
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 14 October 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.956872
 Zonggang Hou1
Zonggang Hou1 Zhenxing Huang1
Zhenxing Huang1 Zhenye Li1
Zhenye Li1 Zhenghai Deng1
Zhenghai Deng1 Gen Li1
Gen Li1 Yaokai Xu1
Yaokai Xu1 Mingran Wang2
Mingran Wang2 Shengjun Sun3
Shengjun Sun3 Yazhuo Zhang4
Yazhuo Zhang4 Hui Qiao2*
Hui Qiao2* Jian Xie1*
Jian Xie1*
Introduction: Insular gliomas have complex anatomy and microvascular supply that make resection difficult. Furthermore, resection of insular glioma is associated with a significant risk of postoperative ischemic complications. Thus, this study aimed to assess the incidence of ischemic complications related to insular glioma resection, determine its risk factors, and describe a single surgeon’s experience of artery-preserving tumor resection.
Methods: We enrolled 75 consecutive patients with insular gliomas who underwent transcortical tumor resection. Preoperative and postoperative demographic, clinical, radiological [including diffusion-weighted imaging (DWI)], intraoperative neurophysiological data, and functional outcomes were analyzed. Motor evoked potentials (MEPs) and radiological characteristics like the relationship between the proximal segment of the lateral lenticulostriate arteries (LLSAs) and the tumor, the flat inner edge sign (the inner edge of the insular glioma is well-defined) or obscure inner edge sign, the distance between the lesion and posterior limb of the internal capsule and the invasion of the superior limiting sulcus by the tumor were analyzed. Strategies such as “residual triangle,” “basal ganglia outline reappearance,” and “sculpting” technique were used to preserve the LLSAs and the main branches of M2 for maximal tumor resection according to the Berger–Sinai classification.
Results: Postoperative DWI showed acute ischemia in 44 patients (58.7%). Moreover, nine patients (12%) had developed new motor deficits, as determined by the treating neurosurgeons. The flat inner edge sign [odds ratio (OR), 0.144; 95% confidence interval (CI), 0.024–0.876) and MEPs (>50%) (OR, 18.182; 95% CI, 3.311–100.00) were significantly associated with postoperative core ischemia, which affected the posterior limb of the internal capsule or corona radiata.
Conclusions: Insular glioma resection was associated with a high incidence of ischemia, as detected by DWI, as well as new motor deficits that were determined by the treating neurosurgeons. Insular glioma patients with obscure inner edge signs and intraoperative MEPs decline >50% had a higher risk of developing core ischemia. With our strategies, maximal safe resection of insular gliomas may be achieved.
The insular lobe is a common site of intrinsic brain tumor growth (1). Improvements in understanding the insular anatomy and the application of various technologies have made insular glioma operable with acceptable morbidity. Postoperative motor deficits remain a significant concern (2), and ischemia's incidence and risk factors after surgery for insular gliomas need further exploration.
For insular tumors, ischemic strokes can arise by lateral lenticulostriate arteries (LLSAs) from M1 segment of the middle cerebral artery (MCA), long insular artery from the M2–M3 junction at the level of the superior insular sulcus, and even long medullary arteries terminating the M4 branches (3–5). Although transitory clipping of the latter two types of arteries under motor evoked potentials (MEPs) monitoring may be helpful (3, 6), the characteristics of the thin, long course and ambiguous origin of these two arteries make their reservation still difficult intraoperatively.
LLSAs supply the internal capsule and often pass along the inner side of insular tumors (7). Although the number of LLSAs varies from 1 to 21, even the occlusion of one branch may cause extensive infarction of the subcortical ganglia and internal capsule, resulting in motor and language deficits (8). Immediate postoperative deficits may be caused by resection-induced contusion, edema, and hypoperfusion. However, permanent deficits are mainly caused by infarctions due to disruption of MCA branches on the lateral surface of the insula and LLSAs within the anterior perforated substance on its mesial side (9, 10). In the transsylvian and transcortical approaches for insular glioma resection, the preservation of these critical blood vessels is essential (11). The characteristics of the relatively large diameter and exact origin of LLSAs, make its reservation possible intraoperatively.
This study tried to determine the incidence of ischemic complications and their risk factors, and describe the strategies which focus on LLSAs and MCA preservation employed to prevent the ischemic complications of insular glioma surgery.
We conducted a single-center, retrospective, noncontrolled study involving 75 insular glioma patients admitted to our department between October 2018 and June 2020. The preoperative and postoperative clinical, radiological, and intraoperative neuromonitoring (IONM) data were analyzed. The Berger–Sanai insular glioma classification was used for tumor classification (12). All tumors were histologically examined and classified according to the World Health Organization (WHO) primary central nervous system tumor classification. This study was approved by our institutional review board.
All surgical procedures were performed via the transcortical approach under general anesthesia, as previously described (13). All surgical procedures were performed under continuous IONM. In these patients, the transcortical windows were limited to the anterior temporal lobe and the pars orbitalis, triangularis of the inferior frontal gyrus, which rarely involved Broca's area, especially for tumors in the dominant hemispheres (10).
LLSAs were subdivided into two segments: (i) proximal segment, which is anterior to the point of entry into the anterior perforated substance, and (ii) distal segment, which is located after the entry point into the anterior perforated substance (14).
We observed our three critical points in the preservation of LLSAs and the main M2 branches of the MCA.
Intraoperatively, we first attempted to identify the origin of LLSAs at the horizontal branch of M1 of the MCA. This procedure was conducted primarily for tumors located in zones I and IV. If the proximal segment of the LLSAs was encased by the tumor (Figure 1A), a small cone-like tumor tissue (Figure 1B) was retained at the level of the anterior perforated substance to preserve the proximal branches of the LLSAs as well as to support the LLSAs and avoid distortion after tumor resection, which could subsequently result in ischemia. The cone-like tumor tissue resembled the shape of a triangle in postoperative imaging; thus, it was termed the “residual triangle” (Figure 1C). The horizontal branch of M1 formed the anterior side of the “residual triangle.” The posterior side extended from the origin of the LLSAs to the entry point of the anterior perforated substance. The superior point was at the origin of the LLSAs from M1 (Figure 1D).
