- Center for Neurosurgery, University Hospital of Cologne, Cologne, Germany
Background: Cerebrospinal fluid leakage (CSFL) following spinal durotomy can lead to severe sequelae. However, while several studies have investigated accidental spinal durotomies, the risk factors and influence of clinical management in planned durotomies remain unclear.
Methods: We performed a retrospective analysis of all patients who underwent planned intradural spinal surgery at our institution between 2010 and 2020. Depending on the occurrence of a CSFL, patients were dichotomized and compared with respect to patient and case-related variables as well as dural closure technique, epidural drainage placement, and timing of mobilization.
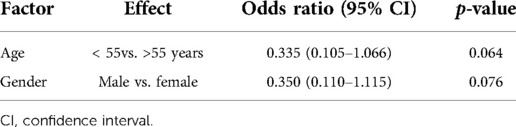
Results: A total of 351 patients were included. CSFL occurred in 4.8% of all cases. Surgical indication, tumor histology, location within the spine, previous intradural surgery, and medical comorbidities were not associated with an increased risk of CSFL development (all p > 0.1). Age [odds ratio (OR), 0.335; 95% confidence interval (CI), 0.105–1.066] and gender (OR, 0.350; 95% CI, 0.110–1.115) were not independently associated with CSFL development. There was no significant association between CSFL development and the dural closure technique (p = 0.251), timing of mobilization (p = 0.332), or placement of an epidural drainage (p = 0.321).
Conclusion: CSFL following planned durotomy pose a relevant and quantifiable complication risk of surgery that should be factored in during preoperative patient counseling. Our data could not demonstrate superiority of any particular dural closure technique but support the safety of both early mobilization within 24 h postoperatively and epidural drainage with reduced or no force of suction.
Introduction
Cerebrospinal fluid leakage (CSFL) is a severe complication following incidental or planned durotomy in spinal procedures. Possible sequelae include infectious complications ranging from wound infections to meningitis, intracranial hypotension and hemorrhage, nerve root compression syndromes, and back pain, as well as the necessity for revision surgery, increased morbidity, a prolonged hospital stay, and higher healthcare costs (1, 2). However, the majority of studies on secondary CSFL investigate the setting of incidental durotomy; hence, there are limited data regarding patient and case-related risk factors for CSFL following planned durotomy, i.e., for the resection of spinal intradural tumors (3, 4). Furthermore, the perioperative management following planned durotomies is highly variable. Different surgical techniques for dural closure are available, the benefit of epidural drainage placement remains unclear, and the utility of postoperative immobilization has been discussed controversially. Thus, we aimed to identify possible risk factors for the occurrence of CSFL following intradural spine surgery in order to improve preoperative patient counseling and to analyze perioperative management strategies.
Materials and methods
This study is a single-center retrospective analysis of 351 consecutive cases of intradural spine surgery conducted at our institution between June 2010 and December 2020, in accordance with the local ethics committee guidelines.
Inclusion and exclusion criteria
All patients who underwent surgery for an intradural spinal pathology at our institution between June 2010 and December 2020 were included in this study. All cases with incomplete data records and patients under the age of 18 years at the time of surgery were excluded, as well as craniocervical pathologies involving the posterior fossa, i.e., Chiari malformations were excluded.
Data collection
A postoperative CSFL was defined as either cerebrospinal fluid (CSF) leakage through the operative wound (CSF fistula) or development of a pseudomeningocele refractory to conservative therapy and requiring revision surgery. The incidence of CSFL within the study cohort was recorded over an observational period of at least 3 months following intradural surgery.
Medical charts were reviewed for the following variables: age, gender, medical comorbidities (hypertension, coronary heart disease, diabetes mellitus type II, chronic obstructive pulmonary disease, history of smoking), obesity (defined as a body mass index > 30), and duration of hospital stay.
In two cases, two different spinal segments distant from each other were operated on during the same procedure and recorded as two separate cases.
Type of surgical indication, tumor histology, surgical approach, and previous intradural operations on the same level were analyzed. Tethered cord syndrome and different forms of spinal dysraphism were summarized as developmental malformations. Tumor histology was determined according to the current WHO classification in effect.
Spinal surgery location was classified as cervical, thoracic, or lumbosacral. In case of a junctional segment, classification was based on the upper vertebra (e.g., a C7/T1 procedure was classified as a cervical case).
