- 1Department of Otorhinolaryngology, Jena University Hospital, Jena, Germany
- 2Department of Medical Statistics, Computer Sciences and Data Sciences, Jena University Hospital, Jena, Germany
Objectives: To determine the decannulation rate (DR) and revision surgery rate after surgery for bilateral vocal fold paralysis (BVFP).
Data Sources: Five databases (MEDLINE, PubMed, Embase, Web of Science, Scopus) were searched for the period 1908–2020.
Methods: The systematic literature review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Data were pooled using a random-mixed-effects model. Randomized controlled trials and non-randomized studies (case-control, cohort, and case series) were included to assess DR and revision surgery rate after different surgical techniques for treatment of BVFP.
Results: The search yielded 857 publications, of which 102 with 2802 patients were included. DR after different types of surgery was: arytenoid abduction (DR 0.93, 95%-confidence interval [CI], 0.86–0.97), endolaryngeal arytenoidectomy (DR 0.92, 95%-CI, 0.86–0.96), external arytenoidectomy (DR 0.94; 95%-CI, 0.71–0.99), external arytenoidectomy and lateralisation (DR 0.87; 95%-CI, 0.73–0.94), laterofixation (DR 0.95; 95%-CI, 0.91–0.97), posterior cordectomy (DR 0.97, 95%-CI, 0.94–0.99), posterior cordectomy and arytenoidectomy (DR 0.98, 95%-CI, 0.93–0.99), posterior cordectomy and subtotal arytenoidectomy (DR 0.98, 95%-CI, 0.88–1.00), posterior cordotomy (DR 0.96, 95%-CI, 0.84–0.99), reinnervation (0.69, 95%-CI, 0.12–0.97), subtotal arytenoidectomy (DR 1.00, 95%-CI, 0.00–1.00) and transverse cordotomy (DR 1.0, 95%-CI, 0.00–1.00). No significant difference between subgroups for DR could be found (Q = 15.67, df = 11, p = 0.1540). The between-study heterogeneity was low (τ2 = 2.2627; τ = 1.5042; I2 = 0.0%). Studies were at high risk of bias.
Conclusion: BLVP is a rare disease and the study quality is insufficient. The existing studies suggest a publication bias and the literature review revealed that there is a lack of prospective controlled studies. There is a lack of standardized measures that takes into account both speech quality and respiratory function and allows adequate comparison of surgical methods.
Introduction
Bilateral vocal fold paralysis (BVFP) is an uncommon condition in which patients are unable to abduct the vocal folds. This results in upper airway obstruction, usually manifested by variable degrees of stridor and/or dyspnoea of varied intensity, often requiring immediate surgical intervention (1). Some cases in which the symptoms worsened over a longer period of time requiring an intervention at a later time (2, 3).. Most of the underlying lesions are iatrogenic damage to the peripheral recurrent laryngeal nerve due to neck surgery (thyroid, parathyroid glands, thymus, oesophagus, and carotid body paragangliomas) as well as cardiosurgical, thoracosurgical, and neurosurgical procedures. Thyroid surgery is the single most common cause of persistent iatrogenic bilateral cord paralysis and accounts for almost a quarter of all cases. The problem occurs in 1% of thyroidectomies (4, 5).
Until the late nineteenth century, tracheotomy was the only surgical method to treat dyspnoea resulting from the bilateral vocal fold paralysis (6). Since the mid-20th century, there have been surgical innovations, mainly minimally invasive endoscopic techniques (7). Recently, there have been experimental trials on reanimating the neurologically impaired larynx by reinnervation procedures or laryngeal pacing (8, 9).
The aim of this meta-analysis was to describe the variety of interventions aimed at restoring the airway patency in BVFP and to compare their success as measured by the decannulation rate (DR) and by the rate of revision surgery (RSR).
Methods
Literature search
Five electronic databases (MEDLINE, PubMed, Embase, Web of Science and Scopus) were screened with following Medical Subject Heading (MeSH) terms: “bilateral vocal fold paralysis”, “bilateral vocal fold palsy”, “BVFP”, “vocal cord paralysis” and “bilateral vocal cord immobility”. All studies published between January 1908 and December 2020 were considered. Moreover, reference lists of identified articles for additional relevant studies were hand-searched.
Selection of cases
Two independent reviewers (K.T.; O.G.L.) reviewed abstracts and full texts. If they came to a different conclusion, a joint decision was made in a discussion. All studies were assessed against the following exclusion criteria: review articles, duplicate patients, absence of essential data (patient count, decannulation rate and operation type), multiple use of same patient dataset and animal studies.
Data extraction
The following data were extracted from the included papers: number of patients, gender, mean age, publication type, intervention, decannulation rate (as primary outcome measure), time between diagnosis and therapy, duration of follow-up, rate of severe complications, rate of reoperation and Oxford Centre for Evidence-based Medicine (CEBM)-Score. Assuming that every patient requiring therapy for respiratory distress would receive a tracheostomy, the decannulation rate referred to all patients included in the respective study.