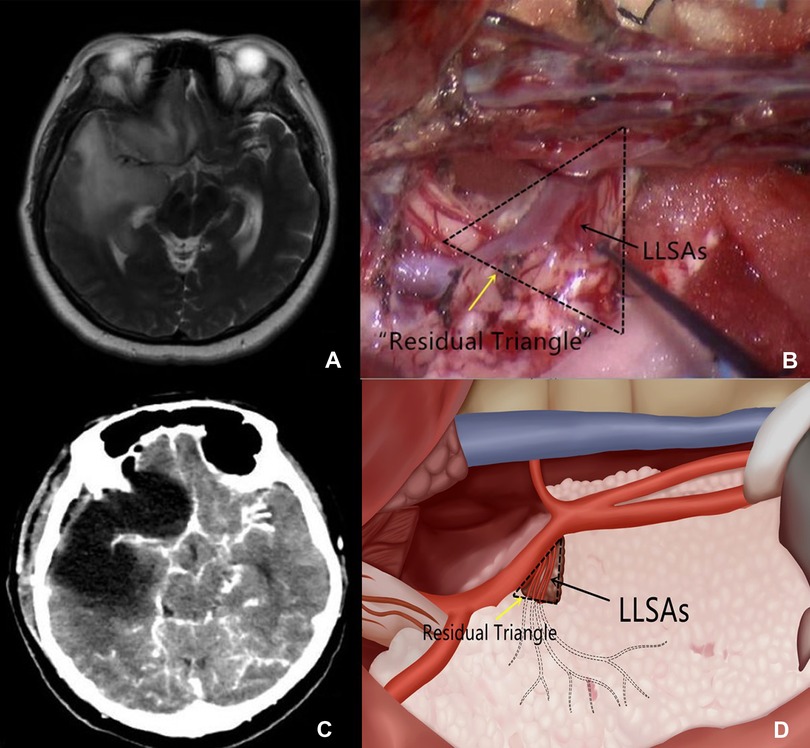
Figure 1. (A) T2-weighted imaging shows that the proximal segment of the LLSAs is encased by the glioma. (B) Intraoperative imaging shows that a small cone-like tumor tissue at the proximal segment of the LLSAs (black arrow) is retained. It also outlines the residual triangle (dotted triangle, yellow arrow). (C) Postoperative CT demonstrates the cone-like tumor tissue supporting the MCA. (D) Schematic depiction of the residual triangles and LLSAs. LLSAs, lateral lenticulostriate arteries; CT, computed tomography; MCA, middle cerebral artery.
Identifying the depth of resection and preserving the distal segment of the LLSAs was another critical point we observed for insular glioma resection. The basal ganglia was usually adjacent to or even invaded by insular gliomas in some cases (Figure 2A). Particularly for tumors that invaded the basal ganglia, the anatomical depth of resection of the basal ganglia (lateral surface of the putamen and the head of the caudate nucleus) (Figure 2B) was dependent on the texture of the basal ganglia and distal branches of the LLSAs. The basal ganglia was orange-yellow and partially crisp in appearance, which was similar to the Chinese tofu kasu and Indonesian nutmeg (15). Intraoperatively, once the structure like this was observed under the microscope, the depth of resection arrived, then the operation continued shallowly to protect the external capsule on the surface of basal ganglia and the distal branches of the LLSA. Then the tumor resection continued at this depth with repeated confirmation of putamen and LLSAs. Thus, the lateral outline of basal ganglia was delineated and termed the “basal ganglia outline reappearance.” Using this method, we prevented direct injury to the pyramidal tract, preserved the distal branches of the LLSA, and safely achieved maximal resection of the tumor.
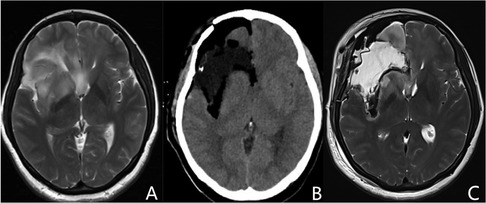
Figure 2. Representative images of the basal ganglia outline reappearance. (A) Preoperative MRI shows that the basal ganglia is invaded by insular gliomas. (B,C) Postoperative CT and MRI T2 imaging demonstrate the artificial profile of the basal ganglia, which is called the basal ganglia outline reappearance. MRI, magnetic resonance imaging; CT, computed tomography.
Finally, as for the insular glioma, insular arteries are the primary providers of blood supply to insular gliomas. The tumor is resected in a subpial fashion with preservation of M2 vessels and coagulation of the insular artery. We used initiative rather than passive hemostasis (15). Like a sculptor, the surgeon needs carefulness and patience. This skill was named as “sculpting” technique. After resecting the tumor, the skeletonized main branches of M2 were preserved and suspended in the operative cavity (13).
All patients underwent preoperative and postoperative magnetic resonance imaging (MRI). Postoperative MRI, including diffusion-weighted imaging (DWI), was performed within 72 h after surgery. Evaluation of the imaging was conducted independently by a neuroradiologist and neurosurgeon, who were blinded to the patient's clinical courses. Four radiological characteristics which might associate with ischemia were evaluated. The relationship between the proximal segment of the LLSAs and the tumor was determined on T2-weighted images. Based on this, we decided whether the proximal segment of the LLSAs was encased by the tumor (Figure 1A). The flat inner edge sign used to describe the inner edge of the insular glioma is well-defined without putamen being involved and can be seen on preoperative T2-weighted images. Figure 3, on the contrary, is the obscure inner edge sign. At the level of the foramen of Monro on axial T2-weighted images, a perpendicular line at the midpoint of the posterior limb of the internal capsule was created, and the length of the line to the tumor was defined as the distance between the lesion and posterior limb of the internal capsule. The tumor's invasion of the superior limiting sulcus was identified via sagittal and coronal MRI. The volume and extent of resection (EOR) were calculated as previously described (13).
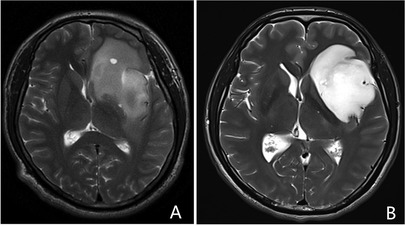
Figure 3. Representative images of the flat inner edge sign of insular gliomas based on the preoperative MRI. (A) Insular glioma with an obscure inner edge sign. (B) Insular glioma with a flat inner sign. MRI, magnetic resonance imaging.
Two experienced technicians performed IONM. Somatosensory evoked potentials (SSEPs) and continued transcranial MEPs of the extremities were monitored in all patients as previously described (16, 17). We defined a decline in MEP amplitude >50% as significant deterioration, provided that technical issues did not cause the decline.
Areas that appeared hyperintense on DWI and hypointense on apparent diffusion coefficient mapping of early postoperative MRI (Figure 4) were classified as ischemic lesions (4, 18). Diffusion restrictions caused by methemoglobin as identified on T2-weighted gradient echo images were excluded.