Surgical technique
The dural exposure was classified according to the surgical report (laminectomy, hemilaminectomy, or laminoplasty). All durotomies were performed either median or paramedian along the longitudinal axis of the dural sac. All meningiomas were resected without removal of the associated dura, and the dural insertion site was coagulated corresponding to Simpson Grade II.
Perioperative management in our study cohort was based on a case-by-case decision according to the intraoperative findings and the preference of the treating surgeon; there were no institutional protocols regarding dural closure technique, epidural drain placement, or timing of mobilization.
Surgical dural closure technique was categorized into the following: (1) standalone suture repair, (2) suture plus a liquid fibrin sealant (TISSUCOL Duo S Immuno®, Baxter International Inc.), (3) suture plus a patch sealant (TachoSil®, Takeda Pharma and Hemopatch®, Baxter International Inc.), (4) suture plus a liquid fibrin sealant and a patch sealant, or (5) suture plus another combination and/or material [e.g., muscle, fascia or a dura replacement material (DuraGen® Plus, Integra LifeSciences; Neuro-Patch®, B. Braun; GORE PRECLUDE® Pericardial Membrane, W. L. Gore / Associates, Inc.].
The additional placement of an epidural drainage as well as the applied force of suction (full, reduced, none) were recorded. The full force of suction applied by the used drainage system was 150 mbar (150 hPa).
The ordered duration of postoperative strict bed rest was summed into an early mobilization (within 24 h) and a late mobilization group (after 24 h).
Procedure-related complications were classified as wound healing disorders without CSFL, epidural hemorrhage, neurological complications (postoperative new or deteriorated deficit, meningitis), urinary tract infections, pneumonia, or miscellaneous.
Statistical analysis
