrasion p = 0.0004; vs. abrasion and desiccation p < 0.0001, vs. abrasion and blood p < 0.0001, vs. simulated bypass p < 0.0001, vs. pericardial excision only p = 0.946). However, this finding may in part be due to the small sample size of this adhesion model (n = 2 studies, n = 13 animals).
An important consideration in the development of pericardial adhesions is the method of entry into the mediastinum, such as via thoracotomy, sternotomy, or other less invasive techniques. However, this was not the focus of our study and thus is a limitation of the presented work.
Animal species for surgical models are typically categorized as small animals (mouse, rat, hamster and rabbit) and large animals (dog, sheep and pig). Advantages of small animals are the ability to have a higher number of replicates, whereas the heart of large animals is more similar in size to human hearts. We found that the differences in adhesion severity in all animal species was similar (Figure 2). Therefore, the major determinant for the choice of the model are animal size, animal cost, and the availability of research tools in the particular model.
By far the most commonly used species to study post-operative pericardial adhesions were rabbits (Table 1). This species was used in almost half of studies (n = 18/41 studies), which included over half of all animals (n = 235/412 animals). The most likely reason for this is that rabbits are the largest of the small animal models. Therefore, this model combines the advantages of the ability to perform more replicates with the advantages of larger operative structures. The next most commonly used species were the large animals dog (n = 8 studies) and pig (n = 8 studies). This indicates that the major focus of many of the studies was to test surgical approaches or mechanical barrier methods to prevent adhesions. Both are relatively expensive animal models. In contrast, only a relatively small number of studies used the small animals mouse, rat or hamster. This is surprising because the research tools to study genetic and molecular mechanisms are by far the most advanced in these animal models. We anticipate that as research moves from mechanical barriers towards molecular therapeutics, the mouse and rat models will become progressively more important.
The kinetics of post-operative adhesion formation is well studied in the peritoneum. The peritoneum requires approximately 7 days to regenerate the mesothelial lining irrespective of the size of the peritoneal injury, as the entire surface of the peritoneum mesothelializes from deep to superficial. This differs from injuries to the skin, which epithelialize from the borders of the defect inward (48). In contrast, healing of pericardial and epicardial injuries are poorly studied. Therefore, the time window during which the pericardium and epicardium are vulnerable to adhesion formation is unclear. Our review found that the earliest time for analysis of pericardial adhesions was 10 days. The most common time course for analysis was 2 weeks. Most studies examined adhesions within the first 8 weeks. The latest time-point for analysis was 12 months. More recently, a study analyzed the formation of pericardial adhesion in relation to angiogenesis in mice models in a 7 day long study (49). This suggests that molecular components of pericardial adhesions may begin before 10 days. Future studies should monitor for pericardial adhesions as early as a few days after induction. Additionally, in the future, investigators may consider analyzing adhesion formation at 2 weeks or earlier since later analysis increases the costs of the experiment.
Pericardial adhesions continue to be a major complication of cardiac surgery. Currently there are multiple methods to prevent adhesions. Mechanical barriers include solid polymers, gels, and liquids (44). Ideally, a mechanical barrier would be present throughout the entire healing process as well as resistant to the immune processes of adhesion formation. It should also be removable or degradable once healing is complete. Antiadhesive agents have also been used; these can work to prevent the adhesion formation pathways or enhance the fibrinolytic ones (44). Finally, physical therapy and movement has been linked to decreased adhesion in joints and the abdomen (44). We have previously described a systematic review of the efficacy of currently available adhesion barriers (50).
While there are many researchers utilizing animal models to investigate post-operative pericardial adhesion formation and prevention, a standardized pericardial adhesion model does not yet exist. Therefore, direct comparisons of interventions to treat post-operative pericardial adhesions are difficult. Our review provides the first survey of the described pericardial adhesion models. This will help investigators make rational choices when choosing a model for pericardial adhesions. Ideally, studies of pericardial adhesions should employ standardized models to allow easy interpretation and comparison. Furthermore, the standardization of pericardial adhesion induction would allow for the study of adhesion prevention techniques.