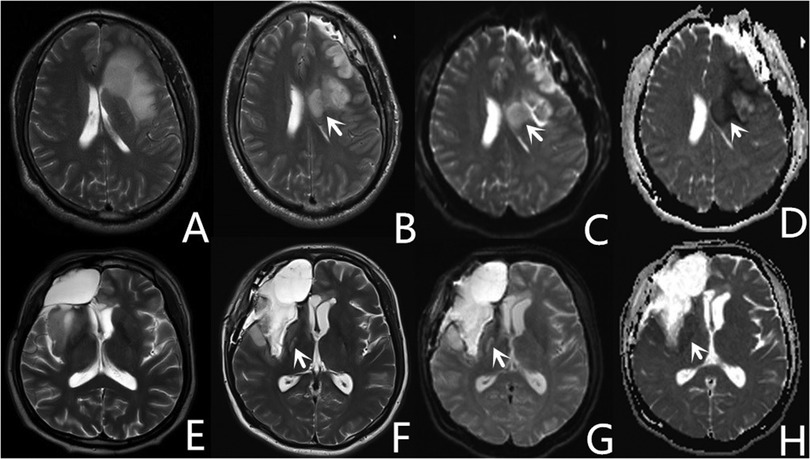
Figure 4. Representative images of two patients with core ischemia. One patient with postoperative ischemia (white arrow) located in the corona radiata (A–D), appears as hyperintense images on diffusion-weighted imaging (C), and hypointense images on apparent diffusion coefficient (D). Another patient with postoperative ischemia (white arrow) located in the posterior limb of the internal capsule (E–H).
The ischemic lesions were further classified into two categories: core ischemia and noncore ischemia. Core ischemia was characterized by circumscribed areas located in the corona radiata (Figures 4B–D) or the posterior limb of the internal capsule (Figures 4F–H). Ischemia in locations other than the corona radiata and the posterior limb of the internal capsule was classified as noncore ischemia.
Postoperative neurological functions, which mainly include motor and language functions, were assessed by two neurosurgeons (ZoH and ZD, with 10 and 15 years of experience, respectively). Next, the data were extracted by ZhH from the medical records and follow-up databases. Any newly developed neurological deterioration was considered a postoperative neurological deficit and evaluated 7 days and 6 months after surgery. Motor function was assessed according to the standardized Medical Research Council (MRC) score.
All statistical analyses were performed using the SPSS for Windows software (version 21.0; IBM Corporation). Significance was set at a P value < 0.05 for the entire analysis. The Kolmogorov–Smirnov and equal variance tests were performed before any other statistical analysis. Continuous data with normal distribution were analyzed using Student's t-test and expressed as mean ± standard deviation. Data with non-normal distribution were analyzed using the Wilcoxon two-sample test and expressed as median (interquartile range). Categorical data were analyzed using Fischer's exact or chi-square tests. Multivariate logistic regression analysis was used to determine the risk factors for the development of core ischemia. To assess the relationship between the four surgical indicators and outcomes of the surgical procedure, we performed logistic multivariate regression analyses. Any variable with a P value < 0.10 in the univariate analysis was considered a potential confounder and further included in the multivariate logistic regression analysis for adjustment.
A total of 75 patients were included in this study (Table 1). The mean age of the patients was 42.6 ± 12.1 years, and 51 patients (68.0%) were male. Two patients underwent surgery due to tumor recurrence. Moreover, 41 patients (54.7%) had right-sided tumors. Preoperative clinical manifestations included seizures (58.7%), incidental findings (20.0%), limb numbness (14.7%), headache (4.0%), and speech deficits (2.6%). A majority of patients had preoperative Karnofsky Performance Scale (KPS) scores ≥ 90 (93.3%) and modified Rankin Scale score < 2 (94.7%). Based on the Berger–Sanai classification (Table 2), 18 (24.0%) tumors were located in zone I, 3 (4.0%) in zone II, 1 (1.3%) in zone III, 1 (1.3%) in zone IV, 8 (10.7%) in zone I + II, 13 (17.3%) in zone I + IV, 3 (4.0%) in zone II + III, and 11 (14.7%) in zone III + IV. Seventeen tumors (22.7%) were classified as giant.
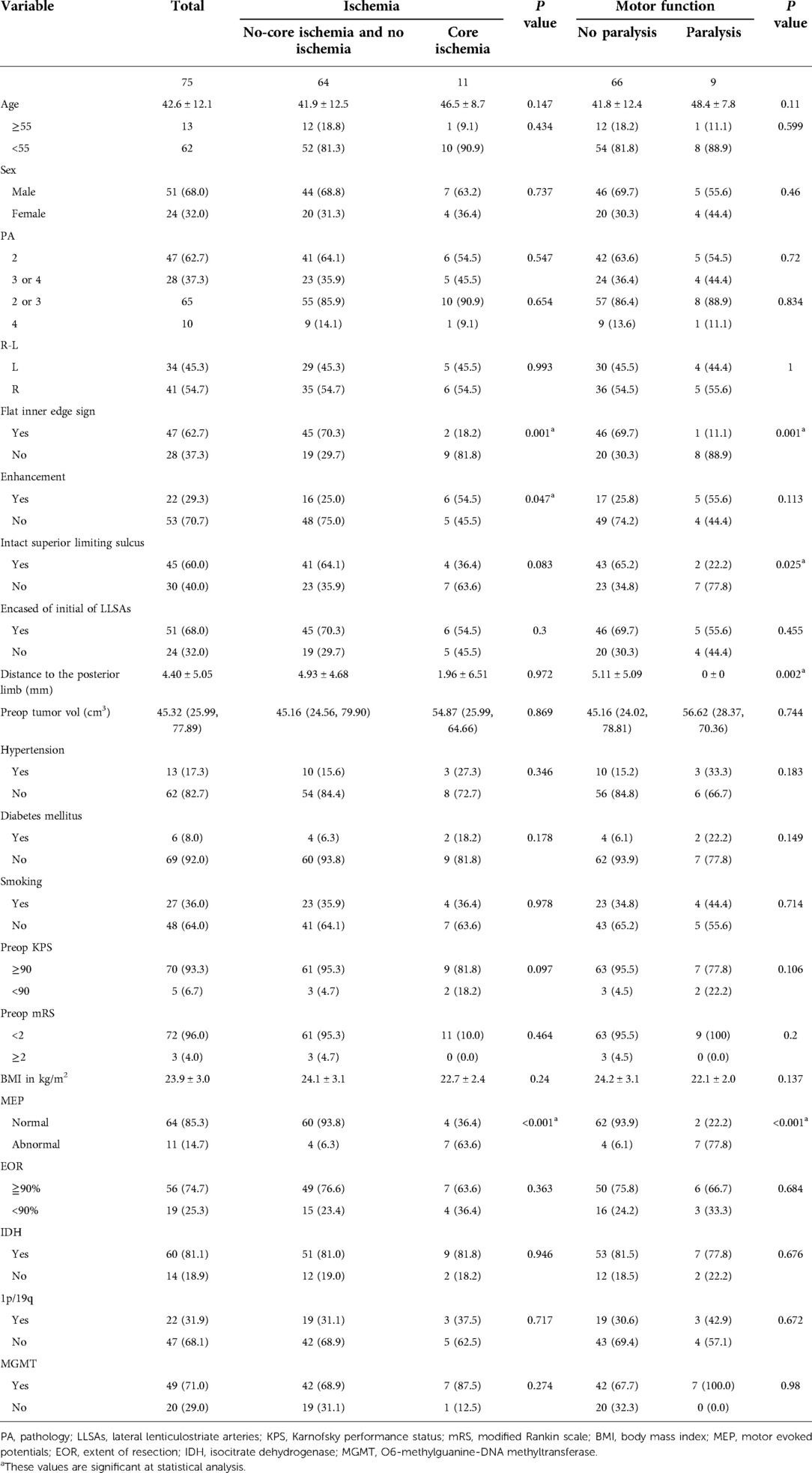
Table 1. Comparison of baseline demographics and clinical characteristics of patients with and without core ischemia and paralysis.