Subgroup analyses depended on the number of patients, so the therapy groups with less than three representatives had to be excluded from the meta-analysis and the remaining therapy approaches were only mentioned descriptively (Supplementary Table S1).
Statistical analyses
The studies were categorised in subgroups by intervention type. Statistical analyses were carried out in R version 4.0.4 (10, 11). The meta package (version 4.18–0) was used to produce the pooled estimates and forest plots. The meta-analysis was conducted for the rate of decannulated patients (DR) and the revision surgery rate (RSR). Actually, these outcome parameters are proportions. Accordingly, parameter estimation was based on a logistic regression model with random effects fit by maximum likelihood (Laplace approximation). Separate estimates of the between-study heterogeneity were used to pool the results within subgroups of different surgical techniques. It was verified whether the parameter “surgical technique” used for grouping had an impact on both target variables (DR and RSR). Publication bias was assessed via Egger's test for funnel plot asymmetry. I² statistics were used to quantify statistical heterogeneity.
Results
Characteristics of the studies
A total of 837 titles were identified by searching the databases and journal that corresponded to the previously mentioned MeSH terms. Of these, 645 did not meet our study inclusion criteria. The remaining 192 articles were screened based on the review of their abstracts. This meant that a further 78 publications, including systematic reviews, animal studies and multiple publications on the same data set, had to be excluded from the study as they did not meet our inclusion criteria. Other reasons for exclusion were that the essential parameters such as the decannulation rate (DR) were not given or, in the case of comparative studies, these could not be assigned to a surgery type. After reviewing the full texts of the remaining papers, 102 articles were selected for meta-analysis (Supplementary Figure S1).
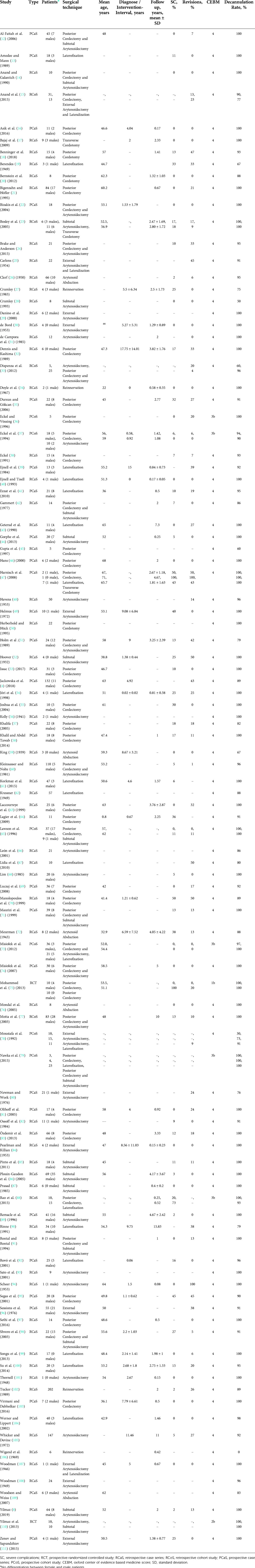
Table 1 displays the characteristics of 102 eligible studies, which were published between 1932 and 2019. Two studies were randomized controlled trials, 30 were prospective case series, 6 prospective cohort studies, 61 retrospective case series and 5 retrospective cohort studies. Sample sizes ranged from 1 to 202, with a total of 2802 patients evaluated across all studies with a weighted mean age of 50.6 years (Figure 1). In the random effects subgroup (surgery type) analysis, we could not find any significant differences with regard to the parameter decannulation rate (Q = 15.67, p = 0.1540). The heterogeneity was low (τ2 = 2.2627; τ = 1.5042; I2 = 0.0%).

Figure 1. Mean age in years (y) of the patient in relation to chosen surgical technique. Dotted line shows weighted mean weight (total number of patients in brackets).
Risk of bias assessment
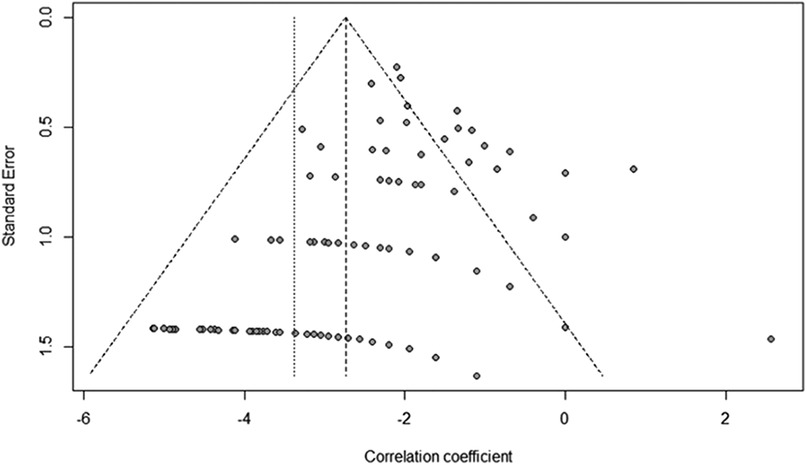
The data was visualized with a funnel plot (Figure 2). Because of result of the Egger's test (t = 9.26, p < 0.0001), a bias was suspected, in particular a publication bias due to the predominant study type (retrospective case series).