Patients were dichotomized according to the occurrence of CSFL. Differences between the two groups were analyzed using descriptive statistics. To compare categorical variables, the Chi-square and Fisher's exact tests were used, when appropriate. Continuous variables were tested for normality using the Kolmogorov–Smirnov test. Group means with normally distributed data were compared using the two-sided unpaired Student's t test and the Mann–Whitney U test in case of non-normally distributed data. Continuous variables are reported as mean and standard deviation (mean ± SD) or as median and range (minimum, maximum). Risk is reported as absolute risk, unless otherwise stated. All calculations were performed using SPSS software (Version 27, IBM SPSS Statistics for Windows, Armonk, NY, USA). A p-value <0.05 was considered as statistically significant.
Results
Patient characteristics
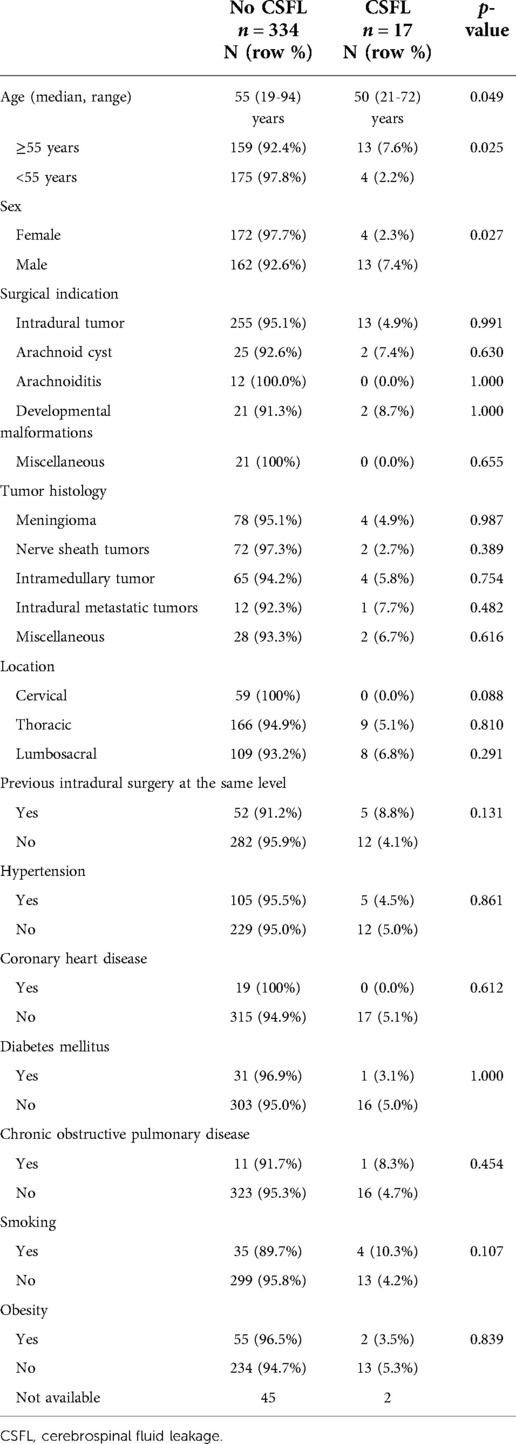
A total of 351 patients who underwent surgery for an intradural spinal pathology were included. Median patient age was 55 years (range: 19–94), and 50.1% were female. The most common surgical indication was resection of an intradural tumor (75.5%). Of these, meningiomas (30.9%), intramedullary tumors (30.2%), and nerve sheath tumors (28.3%) were the most frequent histological diagnoses. The most common surgical site was the thoracic spine (49.9%), followed by lumbosacral (33.3%) and cervical spine (16.8%). In 13.1%, intradural surgery had previously been performed at the same level. Detailed patient characteristics including medical comorbidities are displayed in Table 1.
Perioperative factors
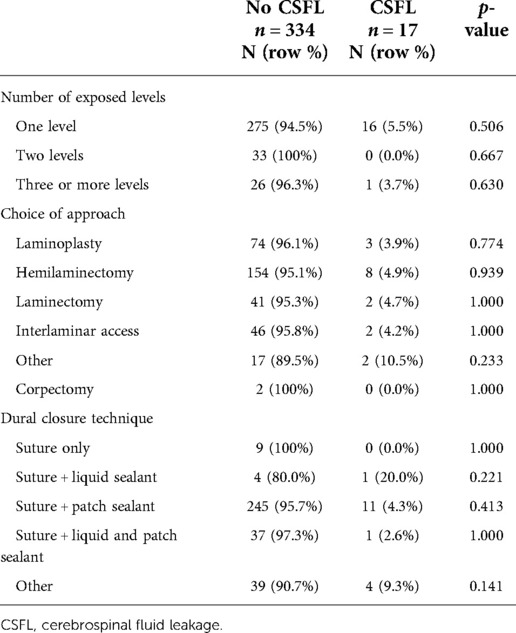
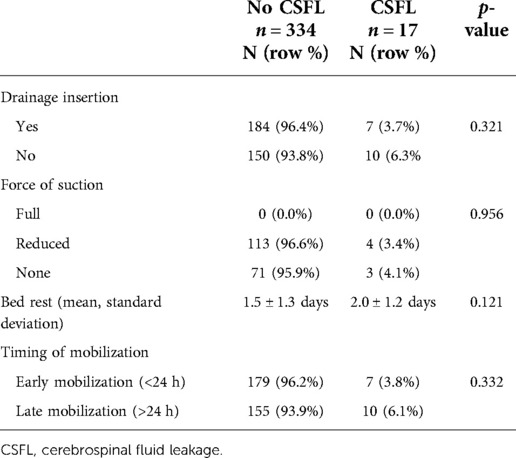
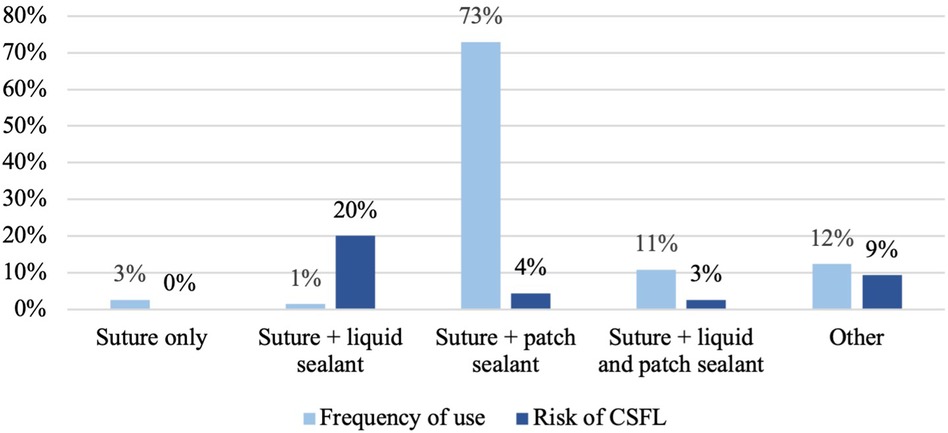
Surgical exposure mainly involved a single level (82.9%) and the majority of cases were operated via a hemilaminectomy (46.2%) or laminoplasty (21.9%). Dural closure was performed using a combination of suture plus a sealant patch in 72.9%, suture plus a liquid sealant as well as a sealant patch in 10.8%, suture plus other materials (e.g. fascia or muscle) in 12.3%, suture plus a liquid sealant in 1.4%, and dural suture was not augmented in 2.6% of all cases (Table 2). An epidural drainage was placed in 191 (54.4%) cases. Of those, reduced suction was applied in 117 (61.3%) and no suction in 74 (38.7%) cases; full suction was never applied. Mean duration of bed rest was 1.6 (±1.3) days. Of all patients, 53.0% were mobilized within 24 h and 47.0% after 24 h (Table 3).