The mean preoperative tumor volume was 57.7 ± 43.4 cm3. The EOR was ≥90% in 56 patients (74.6%). There was no significant difference in the preoperative tumor volume (P > 0.05) or EOR (P > 0.05) between low-grade gliomas (LGGs) and high-grade gliomas (HGGs). The mean EOR was 91.4% in zone I, 86.3% in zone II, 94.0% in zone III, 100% in zone IV, 90.5% in zone I + II, 89.4% in zone I + IV, 92% in zone II + III, 89.1% in zone III + IV, and 89.7% in giant tumors.
The histological composition of tumors was as follows: WHO grade II (62.7%) glioma in 47 patients, grade III glioma (24.0%) in 18 patients, and grade IV glioma (13.3%) in 10 patients. Molecular diagnostic tests revealed the presence of isocitrate dehydrogenase 1/2 mutations in 60 of the 74 cases (71.1%), 1p19q co-deletion in 22 of the 69 cases (31.9%), and O6-methylguanine-DNA methyltransferase promoter methylation in 49 of the 69 cases (71.1%).
Radiotherapy or chemotherapy was recommended postoperatively based on molecular biomarkers and EOR for LGGs. For HGGs, concurrent chemoradiotherapy was performed following the Stupp regimen (19).
Complete short-term and long-term (within 7 days and 6 months postoperatively, respectively) follow-up data were available for all 75 patients. Postoperatively, at 7 days, motor deficits were observed in nine patients (12.0%, according to the Standardized MRC score 3–4/5 in six cases and 0–2/5 in three cases). Facial weakness occurred in six patients (8%). At the 6 months follow-up, two of the nine patients with an early postoperative mild motor deficit demonstrated full recovery, and the other seven patients also improved—five of the seven patients were able to walk (MRC score 4/5, lower limb; MRC score 3–4/5, upper limb), and the remaining two patients unable to walk (MRC score 3/5, both lower and upper limbs). Additionally, facial weakness resolved in five patients with facial. Moreover, the sixth patient with facial weakness also showed improvement.
Language deficits were found in 8 of the 34 patients who underwent surgery on their dominant side, and 4 of 8 patients with aphasia recovered within 7 days. At the 6 month follow-up, improvement in language function was observed in the other four patients with aphasia. However, mild speech fluency disorders persisted in three of these four patients, and mild impairment in speech comprehension stayed in one of these patients.
Of the 75 patients, 44 (58.7%) presented with acute ischemia as detected by postoperative DWI, most of which were located below the resection cavity. Further, the size varied on a case-to-case basis. The median infarction volume was 7.77 cm3 (range 0.69–27.76). The following locations were involved by stroke: ischemia surrounding the resection cavity, 26 (59.1%); the deep-seated ischemia, including the caudate nucleus, corona radiata, and internal capsule, 5 (11.4%); the combination of the latter two, 13 (29.5%). Of the 18 ischemia involving deep structure, head of caudate nucleus and anterior limb of internal capsule ischemia in seven (38.9%) patients, caused by the anterior branch of LLSAs; posterior limb of internal capsule ischemia in three (16.7%) patients, caused by the posterior branch LLSAs; and corona radiata ischemia in eight (44.4%) patients, caused by the long insular artery. The latter 11 patients were determined as core ischemia. Nine of the 11 patients with core ischemia developed new acute motor deficits (Table 1). We also found that six of 11 patients with core ischemia and six of nine patients with paralysis had tumor locations related to zone II.
The retrospective analysis of preoperative MR images revealed the presence of flat inner edge signs in 47 of the 75 tumors (62.7%). Core ischemia developed in two patients, and motor complications developed in one of the 47 patients with flat inner edge signs, which was significantly lower than the number of patients with obscure inner edge signs who developed motor complications (P < 0.05).
The tumor encased the proximal segment of the LLSAs in 51 of the 75 cases. The “residual triangle” was found in 43 patients, accounting for 84.3% of the 51 patients with encased tumors. The “residual triangle” volume ranged from 0.38 to 8.89 cm3, with a mean value of 1.06 cm3, accounting for 1.7% of the preoperative tumor volume. Evidence of the superior limiting sulcus invaded by the tumor was identified in 30 of the 75 patients (40.0%). The distance between the lesion and the posterior limb of the internal capsule was 4.50 ± 5.05 mm. None of these three neuroimaging factors were significantly associated with core ischemia.
IONM data of all 75 patients were available. Eleven patients (14.7%) showed a more than 50% decline in MEP intraoperatively. The decline in MEP was significantly associated with the presence of core ischemia (P < 0.05), postoperative paralysis (P < 0.05), and paralysis at the 6 month follow-up (P < 0.05), but not with noncore ischemia (P > 0.05).
After a multivariate analysis of potential risk factors for core ischemia, flat inner edge sign [odds ratio (OR), 0.144; 95% confidence interval (CI), 0.024–0.876) and MEPs (<50%) (OR, 18.182; 95% CI, 3.311–100.00) had significant associations with postoperative core ischemia (Table 3). Additionally, another multivariate analysis of potential risk factors for paralysis revealed that flat inner edge sign (OR, 0.079; 95% CI, 0.007–0.914) and MEPs (<50%) (OR, 40.00; 95% CI, 5.24–333.33) had significant associations with postoperative paralysis (Table 4).
We found that ischemic complications were common in insular glioma resection in the study. Additionally, patients whose tumors had the obscure inner edge and whose MEPs declined by >50% intraoperatively were at high risk for developing intraoperative strokes that resulted in motor deficits. Further, we described our strategies for arterial preservation during tumor resection.
In our patients, a transcortical approach was used for better exposure and suitability for glioma resection beyond the insula (20). The transcortical windows in our patients were limited to the pars orbitalis and triangularis of the inferior frontal gyrus. Almost all patients with postoperative speech disorders recovered their capacity for simple daily communication, suggesting that language plasticity might play a role (21). Mostly, the pars triangularis does not always involve the Broca’s area, especially for tumors located in the dominant hemispheres (10). To ensure motor function in patients with insular glioma, preservation of the arteries is critical.