Animal model, surgery complications, congenital heart surgeon, cardiac pathologic anatomy.
The authors confirm contribution to the paper as follows: Study conception and design: MAH, OAW, WTH, JHK, MNK, TKR; Data collection: MAH, OAW; Analysis and interpretation of results: MAH, OAW, JHK, TKR; Draft manuscript preparation: MAH, OAW, WTH, JHK, MNK, TKR. All authors contributed to the article and approved the submitted version.
Depictions of animals were created with biorender.com.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Park CB, Suri RM, Burkhart HM, Greason KL, Dearani JA, Schaff HV, et al. Identifying patients at particular risk of injury during repeat sternotomy: analysis of 2555 cardiac reoperations. J Thorac Cardiovasc Surg. (2010) 140(5):1028–35. doi: 10.1016/j.jtcvs.2010.07.086
2. Cohen J. Statistical power analysis for the behavioral sciences. L Erlbaum Associates; New York, New York; (1988).
3. Zhou J, Lee JM, Jiang P, Henderson S, Lee TDG. Reduction in postsurgical adhesion formation after cardiac surgery by application of N,O-carboxymethyl chitosan. J Thorac Cardiovasc Surg. (2010) 140(4):801–6. doi: 10.1016/j.jtcvs.2009.11.030
4. Kojima A, Sakaue T, Okazaki M, Shikata F, Kurata M, Imai Y, et al. A simple mouse model of pericardial adhesions. J Cardiothorac Surg. (2019) 14(1):1–9. doi: 10.1186/s13019-019-0940-9
5. Wang ND, Doty DB, Doty JR, Yuksel U, Flinner R. Bioglue®: a protective barrier after pericardiotomy. J Card Surg. (2007) 22(4):295–9. doi: 10.1111/j.1540-8191.2007.00410.x
6. Moghaddam H J, Aghajani M, Raeis-Abdollahi E, Faghihi M, Dabbagh A, Imani A. Decrease in VEGF-induced pericardial adhesion formation using bevacizumab after surgery. Surg Innov. (2019) 26(1):21–6. doi: 10.1177/1553350618813451
7. Orhan A, Görmüş N, Toy H, Görmüş IS, Çaǧlayan O, Tanyeli Ö. Prevention of retrosternal pericardial adhesions after cardiac surgery with mitomycin C. Hear Lung Circ. (2014) 23(4):357–62. doi: 10.1016/j.hlc.2013.10.080
8. Soga Y, Takai S, Koyama T, Okamoto Y, Ikeda T, Nishimura K, et al. Attenuation of adhesion formation after cardiac surgery with a chymase inhibitor in a hamster model. J Thorac Cardiovasc Surg. (2004) 127(1):72–8. doi: 10.1016/S0022-5223(03)00697-4
9. Feng B, Wang S, Hu D, Fu W, Wu J, Hong H, et al. Bioresorbable electrospun gelatin/polycaprolactone nanofibrous membrane as a barrier to prevent cardiac postoperative adhesion. Acta Biomater. (2019) 83:211–20. doi: 10.1016/j.actbio.2018.10.022
10. Kang H, Chung YS, Kim SW, Choi GJ, Kim BG, Park SW, et al. Effect of temperature-sensitive poloxamer solution/gel material on pericardial adhesion prevention: supine rabbit model study mimicking cardiac surgery. PLoS One. (2015) 10(11):1–12. doi: 10.1371/journal.pone.0143359
11. Krause TJ, Zazanis G. Prevention of pericardial adhesions with N-O carboxymethylchitosan in the rabbit model. J Invest Surg. (2001) 14(2):93–7. doi: 10.1080/08941930152024219
12. Zhou J, Liwski RS, Elson C, Lee TDG. Reduction in postsurgical adhesion formation after cardiac surgery in a rabbit model using N,O-carboxymethyl chitosan to block cell adherence. J Thorac Cardiovasc Surg. (2008) 135(4):777–83. doi: 10.1016/j.jtcvs.2007.09.033