Risk factors for cerebrospinal fluid leak
CSFL occurred in 17 cases (4.8%). In univariate analysis, age (median 55 vs. 50 years, p = 0.025) and gender (2.3% female vs. 7.4% male, p = 0.027) were significantly associated with CSFL development, but neither age [odds ratio (OR), 0.335; 95% confidence interval (CI), 0.105–1.066] nor gender (OR, 0.350; 95% CI, 0.110–1.115) remained as independent risk factors in multivariate analysis (Table 4). The following disease, surgery, and patient-related variables were tested as potential cofactors and found not to influence the risk of CSFL development: surgical indication (all p > 0.1), tumor histology (all p > 0.1), location (all p > 0.05), previous intradural surgery (p = 0.131), medical comorbidities (all p > 0.1), number of exposed levels (all p > 0.1), choice of approach (all p > 0.1), dural closure technique (all p > 0.1, Figure 1), drainage insertion (p = 0.321), force of suction (p = 0.537), and timing of mobilization (p = 0.332).
Complications following early and late mobilization
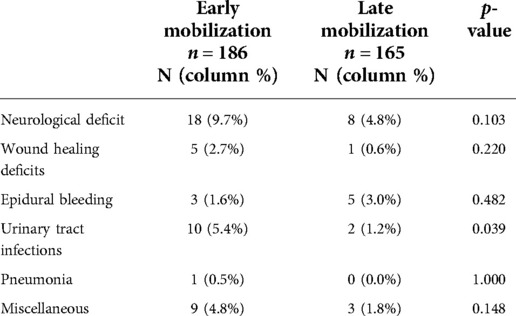
Wound healing disorders occurred in 6 cases (1.6%), epidural hematoma in 8 cases (2.3%), urinary tract infection in 12 cases (3.4%), pneumonia in 1 case (0.3%), and new neurological deficits in 26 cases (7.4%, Table 5). The incidence of urinary tract infections was significantly higher in the early mobilization group (5.4% vs. 1.2%; p = 0.039), and the two groups did not differ with respect to other complications (all p > 0.1).
Discussion
In this series, we report on 351 planned durotomies in adult patients with regard to factors influencing CSFL, making this the second largest series in this regard and the largest to investigate the impact of postoperative mobilization and epidural drainage placement as well as the first to investigate medical comorbidities.
The overall risk of CSFL development was 4.8%, which is in line with previous studies reporting an average risk ranging from 0 to 10% (3–7).
In general, reports regarding risk factors for CSFL following planned durotomies are sparse. Two studies investigating the impact of age and gender on CSFL development found no association, which is in line with the findings of our study. Regarding the impact of location, our study is consistent with previous reports that did not show a significant correlation (6, 8). Of note, no CSFL occurred in the cervical spine in our cohort, possibly attributed to the fact that merely 17% of all intradural surgeries were performed in the cervical spine in our cohort in the first place. Contrary to reports on incidental durotomies, prior epidural surgery does not appear to be a risk factor for CSFL in case of planned durotomies (3, 5, 9, 10).
As this is the first to examine the impact of medical comorbidities on CSFL in planned durotomies, our study first provides evidence of no significant correlations in this regard.