The vascular supply to the insular is mainly derived from the M2 branches that overlie the insular surface (22). The course of the M2 segment along the insular surface constitutes a rich arterial network, which is a substantial obstacle in accessing the insular region (20, 23, 24), especially when the arterial network is encased in, distorted, or even wrapped around by the tumor. LLSAs may also be encased by the tumor and serve as a source of vascular supply to the tumor. The long insular artery and long medullary arteries are usually thin, long course, and have ambiguous beginnings. All these characteristics of insular gliomas make maximal resection difficult and increase the risk of ischemic events. Moreover, the insula is largely and frequently supplied by perforating arteries, with no collateral blood supply (25). New evidence suggests that motor deficits often arise from ischemia of the pyramidal tract due to compromise of the blood vessels supplying to the corona radiata and the internal capsule (18).
Moreover, most insular glioma resections affected LLSAs, long insular arteries, and even long medullary arteries terminating the M4 branches (3–5). Various mechanisms for developing intraoperative brain ischemia have been proposed, including direct vascular damage, vasospasm, and kinking of the arteries by brain retraction (18, 26). Therefore, preservation of these arteries is essential in insular glioma resection.
Ischemic complications of insular glioma resection with a transcortical approach are commonly detected on postoperative MRI, with incidence ranging from 1.4% to 75.4%, and are a significant source of neurological morbidity (12, 21, 26–37). The primary series of procedures for insular glioma resection using the transcortical approach are presented in Table 5. A series of 255 procedures utilizing the transcortical approach had a short-term complication rate of 12.79% and a long-term complication rate of 15.70%, 66.7% of these long-term complications could be attributed to the LLSAs and MCA infarction (30). In 61 of Pallud's 111 cases of insular gliomas who underwent awake transcortical approach, diffusion-weighted hyperintensity was observed in 39 postoperative MRI (75.4%): lenticulostriate artery territory in 5 cases, M2/M3 perforator territory in 2, and anterior choroidal artery territory in 1 (1.6%) (34). These eight deep infarcts were systematically associated with a motor deficit. In Mandonnet's series of procedures using transcortical windows for 12 patients, only 1 patient had severe dysarthria associated with a right facial palsy which resolved at 4 months; however, a slightly reduced speech rate was observed (31). Of these 12 patients, none had small areas of ischemia. In a cohort study by Przybylowski et al., which analyzed 48 patients who underwent a transcortical approach, 10 patients (21%) in the transcortical group showed evidence of ischemia on postoperative MR images (27). At the 4- to 6-week clinical evaluation, eight patients (17%) in the transcortical group demonstrated new or worsened neurological deficits. At 1 year postoperatively, four patients (8%) in the transcortical group had persistent neurological deficits. As for insular glioma surgery in this study, ischemic lesions were found in 44 (58.7%) patients using DWI, and core ischemia was noted in 11 of the 44 patients.
Although the incidence of ischemia in our study was slightly higher than that in previous studies (26), it was still within the known range following insular glioma surgery. According to previous research, the insular tumor location is the most potent risk factor for the development of intraoperative ischemia, affecting almost all patients with ischemia (26). In our study, multivariate analysis showed that core ischemia was significantly associated with an obscure inner edge sign. This may be explained by the flat inner edge between the tumor and basal ganglia, wherein the tumor did not invade the basal ganglia; hence, the tumor could be totally resected, and the risk of LLSA damage was low. The presence of an obscure inner edge sign corresponded to a significantly increased risk of LLSA damage. In our study, four patients with less than 90% tumor removal had core ischemia. All the preoperative MRIs of these four patients showed obscure inner edge signs, which may be the main reason for core ischemia.
Tumor location and size may be a significant factor in postoperative risk, while in our study, tumor preoperative volume was not significantly associated with core ischemia, as shown in Table 1. Preoperative tumor volume was a significant predictor of EOR, which is in line with Eseonu’s study (28). Insular tumors localized in zones II and III present significant surgical difficulties, a minor extent of resection, and a higher rate of permanent morbidity (35). Also shown in our study, zone II is a major risk factor for core ischemia and paralysis.
Preservation of the LLSAs during microsurgical resection of insular gliomas is paramount. A few strategies may be effectively employed to enhance the probability of preventing iatrogenic injury to these vessels. Digital subtraction angiography was used to assess the anatomy of the vessels preoperatively (38), while indocyanine green fluorescence helped in identifying and preserving the long perforating branches of the MCA (39). Intraoperative ultrasonography may be facilitated by using contrast agents to improve LLSA visualization (40). Fluorescence-guided surgery with the use of 5-aminolevulinic acid may also help delineate the tumor at the medial edge of dissection for high-grade lesions (41). Intraoperative MRI may help assess the degree of resection and residual tumor at the medial border, which may allow further tumor resection (42). Subcortical motor mapping is performed in the medial plane of the resection to identify the internal capsule (11). However, these methods have several limitations. Notably, the number of LLSAs determined by each method is variable. Moreover, the identification of LLSAs and basal ganglia under a microscope is also essential. In combination with the surgeon’s experience, modern technology enables the safe resection of insular tumors, regardless of their anatomical and functional complexities.
Our continued experience with insular tumor resection has convinced us that the key to the resection of insular lesions is the preservation of the LLSAs and main branches of the MCA. In our experience, different strategies are used, depending on the tumor's location, according to the Berger–Sinai classification. Preservation of the proximal segment of the LLSAs in the anterior location is paramount. If the tumor encases the proximal segment of the LLSAs, it is difficult to completely resect the tumor and the risk of motor deficits increases (43). Given our experience in such situations, we made the “residual triangle” at the proximal segment of the LLSAs to preserve them. We conducted this procedure to avoid distortion, which may cause ischemia (44). In this study, the multivariate analysis did not show a significant association between the tumor encasement of the proximal segment of the LLSAs and core ischemia. Furthermore, the mean volume of the “residual triangle” was <2% of the tumor volume and had little influence on the EOR. This may confirm the effectiveness of this strategy in preserving proximal LLSAs. Moreover, the preservation of distal LLSAs is essential for tumors that are located posteriorly. Since the basal ganglia might have been invaded by the insular gliomas if there was no clear border between the tumor and basal ganglia, the “basal ganglia outline reappearance” was created based on the texture of the basal ganglia and distal branches of the LLSAs, which not only helped prevent direct injury to the posterior limb of the internal capsule but also preserved the distal branches of the LLSAs. When the texture of the basal ganglia and distal branches of the LLSAs was identified to guide the depth of resection, direct injury to the white matter motor fibers was unusual (18), and the tumor could be maximally and safely resected. Finally, in all tumor resection procedures, preservation of the main branches of M2 is essential. In a subpial fashion, the “sculpting” technique was used to outline the frame of the main branches of M2. In this way, we could cut off the insular arteries and preserve the arteries that merely passed through the tumor, i.e., the branches of M2.