Association of surgical techniques to decannulation rate
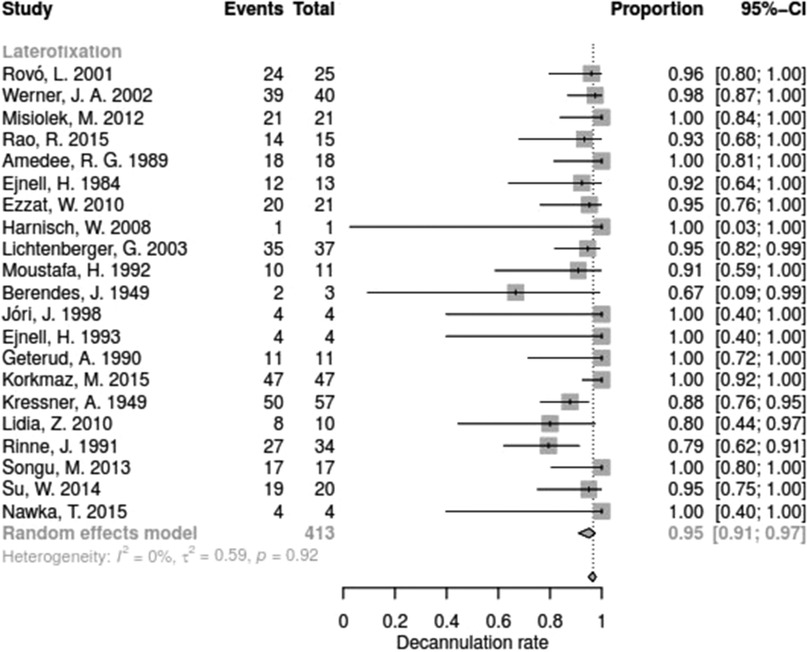
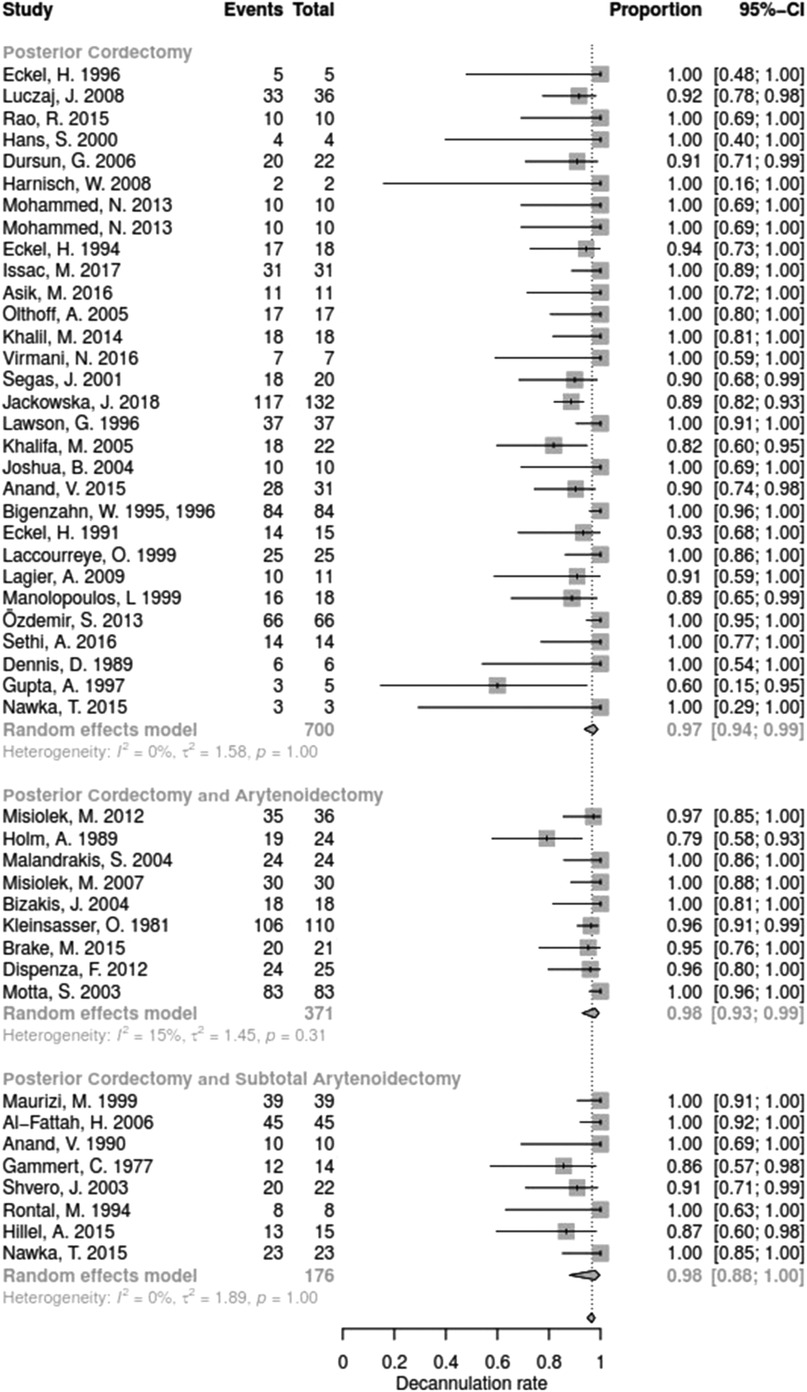
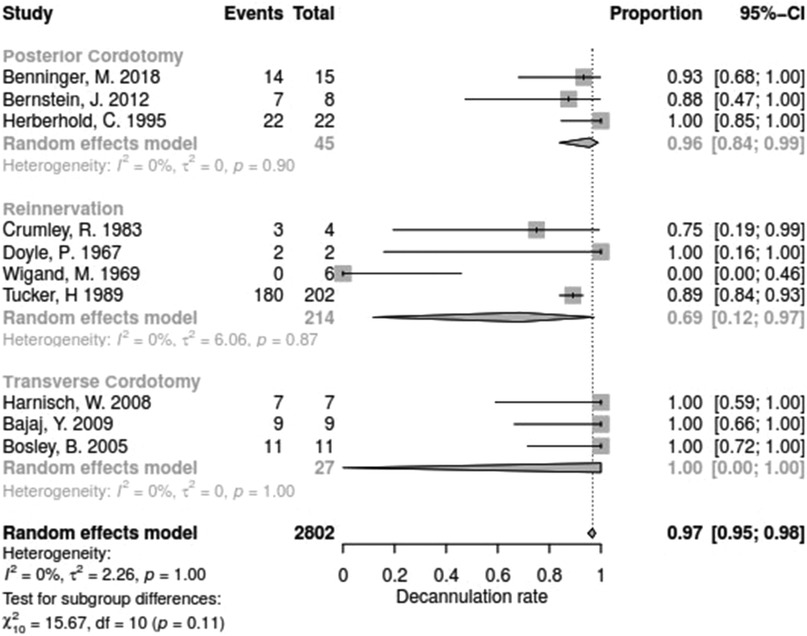
The decannulation rate varied from 50% to 100% between the studies. No significant difference between surgical techniques could be determined with regards for DR (Q = 15.67, df = 11, p = 0.1540).
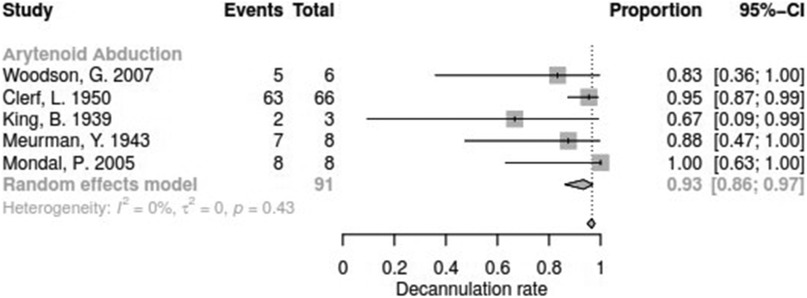
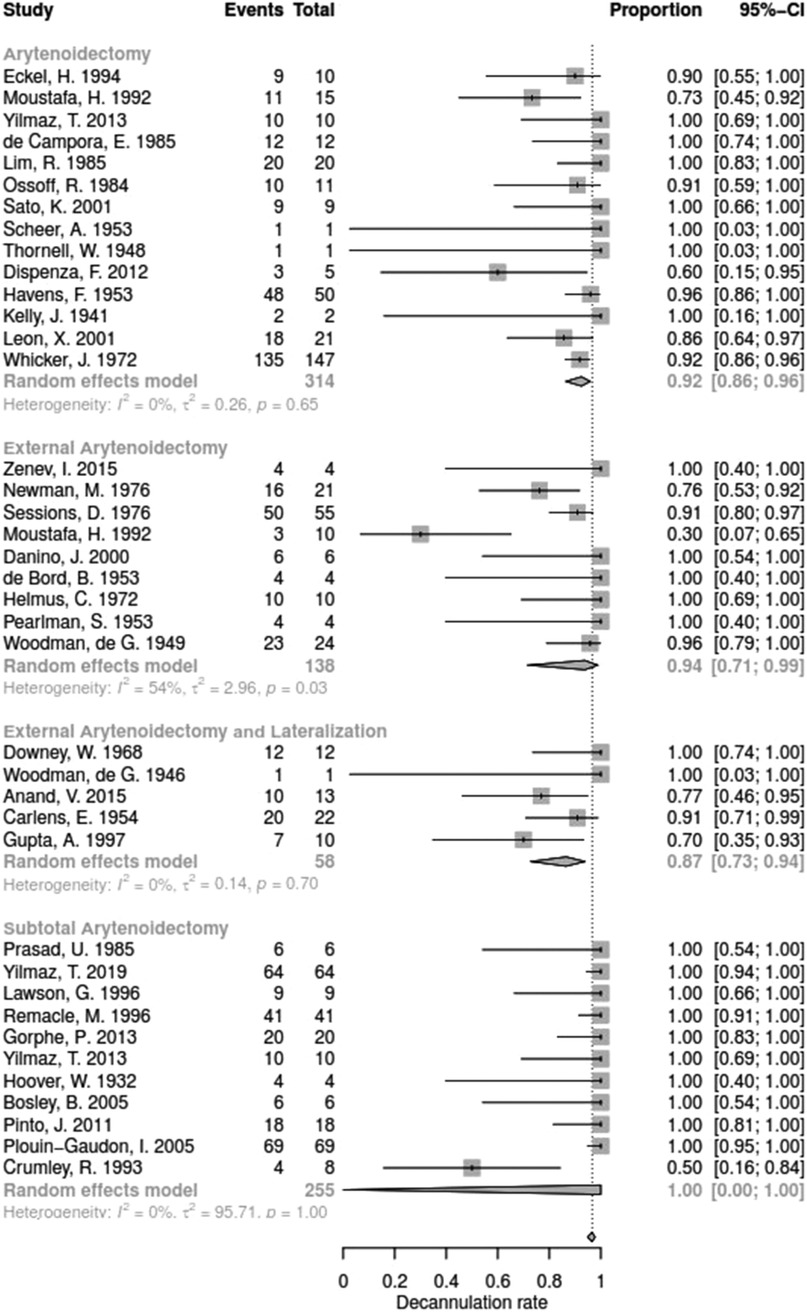
Data for arytenoid abduction was available in five studies with a total of 91 patients (DR = 0.93; 95%-CI, 0.86–0.97, Figure 3). Fourteen studies reported on 314 patients treated by arytenoidectomy (DR = 0.92; 95%-CI, 0.86–0.96) and 11 studies on 255 patients treated by subtotal arytenoidectomy (DR = 1.0; 95%-CI, 0.00–1.00). Nine studies reported on 138 patients treated by external arytenoidectomy (DR = 0.94; 95%-CI, 0.71–0.99) and five studies reported on 58 patients treated by external arytenoidectomy and additional lateralization (DR = 0.87; 