While the choice of surgical approach and degree of dural exposure and bone removal was not found to influence the risk for CSFL, which is in line with previous findings (6), there was no dedicated analysis comparing open and minimally invasive approaches in our study due to the small number of patients being treated with minimally invasive approaches. However, minimally invasive techniques may offer advantages over open procedures in terms of reduced perioperative complications, including CSFL, for both accidental and planned durotomies (11–13).
Although additional (liquid or patch) sealants are frequently used to reinforce dural suture (97.4% in our study), their benefit remains uncertain (11).
Use of fibrin sealants has been discouraged by several authors following both planned and unplanned durotomies due to a lack of effectiveness in preventing CSFL (10, 14–16), even though two studies reported favorable results (4, 17). The effectiveness and the necessity of patch sealants to prevent CSFL are not sufficiently studied in planned durotomies. Favorable results were reported by Montano et al. who reported no CSFL requiring revision surgery after dural closure with TachoSil® in 35 intradural procedures (18). In contrast, other studies investigating patch sealants found no clinical benefit in terms of CSFL risk reduction, thus questioning their application (3, 6, 11). Overall, further research is warranted to investigate the utility of sealants in planned durotomies. Based on the currently available data as well as our findings, the use of additional sealants in case of adequate dural closure by suture may not provide further benefit.
The placement of an epidural drainage following durotomy is controversially discussed (16, 19). Our data showed no effect on CSFL development, suggesting a generally safe applicability with reduced or no force of suction. It does not allow further conclusions such as previous reports advocating for epidural drainage placement in order to reduce the risk of CSFL by influencing epidural pressure gradients and thus supporting wound healing (16, 20). Furthermore, our results are inconsistent with reports of increased complication rates, including CSFL, following epidural drainage in both planned and accidental durotomy (3, 21–24).
Bed rest following spinal durotomy remains a common measure, although in case of accidental durotomy, studies failed to show any benefit in terms of CSFL reduction. Accordingly, our series of planned durotomies showed no impact of early or late mobilization on the development of CSFL, consistent with the only other study currently available on this topic (4). Consequently, early mobilization after planned durotomy appears to be beneficial, as it is associated with a reduction in medical complications such as ileus, pneumonia, and deep vein thrombosis, as well as a reduced socioeconomic financial burden due to overall shorter hospital stays (25, 26). Of note, the overall complication rate did not differ significantly between the early and late mobilization groups in our study, possibly due to the small number of patients immobilized for more than three days in our cohort. Accordingly, we attribute the higher rate of urinary tract infections in the early mobilization group to the retrospective study design.
This study carries several limitations. First, our study is limited by its retrospective design and single-center patient population. Second, the risk of CSFL development might be underestimated due to the rather strict definition of a CSFL used in this study. CSFL was defined as cerebrospinal fluid leakage through the operative wound refractory to conservative therapy and necessitating operative revision surgery, which is in contrast with other publications that included conservatively managed cases as well. Third, the technique of dural closure, epidural drain placement, and timing of mobilization were not based on standardized protocols but at the discretion of the treating surgeon, potentially causing a selection bias. Finally, case numbers for certain techniques of dural closure were small, limiting statistical power.