Regarding the possible limitations of these strategies, the volume of the “residual triangle” should be left as small as possible due to the possibility of tumor relapse. Skrap et al. also advocated that they would leave a minimal part of the tumor around the LLSAs in case they were encased by the tumor (29). In the process of creating the “basal ganglia outline reappearance,” vasospasm might occur in the distal branches of the LLSAs, especially in patients with arteriosclerosis; in our study, ischemia related to LLSAs occurred in 10 patients, 7 of whose ischemia located in the head of caudate nucleus and anterior limb of internal capsule, which caused by the anterior branch of LLSAs; thus, a thin layer of the tumor or the external capsule left on the surface of basal ganglia was necessary.
While injury to the LLSAs accounts for the majority of postoperative hemiparesis following insular glioma resection (45), injury to the long insular artery is not infrequently implicated. Long insular artery and long medullary arteries terminating the M4 branches, which are characteristics of the thin, long course, and ambiguous origin, are difficult to be preserved intraoperatively. As in our study, eight deep-seated ischemia were caused by the long insular artery. Besides the subpial resection and transitory clipping of these vessels under MEP monitoring, Ikegaya et al. describe a strategy in which a small piece of gray matter is spared at the bottom of the peri-insular sulcus, where long insular artery and long medullary arteries pass en route to the pyramidal tract (46), which is the same principle as our “residual triangle.”
Intraoperative detection of an impending stroke is essential because a prompt response may make the decline transient instead of causing permanent deficits (26). Although intraoperative MRI is efficient in estimating the EOR, its use in detecting ischemic lesions is not recommended (47). Multiple studies verified that IONM parameters, including MEP and SSEP, are helpful and reliable for predicting and preventing ischemic brain injury during neurosurgery (48). Generally, MEP showed better diagnostic accuracy than SSEP (49). The monitoring of intraoperative MEPs revealed early ischemia and aided in preventing ischemia from becoming permanent through a therapeutic response such as holding surgery, irrigating with warm saline, and releasing retraction of the brain parenchyma (18, 50). In this study, a 50% MEP decline was significantly associated with core ischemia, postoperative paralysis, and 6 months of paralysis but not noncore ischemia. Thus, continuous IONM is essential during insular glioma resection. In our study, 4 of 11 patients with MEP abnormalities had no paralysis. Of these four patients, two with paralysis immediately after surgery recovered fully within 7 days. The other two patients may be false-positive. Abboud et al. reported a false-positive rate of 1.1% of MEPs for outcome prognostication during surgery for supratentorial lesions (51). As to 4 of 11 patients with MEP normal who had core ischemia, three of these four patients were indeed with a decline in MEP amplitude >50% intraoperatively, while withholding surgery, irrigating with warm saline, and releasing retraction of the brain parenchyma, the amplitude of these three patients recovered. Although these three patients had no paralysis, they did have core ischemia. The other patient may be with false negative, which is caused by edema in peri-resectional regions (52). After all, there is no immediate postoperative MRI scan.
Recently, awake craniotomy with intraoperative cortico-subcortical direct electrical stimulation has become an increasingly accepted and recommended technique worldwide, particularly for lesions in the dominant hemisphere (53, 54). In our study, although 4 of the 34 patients with left-sided tumors had long-term aphasia, these patients could still carry out a basic conversation. This study aimed to emphasize skill techniques for vascular preservation. If these vascular preservation strategies are applied in awake craniotomy, intraoperative direct electrical stimulation, and even network-level approaches (55), maximal insular tumor resection may be achieved, and better cognitive and emotional functional outcomes may be preserved.
The main limitations of our study are its retrospective nature and small sample size. Moreover, neurological functions assessed by the neurosurgeon might leave room for observation bias. Moreover, our strategies for vascular preservation during insular glioma resection require a longer time and more cases. Finally, instead of using a rough KPS, which was the limitation of our study, a detailed description of neurocognitive and other neurological deficits, outcomes, quality of life, and daily life activities would be added in our subsequent work. The mapping of higher-order functions during the awake procedure may address these questions in the future.
Insular glioma resection is associated with a high incidence of ischemia, as detected by DWI, as well as new motor deficits that were determined by the treating neurosurgeons. Insular glioma patients with obscure inner edge signs and intraoperative MEP decline >50% had a higher risk of developing core ischemia. With strategies of LLSAs and main branches of MCA preservation, such as “residual triangle” at the proximal segment of the LLSAs, “basal ganglia outline reappearance” at the distal segment of LLSAs, and “sculpting” technique for the main branches of M2, maximal safe resection of insular gliomas may be achieved. Nevertheless, ischemia and motor deficits occurred, even with artery-preserving strategies intended to minimize such risks. Both function and vessel protection strategies need further exploration.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Beijing Tiantan Hospital, Capital Medical University. Written informed consent for participation was not required for this study in accordance with national legislation and institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JX and HQ contributed to the conceptualization. ZH and ZD contributed to the neurological function evaluation. ZH contributed to the formal analysis and project administration. ZH, ZH, ZL, GL, YX, MW, and SS contributed to the methodology. YZ, JX, and HQ contributed to the supervision. ZH contributed to the writing of the original draft. JX and HQ contributed to the review and editing. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (ID: 81771792 and 82172028), Beijing Municipal Science and Technology Commission (Z171100000117002), and Beijing Health Care Research Project (19-6).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MCA, middle cerebral artery; LLSAs, lateral lenticulostriate arteries; IONM, intraoperative neuromonitoring; MEPs, motor evoked potentials; WHO, World Health Organization; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; EOR, extent of resection; KPS, Karnofsky Performance Scale