95%-CI, 0.73–0.94) (Figure 4). Laterofixation was reported in 21 studies involving 413 patients (DR = 0.95; 95%-CI, 0.91–0.97, Figure 5). Seven hundred (700) patients who underwent posterior cordectomy were reported in 30 studies (DR = 0.97; 95%-CI, 0.94–0.99). In 371 patients reported in nine studies, a arytenoidectomy was also performed (DR = 0.98; 95%-CI, 0.93–0.99). Additional subtotal arytenoidectomy was performed instead in 176 patients reported in eight studies (DR = 0.98; 95%-CI, 0.88–1.00) (Figure 6). Posterior cordotomy was described in three studies involving a total of 45 patients (DR = 0.96; 95%-CI, 0.84–0.99, Figure 7). Transverse cordotomy was the intervention in three studies with a total of 27 patients (DR = 1.00; 95%-CI, 0.00–1.00). In the reinnervation group, there was the lowest decannulation rate (DR = 0.69; 95%-CI, 0.12–0.97, Figure 7). Patients receiving reinnervation surgery were younger than patients receiving other types of surgery (cf. Figure 1).
Association of surgical technique to revision surgery rate
The revision surgery rate (RSR) varied from 0% to 100% between the studies. The difference between surgical techniques in terms of RSR was significant (Q = 38.58, df = 10, p < 0.0001). In 98 studies, RSR was reported for 2453 patients. The heterogeneity was low (τ2 = 1.9139; τ = 1.3834; I2 = 32.8%)
RSR for arytenoid abduction was reported in four studies and 85 patients (RSR = 0.06; 95%-CI, 0.02–0.13; Supplementary Figure S2). The 257 patients in the subgroup arytenoidectomy included nine studies with a pooled RSR of 0.06 (95%-CI, 0.01–0.28). Furthermore, the external arytenoidectomy was described in six studies (98 patients; RSR = 0.08; 95%-CI, 0.01–0.53). In combination with lateralization, the external arytenoidectomy was mentioned in four studies (48 patients; RSR = 0.10; 95%-CI, 0.04–0.23; Supplementary Figure S3). Laterofixation had a pooled revision rate of 0.11 (RSR = 0.11; 95%-CI, 0.04–0.23) for 290 patients in 17 studies (Supplementary Figure S4).