Conclusion
CSFL following planned durotomy pose a relevant complication risk of surgery that can be quantified for preoperative patient-specific counseling and consent. To minimize the risk of CSFL, our data highlight the importance of adequate dural closure by suture, and the use of additional sealants remains optional. In contrast to previous treatment recommendations, our results indicate the safety of both epidural drainage with reduced or no force of suction and early mobilization within 24 h following intradural surgery to prevent further complications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VN, ML, and MP contributed to the initial conception and study design. ML, MP, ST, and NvS acquired and curated the database. ML and MP conducted the formal analysis and wrote the manuscript. ST, NvS, JP, RG, and VN reviewed and edited the manuscript. RG and VN supervised the project. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge publishing funding support from the DFG (German Research Foundation, 491454339).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Weber C, Piek J, Gunawan D. Health care costs of incidental durotomies and postoperative cerebrospinal fluid leaks after elective spinal surgery. Eur Spine J. (2015) 24:2065–8. doi: 10.1007/s00586-014-3504-7
2. Takenaka S, Makino T, Sakai Y, Kashii M, Iwasaki M, Yoshikawa H, Kaito T. Dural tear is associated with an increased rate of other perioperative complications in primary lumbar spine surgery for degenerative diseases. Medicine (Baltimore). (2019) 98:e13970. doi: 10.1097/MD.0000000000013970
3. Sellin JN, Kolcun JPG, Levi AD. Cerebrospinal fluid leak and symptomatic pseudomeningocele after intradural spine surgery. World Neurosurg. (2018) 120:497–502. doi: 10.1016/j.wneu.2018.08.112
4. Tan LA, Takagi I, Straus D, O’Toole JE. Management of intended durotomy in minimally invasive intradural spine surgery: clinical article. J Neurosurg Spine. (2014) 21:279–85. doi: 10.3171/2014.3.SPINE13719
5. Hoover JM, Clarke MJ, Wetjen NM, Mandrekar J, Puffer RC, Krauss W. Complications necessitating a return to the operating room following intradural spine surgery. World Neurosurg. (2012) 78:344–7. doi: 10.1016/j.wneu.2011.12.085
6. Koechlin NO, Burkhardt JK, Scherer M, Krayenbühl N, Sarnthein J, Bernays RL, Bozinov O. Cerebrospinal fluid leaks after planned intradural spine surgery: a single-center analysis of 91 cases. J Neurol Surg A Cent Eur Neurosurg. (2013) 74:216–22. doi: 10.1055/s-0032-1304809
7. Fisahn C, Sanders FH, Moisi M, Page J, Oakes PC, Wingerson M, Dettori J, Tubbs RS, Chamiraju R, Nora P, Newell D, Delashaw J, Oskouian RJ, Chapman JR. Descriptive analysis of unplanned readmission and reoperation rates after intradural spinal tumor resection. J Clin Neurosci. (2017) 38:32–6. doi: 10.1016/j.jocn.2016.12.013
8. Safaee MM, Lyon R, Barbaro NM, Chou D, Mummaneni PV, Weinstein PR, Chin CT, Tihan T, Ames CP. Neurological outcomes and surgical complications in 221 spinal nerve sheath tumors. J Neurosurg Spine. (2017) 26:103–11. doi: 10.3171/2016.5.SPINE15974
9. Patel J, Kundnani V, Kuriya S. Dural leak: is it deterrent to outcomes in spine surgery?: 10 years retrospective analysis of incidence, management protocol, and surgical outcomes. Spine. (2020) 45:1615–21. doi: 10.1097/BRS.0000000000003662
10. Jankowitz BT, Atteberry DS, Gerszten PC, Karausky P, Cheng BC, Faught R, Welch WC. Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J. (2009) 18:1169–74. doi: 10.1007/s00586-009-0928-6
11. Kinaci A, Moayeri N, van der Zwan A, van Doormaal TPC. Effectiveness of sealants in prevention of cerebrospinal fluid leakage after spine surgery: a systematic review. World Neurosurg. (2019) 127:567–75. doi: 10.1016/j.wneu.2019.02.236
12. Wong AP, Shih P, Smith TR, Slimack NP, Dahdaleh NS, Aoun SG, El Ahmadieh TY, Smith ZA, Scheer JK, Koski TR, Liu JC, Fessler RG. Comparison of symptomatic cerebral spinal fluid leak between patients undergoing minimally invasive versus open lumbar foraminotomy, discectomy, or laminectomy. World Neurosurg. (2014) 1:634–40. doi: 10.1016/j.wneu.2013.11.012