13. Hong JH, Choe JW, Kwon GY, Cho DY, Sohn DS, Kim SW, et al. The effects of barrier materials on reduction of pericardial adhesion formation in rabbits: a comparative study of a hyaluronan-based solution and a temperature sensitive poloxamer solution/gel material. J Surg Res. (2011) 166(2):206–13. doi: 10.1016/j.jss.2010.09.034
14. Shen J, Xu ZW. Combined application of acellular bovine pericardium and hyaluronic acid in prevention of postoperative pericardial adhesion. Artif Organs. (2014) 38(3):224–30. doi: 10.1111/aor.12140
15. Hu C, Tang F, Wu Q, Guo B, Long WA, Ruan R, et al. Novel trilaminar polymeric antiadhesion membrane prevents postoperative pericardial adhesion. Ann Thorac Surg. (2021) 111(1):184–9. doi: 10.1016/j.athoracsur.2020.03.011
16. Kaushal S, Patel SK, Goh SK, Sood A, Walker BL, Backer CL. A novel combination of bioresorbable polymeric film and expanded polytetrafluoroethylene provides a protective barrier and reduces adhesions. J Thorac Cardiovasc Surg. (2011) 141(3):789–95. doi: 10.1016/j.jtcvs.2010.11.043
17. Biçer M, Bayram AS, Gürbüz O, Senkaya I, Yerci O, Tok M, et al. Assesssment of the efficacy of the bio-absorbable oxidized regenerated cellulose for prevention of post-operative pericardial adhesion in the rabbit model. J Int Med Res. (2008) 36(6):1311–8. doi: 10.1177/147323000803600619
18. Colak N, Nazli Y, Tasoglu I, Bayrak R, Alpay MF, Aksoy ON, et al. The effect of mitomycin-C in reducing pericardial adhesion after cardiac surgery in rabbits. Can J Cardiol. (2013) 29(6):712–7. doi: 10.1016/j.cjca.2012.05.005
19. Colak N, Nazli Y, Alpay MF, Aksoy ON, Akkaya IO, Bayrak R, et al. Effect of topical N-acetylcysteine in the prevention of postoperative pericardial adhesion formation in a rabbit model. Cardiovasc Pathol. (2013) 22(5):368–72. doi: 10.1016/j.carpath.2013.02.001
20. Okuyama N, Rodgers KE, Wang CY, Girgis W, Oz M, St Amand K, et al. Prevention of retrosternal adhesion formation in a rabbit model using bioresorbable films of polyethylene glycol and polylactic acid. J Surg Res. (1998) 78(2):118–22. doi: 10.1006/jsre.1998.5317
21. Chen Z, Zheng J, Zhang J, Li S. A novel bioabsorbable pericardial membrane substitute to reduce postoperative pericardial adhesions in a rabbit model. Interact Cardiovasc Thorac Surg. (2015) 21(5):565–72. doi: 10.1093/icvts/ivv213
22. Connors RC, Muir JJ, Liu Y, Reiss GR, Kouretas PC, Whitten MG, et al. Postoperative pericardial adhesion prevention using carbylan-SX in a rabbit model. J Surg Res. (2007) 140(2):237–42. doi: 10.1016/j.jss.2007.03.014
23. Kuschel TJ, Gruszka A, Hermanns-Sachweh B, Elyakoubi J, Sachweh JS, Vazquez-Jimenez JF, et al. Prevention of postoperative pericardial adhesions with TachoSil. Ann Thorac Surg. (2013) 95(1):183–8. doi: 10.1016/j.athoracsur.2012.08.057
24. Iskesen I, Aksoy O, Cerrahoglu M, Sirin H. The effect of piroxicam on the prevention of postoperative retrosternal and pericardial adhesions. Acta Cardiol. (2007) 62(6):559–64. doi: 10.2143/AC.62.6.2024014
25. Robison RJ, Brown JW, Deschner WP, Highes B, King H. Prevention of pericardial adhesions with dextran 70. Ann Thorac Surg. (1984) 37(6):488–90. doi: 10.1016/S0003-4975(10)61138-1