Twenty-six (112) studies have reported on RSRs in posterior cordectomy (RSR = 0.13; 95%-CI, 0.08–0.21) in 671 patients. A further nine studies combined this with arytenoidectomy (371 patients; RSR = 0.02; 95%-CI, 0.00–0.14) and another seven studies with subtotal arytenoidectomy (153 patients; revision rate 0.09; 95%-CI, 0.05–0.14: Supplementary Figure S5). The RSRs for subtotal arytenoidectomy alone have been described in 10 studies (RSR = 0.03; 95%-CI, 0.01–0.15) in 245 patients.
For the reinnervation subgroup (208 patients; RSR = 0.25; 95%-CI, 0.20–0.32) and the transverse cordotomy group (27 patients; RSR = 0.12; 95%-CI, 0.02–0.48), there were only three studies each that included statements about the RSRs (Supplementary Figure S6).
Discussion
All surgical techniques investigated in this meta-analysis, except for the reinnervation techniques, showed high DR (87%–100%). No significant difference was found between the techniques in terms of DR. Since progressive atrophy of the laryngeal muscles is likely if the denervation is permanent and ankyloses of the non-moving cricoarytenoid joint is possible, timely treatment is essential. In both Tucker's and Wigand's techniques of recurrent nerve reinnervation, the failures were mostly due to already atrophic muscles or ankyloses of the cricoarytenoid joint so that no lasting success could be (102, 106). Here, it is necessary to weigh up the possibility of a spontaneous remission in the first 6 months. Due to the assumed publication bias, an overestimation of the positive results regarding DR should be considered.
The RSR showed a wider spread of efficacy (revision rate 0%–100%). The transverse cordotomy technique showed the lowest RSR of 1%. The reinnervation technique had the highest RSR of 25%, whereby account must be taken that the group of patients who underwent reinnervation surgery had the lowest mean age with 22 years. A possible explanation for the higher revision rate in younger patients treated with reinnervation could be increased activity, which is associated with a higher demand for respiratory function. The reinnervation techniques became more sophisticated in the recent years (113). More publications with larger case numbers achieving even better results can be expected in the future. Concerning the transverse cordotomy technique, it has to be taken into account that this is a bilateral procedure, so a larger glottal gap and consequently easier breathing is likely, which, however, has a disadvantageous effect on speech, but this was not investigated here.
Various explanations for variability of RSR could be found. On the one hand, all surgical techniques can lead to very variable degree of granuloma and oedema formation. Larger glottis gaps may increase the risk of aspiration (6). In addition, the surgeon must find in each individual case a compromise between sufficient airflow and speech preservation, which may justify a cautious approach to surgeries with as less resection extent as possible (114), and thereby higher risk of revision surgery. Some variability of the outcome within the same surgical technique can be explained by the learning curve of the individual surgeon or by the different experience within the involved head and neck surgeons. It has also be taken into account, that some of the analysed techniques were easier to perform that other ones. In summary, the inter-individual variability of the outcome of each technique seems to be higher than the variability between different surgical techniques. For the evaluation of the success of a surgical technique, it is also important that a sufficiently long follow-up period after surgery is given, as scar and granulation tissue can subsequently cause deterioration in breathing. This factors could not be investigated further in this meta-analysis, as most publications did not specify the follow-up period. For those who provided information, their mean value varied between 0.08 and 15.83 years (cf. Table 1).
A meta-analysis by Thorpe and Kanotra examined only paediatric patients (115). They compared the decannulation rates of four different surgical techniques (suture lateralization, cricoid split, arytenoidectomy and cordectomy/cordotomy). In addition, no difference in decannulation rates was found between the surgical techniques, but glottic widening surgery after tracheostomy was found to increase the decannulation rate. A meta-analysis on types and timing of surgery for BVFP after thyroidectomy revealed that outcome as more variable after bilateral posterior cordectomy than after early laterofixation and combined laser arytenoidectomy with posterior cordectomy after 12 months (116). Unfortunately, the definition of the outcome criteria remains unclear in this study. Furthermore, there were systematic reviews that attempted to collect the multitude of surgical procedures and provide an overview and decision support (1, 6, 27, 117). A superior surgical technique could not be identified, so it was advised to choose a surgical technique within the expertise of the surgeon and a method that is adapted to the patient's wishes and needs (e.g. higher risk of aspiration in arytenoidectomy) (1, 117). Eckel in his systematic review also chose the decannulation rate as a measure of respiratory disability (with DR of 100%–69.4%) (1).
As long as approaches to restore vocal cord function in permanent BVLP using reinnervation (34, 106, 118), or laryngeal pacemakers (119–121), only have been tried in very small clinical trials, it remains open if these techniques can find the right balance between restoring the airway and maintaining an adequate voice (110). For example, a large glottis gap in the mobile part of the vocal folds leads to impaired voice, but a glottis gap that is too small leads to respiratory distress7 and makes a decannulation impossible. An unlikely functionally complete reinnervation after recurrent nerve reconstruction surgery could only be expected if the original nerve fibres responsible for adduction and abduction, respectively, are reconnected correctly to the respective original target muscles (1).