13. Wong AP, Lall RR, Dahdaleh NS, Lawton CD, Smith ZA, Wong RH, Harvey MJ, Lam S, Koski TR, Fessler RG. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg Focus. (2015) 39:E11. doi: 10.3171/2015.5.FOCUS15129
14. Won YI, Kim CH, Chung CK, Jahng TA, Park SB. The use fibrin sealant after spinal intradural tumor surgery: is it necessary? Korean J Spine. (2016) 13:24–9. doi: 10.14245/kjs.2016.13.1.24
15. Bakhsheshian J, Strickland BA, Patel NN, Jakoi AM, Minneti M, Zada G, Acosta FL, Hsieh PC, Wang JC, Liu JC, Pham MH. The use of a novel perfusion-based cadaveric simulation model with cerebrospinal fluid reconstitution comparing dural repair techniques: a pilot study. Spine J. (2017) 17:1335–41. doi: 10.1016/j.spinee.2017.04.007
16. Hassanzadeh H, Bell J, Bhatia M, Puvanesarajah V. Incidental durotomy in lumbar spine surgery; risk factors, complications, and perioperative management. J Am Acad Orthop Surg. (2021) 29:279–86. doi: 10.5435/JAAOS-D-20-00210
17. Hodges SD, Humphreys SC, Eck JC, Covington LA. Management of incidental durotomy without mandatory bed rest: a retrospective review of 20 cases. Spine. (1999) 24:2062–4. doi: 10.1097/00007632-199910010-00017
18. Montano N, Pignotti F, Auricchio AM, Fernandez E, Olivi A, Papacci F. Results of TachoSil® associated with fibrin glue as dural sealant in a series of patients with spinal intradural tumors surgery. Technical note with a review of the literature. J Clin Neurosci. (2019) 61:88–92. doi: 10.1016/j.jocn.2018.10.138
19. Gautschi OP, Stienen MN, Smoll NR, Corniola MV, Tessitore E, Schaller K. Incidental durotomy in lumbar spine surgery—a three-nation survey to evaluate its management. Acta Neurochir. (2014) 156:1813–20. doi: 10.1007/s00701-014-2177-7
20. Khan MH, Rihn J, Steele G, Davis R, Donaldson WF 3rd, Kang JD, Lee JY. Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: a review of 3,183 consecutive degenerative lumbar cases. Spine. (2006) 31:2609–13. doi: 10.1097/01.brs.0000241066.55849.41
21. Niu T, Lu DS, Yew A, Lau D, Hoffman H, McArthur D, Chou D, Lu DC. Postoperative cerebrospinal fluid leak rates with subfascial epidural drain placement after intentional durotomy in spine surgery. Global Spine J. (2016) 6:780–5. doi: 10.1055/s-0036-1582392
22. Hughes SA, Ozgur BM, German M, Taylor WR. Prolonged Jackson-Pratt drainage in the management of lumbar cerebrospinal fluid leaks. Surg Neurol. (2006) 65:410–4. doi: 10.1016/j.surneu.2005.11.052
23. Fang Z, Jia YT, Tian R, Liu Y. Subfascial drainage for management of cerebrospinal fluid leakage after posterior spine surgery—a prospective study based on Poiseuille's law. Chin J Traumatol. (2016) 19:35–8. doi: 10.1016/j.cjtee.2016.01.008
24. Sohn S, Chung CK, Kim CH. Is closed-suction drainage necessary after intradural primary spinal cord tumor surgery? Eur Spine J. (2013) 22:577–83. doi: 10.1016/j.cjtee.2016.01.008
25. Farshad M, Aichmair A, Wanivenhaus F, Betz M, Spirig J, Bauer DE. No benefit of early versus late ambulation after incidental durotomy in lumbar spine surgery: a randomized controlled trial. Eur Spine J. (2020) 29:141–6. doi: 10.1007/s00586-019-06144-5
Keywords: cerebrospinal fluid leak, drainage, postoperative complications, mobilization, spine
Citation: Lenschow M, Perrech M, Telentschak S, von Spreckelsen N, Pieczewski J, Goldbrunner R and Neuschmelting V (2022) Cerebrospinal fluid leaks following intradural spinal surgery—Risk factors and clinical management. Front. Surg. 9:959533. doi: 10.3389/fsurg.2022.959533
Received: 1 June 2022; Accepted: 29 August 2022;
Published: 20 September 2022.
Edited by:
Jeremy Steinberger, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Sami Ridwan, Klinikum Ibbenbueren, GermanyTeresa Somma, Federico II University Hospital, Italy
Brian Dalm, The Ohio State University, United States
© 2022 Lenschow, Perrech, Telentschak, Von Spreckelsen, Pieczewski, Goldbrunner and Neuschmelting. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moritz Lenschow bW9yaXR6LmxlbnNjaG93QHVrLWtvZWxuLmRl
†ORCID Moritz Lenschow orcid.org/0000-0003-0788-4681 Volker Neuschmelting orcid.org/0000-0001-7527-6990
‡These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Moritz Lenschow*†,‡
Moritz Lenschow*†,‡