26. Walker AS, Blue MA, Brandon TA, Emmanual J, Guilbeau EJ. Performance of a hydrogel composite pericardial substitute after long-term implant studies. ASAIO J. (1992) 38(3):M550–M554. doi: 10.1097/00002480-199207000-00095
27. Tsukihara H, Takamoto S, Kitahori K, Matsuda K, Murakami A, Novick RJ, et al. Prevention of postoperative pericardial adhesions with a novel regenerative collagen sheet. Ann Thorac Surg. (2006) 81(2):650–7. doi: 10.1016/j.athoracsur.2005.07.022
28. Seeger JM, Kaelin LD, Staples EM. Prevention of postoperative pericardial adhesions using tissue-protective solutions. J Surg Res. (1997) 68(1):63–66. doi: 10.1006/jsre.1996.4990
29. Soga Y, Takai S, Koyama T, Okamoto Y, Ikeda T, Nishimura K, et al. Attenuating effects of chymase inhibitor on pericardial adhesion following cardiac surgery. J Card Surg. (2007) 22(4):343–7. doi: 10.1111/j.1540-8191.2007.00420.x
30. Okuyama N, Wang CY, Rose EA, Rodgers KE, Pines E, diZerega GS, et al. Reduction of retrosternal and pericardial adhesions with rapidly resorbable polymer films. Ann Thorac Surg. (1999) 68(3):913–8. doi: 10.1016/S0003-4975(99)00556-1
31. Mitchell JD, Lee R, Hodakowski GT, Neya K, Harringer W, Valeri CR, et al. Prevention of postoperative pericardial adhesions with a hyaluronic acid coating solution: experimental safety and efficacy studies. J Thorac Cardiovasc Surg. (1994) 107(6):1481–8. doi: 10.1016/S0022-5223(12)70149-6
32. Milgalter E, Uretzky G, Siberman S, Appelbaum Y, Shimon DV, Kopolovic J, et al. Pericardial meshing: an effective method for prevention of pericardial adhesions and epicardial reaction after cardiac operations. J Thorac Cardiovasc Surg. (1985) 90(2):281–6. doi: 10.1016/s0022-5223(19)38631-3
33. Lemmer JH, Faber LA, Mariano DJ, Drews TA, Kienzle MG. Pericardial influence on internal defibrillation energy requirements. J Thorac Cardiovasc Surg. (1991) 101(5):839–42. doi: 10.1016/s0022-5223(19)36654-1
34. Amigo N, Riganti JM, Ramirez M, Andrea L, Renda P, Lovera R, et al. Urinary bladder matrix scaffolds promote pericardium repair in a porcine model. J Surg Res. (2020) 249:216–24. doi: 10.1016/j.jss.2019.12.033
35. Qazi S, Kjaergaard B, Yang F, Shen G, Wang S, Zhang N, et al. No effect of rapamycin on cardiac adhesion formation: a drug-loaded bioresorbable polylactone patch in a porcine cardiac surgical model. Eur Surg Res. (2016) 56(1-2):76–85. doi: 10.1159/000441914
36. Taksaudom N, Ketwong M, Lertprasertsuke N, Kongkaew A. Postoperative pericardial adhesion prevention using collagen membrane in pigs: a pilot study. Open J Cardiovasc Surg. (2017) 9:117906521772090. doi: 10.1177/1179065217720909
37. Lopes JB, Dallan LAO, Moreira LFP, Campana Filho SP, Gutierrez PS, Lisboa LA, et al. Synergism between keratinocyte growth factor and carboxymethyl chitosan reduces pericardial adhesions. Ann Thorac Surg. (2010) 90(2):566–72. doi: 10.1016/j.athoracsur.2010.03.086
38. Alizzi AM, Summers P, Boon VH, Tantiongo JP, Thompson T, Leslie BJ, et al. Reduction of post-surgical pericardial adhesions using a pig model. Hear Lung Circ. (2012) 21(1):22–9. doi: 10.1016/j.hlc.2011.10.002