BVFP is a rare disease and as a result, the published clinical case series often have too few cases to draw statistically significant conclusions (7). The heterogeneity among the chosen clinical parameters to determine the success of a therapy (e.g. forced expiratory pressure in 1 s [FEV1] or maximum phonation time) complicated the comparison. The most common detectable parameter was the DR. In the selected 102 studies, only 13% reported on FEV1, 17% on maximum phonation time and 9% on jitter/shimmer. That is why DR was chosen as a parameter to measure success in this meta-analysis, even though it only examines part of the problem (airway). This strategy provided only limited information about the extent to which respiratory function has been restored (e.g. whether sport is possible or only everyday activities) and no information about vocal function.
Although all relevant studies were included, a risk of publication bias could not be excluded, which was unavoidable due to the rarity of the disease and a lack of high-quality studies (the predominant study type is retrospective case series).
BVFP often already occurs due to neurapraxia of the recurrent laryngeal nerve, so that regeneration is possible (112). Up to which time this can still take place is not fully clarified. A period between 6 and 12 months is discussed (6, 112, 114, 122, 123). Only after this period a permanent paralysis of the vocal folds can be assumed. The mean time between diagnosis and intervention varied in this meta-analysis between 0.08 and 17.75 years (cf. Table 1). In most cases, a unilateral improvement in the function of the vocal cords would be sufficient (92, 104). In unilateral interventions, it cannot be ruled out with certainty that the success is not due to a spontaneous remission of the opposite side without further diagnostics in the period up to 12 months. However, atrophy can also occur within 12 months, jeopardising the success of, for example, reinnervation.
Conclusions
In conclusion, no significant difference in decannulation rates was found between the surgical techniques studied. Since the first clinical experiments around 1908 by Citelli (124), more than 100 years of research have passed and no common therapy standard and measurement of success has been established. There is an urgent need for prospective, randomised clinical trials and the definition of parameters for an objective evaluation of the success of therapy beyond decannulation and revision surgery rate in terms of voice quality, swallowing function, and adequate airway. This applies to patients who require therapy immediately after first occurrence of BLVP as well as to patients with increasing symptoms in the later time course of the disease.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. OG-L designed the study. PS and OG-L provided information about literature search strategies, and assisted with the literature search. Thereafter, KT performed the literature research and performed the analysis. PS assisted with the meta-analysis. KT performed the first draft of the manuscript. PS and OG-L revised the manuscript. All authors contributed to the article and approved the submitted version.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.956338/full#supplementary-material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eckel HE, Sittel C. Beidseitige Rekurrenslähmung. HNO. (2001) 49:166–79. doi: 10.1007/s001060050729
2. Benninger MS, Gillen JB, Altman JS. Changing etiology of vocal fold immobility. Laryngoscope. (1998) 108(9):1346–50. doi: 10.1097/00005537-199809000-00016
3. Iro H. Die beidseitige Rekurrensparese - Glottiserweiternde Eingriffe. HNO Praxis Heute. (1999) 19:71–93. doi: 10.1007/978-3-642-58490-9_4
4. Jackowska J, Sjogren EV, Bartochowska A, Czerniejewska-Wolska H, Piersiala K, Wierzbicka M. Outcomes of CO2 laser-assisted posterior cordectomy in bilateral vocal cord paralysis in 132 cases. Lasers Med Sci. (2018) 33:1115–21. doi: 10.1007/s10103-018-2478-9
5. Yumoto E, Minoda R, Hyodo M,TY. Causes of recurrent laryngeal nerve paralysis. Auris Nasus Larynx. (2002) 29(1):41–5. doi: 10.1016/s0385-8146(01)00122-5
6. Sapundzhiev N, Lichtenberger G, Eckel HE, Friedrich G, Zenev I, Toohill RJ, et al. Surgery of adult bilateral vocal fold paralysis in adduction: history and trends. Eur Arch Oto-Rhino-Laryngol. (2008) 265:1501–14. doi: 10.1007/s00405-008-0665-1
7. Szakács L, Sztanó B, Matievics V, Bere Z, Bach A, Castellanos PF, et al. A comparison between transoral glottis-widening techniques for bilateral vocal fold immobility. Laryngoscope. (2015) 125:2522–9. doi: 10.1002/lary.25401
8. Yılmaz T. Endoscopic partial arytenoidectomy for bilateral vocal fold paralysis: medially based mucosal flap technique. J Voice. (2019) 33:751–8. doi: 10.1016/j.jvoice.2018.04.007