1. Duffau H, Capelle L. Preferential brain locations of low-grade gliomas. Cancer. (2004) 100(12):2622–6. doi: 10.1002/cncr.20297
2. Gempt J, Förschler A, Buchmann N, Pape H, Ryang YM, Krieg SM, et al. Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg. (2013) 118(4):801–8. doi: 10.3171/2012.12.jns12125
3. Loit MP, Rheault F, Gayat E, Poisson I, Froelich S, Zhi N, et al. Hotspots of small strokes in glioma surgery: an overlooked risk? Acta Neurochir. (2019) 161(1):91–8. doi: 10.1007/s00701-018-3717-3
4. Kumabe T, Higano S, Takahashi S, Tominaga T. Ischemic complications associated with resection of opercular glioma. J Neurosurg. (2007) 106(2):263–9. doi: 10.3171/jns.2007.106.2.263
5. Delion M, Mercier P. Microanatomical study of the insular perforating arteries. Acta Neurochir. (2014) 156(10):1991–7, discussion 1997–8. doi: 10.1007/s00701-014-2167-9
6. Iwasaki M, Kumabe T, Saito R, Kanamori M, Yamashita Y, Sonoda Y, et al. Preservation of the long insular artery to prevent postoperative motor deficits after resection of insulo-opercular glioma: technical case reports. Neurol Med Chir. (2014) 54(4):321–6. doi: 10.2176/nmc.cr2012-0361
7. Hentschel SJ, Lang FF. Surgical resection of intrinsic insular tumors. Neurosurgery. (2005) 57(1 Suppl):176–83, discussion 183. doi: 10.1227/01.neu.0000163603.70972.ab
8. Moshel YA, Marcus JD, Parker EC, Kelly PJ. Resection of insular gliomas: the importance of lenticulostriate artery position. J Neurosurg. (2008) 109(5):825–34. doi: 10.3171/jns/2008/109/11/0825
9. De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. (2012) 30(20):2559–65. doi: 10.1200/JCO.2011.38.4818
10. Dziedzic TA, Bala A, Marchel A. Anatomical aspects of the insula, opercula and peri-insular white matter for a transcortical approach to insular glioma resection. Neurosurg Rev. (2021) 45:793–806. doi: 10.1007/s10143-021-01602-5
11. Przybylowski CJ, Hervey-Jumper SL, Sanai N. Surgical strategy for insular glioma. J Neurooncol. (2021) 151(3):491–7. doi: 10.1007/s11060-020-03499-4
12. Sanai N, Polley MY, Berger MS. Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. (2010) 112(1):1–9. doi: 10.3171/2009.6.jns0952
13. Li Z, Li G, Liu Z, Pan Y, Hou Z, Wu L, et al. Transcortical approach for insular gliomas: a series of 253 patients. J Neurooncol. (2020) 147(1):59–66. doi: 10.1007/s11060-020-03390-2
14. Pitskhelauri DI, Bykanov AE. Complication avoidance: resection of the insular glioma complicated by iatrogenic injury to the lenticulostriate artery. Acta Neurochir. (2021) 163(11):3093–6. doi: 10.1007/s00701-021-04806-2
15. Rey-Dios R, Cohen-Gadol AA. Technical nuances for surgery of insular gliomas: lessons learned. Neurosurg Focus. (2013) 34(2):E6. doi: 10.3171/2012.12.FOCUS12342
16. Li Z, Fan X, Wang M, Tao X, Qi L, Ling M, et al. Prediction of postoperative motor deficits using motor evoked potential deterioration duration in intracranial aneurysm surgery. Clin Neurophysiol. (2019) 130(5):707–13. doi: 10.1016/j.clinph.2019.02.010
17. Wang M, Li Z, Fan X, Tao X, Qi L, Ling M, et al. A prediction of postoperative neurological deficits following intracranial aneurysm surgery using somatosensory evoked potential deterioration duration. Neurosurg Rev. (2020) 43(1):293–9. doi: 10.1007/s10143-019-01077-5
18. Gempt J, Krieg SM, Hüttinger S, Buchmann N, Ryang YM, Shiban E, et al. Postoperative ischemic changes after glioma resection identified by diffusion-weighted magnetic resonance imaging and their association with intraoperative motor evoked potentials. J Neurosurg. (2013) 119(4):829–36. doi: 10.3171/2013.5.jns121981
19. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
20. Benet A, Hervey-Jumper SL, Sánchez JJG, Lawton MT, Berger MS. Surgical assessment of the insula. Part 1: surgical anatomy and morphometric analysis of the transsylvian and transcortical approaches to the insula. J Neurosurg. (2016) 124(2):469–81. doi: 10.3171/2014.12.JNS142182
21. Duffau H, Moritz-Gasser S, Gatignol P. Functional outcome after language mapping for insular World Health Organization grade II gliomas in the dominant hemisphere: experience with 24 patients. Neurosurg Focus. (2009) 27(2):E7. doi: 10.3171/2009.5.FOCUS0938
22. Tanriover N, Rhoton AL Jr, Kawashima M, Ulm AJ, Yasuda A. Microsurgical anatomy of the insula and the sylvian fissure. J Neurosurg. (2004) 100(5):891–922. doi: 10.3171/jns.2004.100.5.0891
23. Hervey-Jumper SL, Li J, Osorio JA, Lau D, Molinaro AM, Benet A, et al. Surgical assessment of the insula. Part 2: validation of the Berger-Sanai zone classification system for predicting extent of glioma resection. J Neurosurg. (2016) 124(2):482–8. doi: 10.3171/2015.4.JNS1521
24. Türe U, Yaşargil MG, Al-Mefty O, Yaşargil DC. Arteries of the insula. J Neurosurg. (2000) 92(4):676–87. doi: 10.3171/jns.2000.92.4.0676
25. Phan TG, Donnan GA, Wright PM, Reutens DC. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke. (2005) 36(5):986–91. doi: 10.1161/01.STR.0000163087.66828.e9
26. Berger A, Tzarfati G, Costa M, Serafimova M, Korn A, Vendrov I, et al. Incidence and impact of stroke following surgery for low-grade gliomas. J Neurosurg. (2019) 134(1):153–61. doi: 10.3171/2019.10.jns192301
27. Przybylowski CJ, Baranoski JF, So VM, Wilson J, Sanai N. Surgical morbidity of transsylvian versus transcortical approaches to insular gliomas. J Neurosurg. (2019) 132(6):1731–8. doi: 10.3171/2018.12.Jns183075
28. Eseonu CI, ReFaey K, Garcia O, Raghuraman G, Quinones-Hinojosa A. Volumetric analysis of extent of resection, survival, and surgical outcomes for insular gliomas. World Neurosurg. (2017) 103:265–74. doi: 10.1016/j.wneu.2017.04.002
29. Skrap M, Mondani M, Tomasino B, Weis L, Budai R, Pauletto G, et al. Surgery of insular nonenhancing gliomas: volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases. Neurosurgery. (2012) 70(5):1081–93, discussion 1093–4. doi: 10.1227/NEU.0b013e31823f5be5
30. Hameed NUF, Qiu T, Zhuang D, Lu J, Yu Z, Wu S, et al. Transcortical insular glioma resection: clinical outcome and predictors. J Neurosurg. (2018) 131(3):706–16. doi: 10.3171/2018.4.jns18424