39. Lopes JB, Dallan LAO, Campana-Filho SP, Lisboa LA, Gutierrez PS, Moreira LF, et al. Keratinocyte growth factor: a new mesothelial targeted therapy to reduce postoperative pericardial adhesions. Eur J Cardio-Thoracic Surg. (2009) 35(2):313–8. doi: 10.1016/j.ejcts.2008.09.046
40. Pätilä T, Jokinen JJ, Salminen J, Kankuri E, Harjula A. Polyglycolic acid glue does not prevent intrapericardial adhesions in a short-term follow-up. J Surg Res. (2008) 148(2):181–4. doi: 10.1016/j.jss.2007.07.002
41. Malm T, Bowald S, Bylock A, Busch C. Prevention of postoperative pericardial adhesions by closure of the pericardium with absorbable polymer patches: an experimental study. J Thorac Cardiovasc Surg. (1992) 104(3):600–7. doi: 10.1016/s0022-5223(19)34724-5
42. Bel A, Ricci M, Piquet J, Bruneval P, Perier MC, Gagnieu C, et al. Prevention of postcardiopulmonary bypass pericardial adhesions by a new resorbable collagen membrane. Interact Cardiovasc Thorac Surg. (2012) 14(4):469–73. doi: 10.1093/icvts/ivr159
43. Bel A, Kachatryan L, Bruneval P, Peyrard S, Gagnieu C, Fabiani JN, et al. A new absorbable collagen membrane to reduce adhesions in cardiac surgery⋆. Interact Cardiovasc Thorac Surg. (2010) 10(2):213–6. doi: 10.1510/icvts.2009.215251
44. Capella-Monsonís H, Kearns S, Kelly J, Zeugolis DI. Battling adhesions: from understanding to prevention. BMC Biomed Eng. (2019) 1:5. doi: 10.1186/s42490-019-0005-0
45. Rout UK, Saed GM, Diamond MP. Expression pattern and regulation of genes differ between fibroblasts of adhesion and normal human peritoneum. Reprod Biol Endocrinol. (2005) 3:1–14. doi: 10.1186/1477-7827-3-1
46. Lucas PA, Warejcka DJ, Young HE, Lee BY. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-β1. J Surg Res. (1996) 65(2):135–8. doi: 10.1006/jsre.1996.0355
47. Thaler K, Mack JA, Berho M, Grotendorst G, Wexner SD, Abramson SR. Coincidence of connective tissue growth factor expression with fibrosis and angiogenesis in postoperative peritoneal adhesion formation. Eur Surg Res. (2005) 37(4):235–41. doi: 10.1159/000087869
48. Duron JJ. Postoperative intraperitoneal adhesion pathophysiology. Color Dis. (2007) 9(Suppl. 2):14–24. doi: 10.1111/j.1463-1318.2007.01343.x
49. Namiguchi K, Sakaue T, Okazaki M. Unique angiogenesis from cardiac arterioles during pericardial adhesion formation. Front Cardiovasc Med. (2022) 8:761591. doi: 10.3389/fcvm.2021.761591
Keywords: adhesions, cardiac surgery, animal model, post-operative adhesions, pericardial adhesions
Citation: Hill MA, Walkowiak OA, Head WT, Kwon JH, Kavarana MN and Rajab TK (2022) A review of animal models for post-operative pericardial adhesions. Front. Surg. 9:966410. doi: 10.3389/fsurg.2022.966410
Received: 10 June 2022; Accepted: 22 August 2022;
Published: 12 September 2022.
Edited by:
Bernhard Winkler, Vienna Health Association, AustriaReviewed by:
Tomohisa Sakaue, Ehime University, Japan© 2022 Hill, Walkowiak, Head, Kwon, Kavarana and Rajab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taufiek Konrad Rajab cmFqYWJ0QG11c2MuZWR1
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.