31. Mandonnet E. Transopercular resection of IDH-mutated insular glioma: a critical appraisal of an initial experience. World Neurosurg. (2019) 132:e563–e76. doi: 10.1016/j.wneu.2019.08.071
32. Leroy HA, Strachowksi O, Tuleasca C, Vannod-Michel Q, Le Rhun E, Derre B, et al. Microsurgical resection of fronto-temporo-insular gliomas in the non-dominant hemisphere, under general anesthesia using adjunct intraoperative MRI and no cortical and subcortical mapping: a series of 20 consecutive patients. Sci Rep. (2021) 11(1):6994. doi: 10.1038/s41598-021-86165-7
33. Zarino B, Sirtori MA, Meschini T, Bertani GA, Caroli M, Bana C, et al. Insular lobe surgery and cognitive impairment in gliomas operated with intraoperative neurophysiological monitoring. Acta Neurochir. (2021) 163(5):1279–89. doi: 10.1007/s00701-020-04643-9
34. Pallud J, Zanello M, Moiraghi A, Peeters S, Trancart B, Edjlali M, et al. Surgery of insular diffuse gliomas—part 1: transcortical awake resection is safe and independently improves overall survival. Neurosurgery. (2021) 89(4):565–78. doi: 10.1093/neuros/nyab254
35. Rossi M, Gay L, Conti Nibali M, Sciortino T, Ambrogi F, Leonetti A, et al. Challenging giant insular gliomas with brain mapping: evaluation of neurosurgical, neurological, neuropsychological, and quality of life results in a large mono-institutional series. Front Oncol. (2021) 11:629166. doi: 10.3389/fonc.2021.629166
36. Panigrahi M, Doshi S YBKC, Vooturi S. Avoiding complications in surgical resection of insular gliomas—single surgeon experience. Neurol India. (2021) 69(4):904–9. doi: 10.4103/0028-3886.325334
37. Duffau H. Awake surgery for left posterior insular low-grade glioma through the parietorolandic operculum: the need to preserve the functional connectivity. A case series. Front Surg. (2021) 8:824003. doi: 10.3389/fsurg.2021.824003
38. Yasargil MG, von Ammon K, Cavazos E, Doczi T, Reeves JD, Roth P. Tumours of the limbic and paralimbic systems. Acta Neurochir. (1992) 118(1-2):40–52. doi: 10.1007/BF01400725
39. Shah A, Rangarajan V, Kaswa A, Jain S, Goel A. Indocyanine green as an adjunct for resection of insular gliomas. Asian J Neurosurg. (2016) 11(3):276–81. doi: 10.4103/1793-5482.175626
40. Steno A, Jezberova M, Holly V, Timarova G, Steno J. Visualization of lenticulostriate arteries during insular low-grade glioma surgeries by navigated 3D ultrasound power Doppler: technical note. J Neurosurg. (2016) 125(4):1016–23. doi: 10.3171/2015.10.JNS151907
41. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. (2006) 7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9
42. Scherer M, Jungk C, Younsi A, Kickingereder P, Muller S, Unterberg A. Factors triggering an additional resection and determining residual tumor volume on intraoperative MRI: analysis from a prospective single-center registry of supratentorial gliomas. Neurosurg Focus. (2016) 40(3):E4. doi: 10.3171/2015.11.FOCUS15542
43. Kawaguchi T, Kumabe T, Saito R, Kanamori M, Iwasaki M, Yamashita Y, et al. Practical surgical indicators to identify candidates for radical resection of insulo-opercular gliomas. J Neurosurg. (2014) 121(5):1124–32. doi: 10.3171/2014.7.jns13899
44. Hirono S, Ozaki K, Ito D, Matsutani T, Iwadate Y. Hammock middle cerebral artery and delayed infarction in lenticulostriate artery after staged resection of giant insular glioma. World Neurosurg. (2018) 117:80–3. doi: 10.1016/j.wneu.2018.05.226
45. Ghali MGZ. Preservation of the lenticulostriate arteries during insular glioma resection. Asian J Neurosurg. (2020) 15(1):16–21. doi: 10.4103/ajns.AJNS_146_18
46. Ikegaya N, Takahashi A, Kaido T, Kaneko Y, Iwasaki M, Kawahara N, et al. Surgical strategy to avoid ischemic complications of the pyramidal tract in resective epilepsy surgery of the insula: technical case report. J Neurosurg. (2018) 128(4):1173–7. doi: 10.3171/2017.1.jns161278
47. Masuda Y, Akutsu H, Ishikawa E, Matsuda M, Masumoto T, Hiyama T, et al. Evaluation of the extent of resection and detection of ischemic lesions with intraoperative MRI in glioma surgery: is intraoperative MRI superior to early postoperative MRI? J Neurosurg. (2018) 131(1):209–16. doi: 10.3171/2018.3.jns172516
48. Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: intraoperative changes and postoperative outcome. J Neurosurg. (2006) 105(5):675–81. doi: 10.3171/jns.2006.105.5.675
49. Thomas B, Guo D. The diagnostic accuracy of evoked potential monitoring techniques during intracranial aneurysm surgery for predicting postoperative ischemic damage: a systematic review and meta-analysis. World Neurosurg. (2017) 103:829–40.e3. doi: 10.1016/j.wneu.2017.04.071
50. Neuloh G, Simon M, Schramm J. Stroke prevention during surgery for deep-seated gliomas. Neurophysiol Clin. (2007) 37(6):383–9. doi: 10.1016/j.neucli.2007.09.002
51. Abboud T, Mader M, Komboz F, Martens T, Mielke D, Rohde V. False-positive results in transcranial motor evoked potentials for outcome prognostication during surgery for supratentorial lesions. Neurosurg Rev. (2022) 45:2869–75. doi: 10.1007/s10143-022-01803-6
52. Lee JJ, Kim YI, Hong JT, Sung JH, Lee SW, Yang SH. Intraoperative monitoring of motor-evoked potentials for supratentorial tumor surgery. J Korean Neurosurg Soc. (2014) 56(2):98–102. doi: 10.3340/jkns.2014.56.2.98
53. Duffau H. Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir. (2012) 154(4):575–84. doi: 10.1007/s00701-011-1216-x
54. Zigiotto L, Annicchiarico L, Corsini F, Vitali L, Falchi R, Dalpiaz C, et al. Effects of supra-total resection in neurocognitive and oncological outcome of high-grade gliomas comparing asleep and awake surgery. J Neurooncol. (2020) 148(1):97–108. doi: 10.1007/s11060-020-03494-9
Keywords: insular gliomas, surgery, middle cerebral artery, lateral lenticulostriate arteries, ischemia, motor evoked potentials, surgical technique
Citation: Hou Z, Huang Z, Li Z, Deng Z, Li G, Xu Y, Wang M, Sun S, Zhang Y, Qiao H and Xie J (2022) Incidence of ischemic complications and technical nuances of arteries preservation for insular gliomas resection. Front. Surg. 9:956872. doi: 10.3389/fsurg.2022.956872
Received: 30 May 2022; Accepted: 12 September 2022;
Published: 14 October 2022.
Edited by:
Philipp Taussky, Harvard Medical School, United StatesReviewed by:
Takashi Maruyama, Tokyo Women's Medical University, Japan© 2022 Hou, Huang, Li, Deng, Li, Xu, Wang, Sun, Zhang, Qiao and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xie eGllamlhbndAeWVhaC5uZXQ= Hui Qiao cHJvcWlhb0BzaW5hLmNvbQ==
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.