9. Granitzka T, Guntinas-Lichius O, Hagen R, Müller A, Gugatschka MCP. Internationales Register zur beidseitigen Stimmlippenlähmung: Globale Epidemiologie und aktuelle Versorgungsmöglichkeiten. Laryngo-, Rhino-, Otologie. (2018) 97(2):292–3. doi: 10.1055/s-0038-1640748
10. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (Nov 2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117
11. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing Boston, USA (2022). https://www.R-project.org/
12. Al-Fattah HA, Hamza A, Gaafar A, Tantawy A. Partial laser arytenoidectomy in the management of bilateral vocal fold immobility: a modification based on functional anatomical study of the cricoarytenoid joint. Otolaryngology. (2006) 134:294–301. doi: 10.1016/j.otohns.2005.08.028
13. Amedee RG, Mann WJ. A functional approach to lateral fixation in bilateral abductor cord paralysis. Otolaryngology. (1989) 100:542–5. doi: 10.1177/019459988910000603
14. Anand VK, Galantich PT. Advantages of endoscopic laser arytenoidectomy using yag laser scalpel. J Voice. (1990) 4:165–8. doi: 10.1016/S0892-1997(05)80142-0
15. Anand V, Kumaran BR, Chenniappan S. Cordoplasty: a new technique for managing bilateral vocal cord paralysis and its comparison with posterior cordotomy and external procedure in a large study group. Indian J Otolaryngol Head Neck Surg. (2015) 67:40–6. doi: 10.1007/s12070-014-0740-4
16. Asik MB, Karasimav O, Birkent H, Merati AL, Gerek M, Yildiz Y. Impact of unilateral carbon dioxide laser posterior transverse cordotomy on vocal and aerodynamic parameters in bilateral vocal fold paralysis. J Laryngol Otol. (2016) 130:373–9. doi: 10.1017/S0022215116000700
17. Bajaj Y, Sethi N, Shayah A, Harris AT, Henshaw P, Coatesworth AP, et al. Vocal fold paralysis: role of bilateral transverse cordotomy. J Laryngol Otol. (2009) 123:1348–51. doi: 10.1017/S0022215109990983
18. Benninger MS, Xiao R, Osborne K, Bryson PC. Outcomes following cordotomy by coblation for bilateral vocal fold immobility. JAMA Otolaryngol. (2018) 144:149–55. doi: 10.1001/jamaoto.2017.2553
19. Berendes J. Ein retrolaryngealer Weg zur Erweiterung der bei beiderseitiger Posticuslähmung verengten Glottis. Archiv für Ohren-, Nasen- und Kehlkopfheilkunde. (1949) 155:586–90. doi: 10.1007/BF02106159
20. Bernstein JM, Jones SM, Jones PH. Unilateral transverse cordotomy for bilateral abductor vocal fold immobility. J Laryngol Otol. (2012) 126:913–7. doi: 10.1017/S0022215112001521
21. Bigenzahn W, Höfler H. Mikrolaryngeale Laserchirurgie: Glottiserweiterung bei beidseitiger Stimmlippenlähmung. Eur Surg. (1995) 27:211–3. doi: 10.1007/BF02616525
22. Bizakis JG, Papadakis CE, Karatzanis AD, Skoulakis CE, Kyrmizakis DE, Hajiioannou JK, et al. The combined endoscopic CO(2) laser posterior cordectomy and total arytenoidectomy for treatment of bilateral vocal cord paralysis. Clin Otolaryngol Allied Sci. (2004) 29:51–4. doi: 10.1111/j.1365-2273.2004.00779.x
23. Bosley B, Rosen CA, Simpson CB, McMullin BT, Gartner-Schmidt JL. Medial arytenoidectomy versus transverse cordotomy as a treatment for bilateral vocal fold paralysis. Ann Otol Rhinol Laryngol. (2005) 114:922–6. doi: 10.1177/000348940511401205
24. Brake MK, Anderson J. Bilateral vocal fold immobility: a 13 year review of etiologies, management and the utility of the empey index. J Otolaryngol Head Neck Surg. (2015) 44:27. doi: 10.1186/s40463-015-0080-8
25. Carlens E. Bilateral abductor paralysis of the larynx medical and surgical aspects. Acta Otolaryngol. (1954) 43:57–62. doi: 10.3109/00016485409130274
26. Clerf LH. The surgical treatment of bilateral posticus paralysis of the larynx. Trans Am Laryngol Rhinol Otol Soc. (1950) 54th Meeti:64–74. doi: 10.1288/00005537-195002000-00002
27. Cheung EJ, McGinn JD. The surgical treatment of bilateral vocal fold impairment. Oper Tech Otolaryngol Head Neck Surg. (2007) 18:144–55. doi: 10.1016/j.otot.2007.03.007
28. Crumley RL. Endoscopic laser medial arytenoidectomy for airway management in bilateral laryngeal paralysis. Ann Otol Rhinol Laryngol. (1993) 102:81–4. doi: 10.1177/000348949310200201
29. Danino J, Goldenberg D, Joachims HZ. Submucosal arytenoidectomy: new surgical technique and review of the literature. J Otolaryngol. (2000) 29:13–6. PMID: 10709166
30. de Bord BA. Paralytic stenosis of the larynx; a modification of the intralaryngeal approach to arytenoidectomy. Laryngoscope. (1953) 63:757–77. doi: 10.1288/00005537-195309000-00001
31. de Campora E, Camaioni A, Corradini C, D’Agnone N, Calabrese V, Croce A. Thornell’s approach for arytenoidectomy in the surgical treatment of bilateral abductor paralysis; personal experience and results. J Laryngol Otol. (1985) 99:379–82. doi: 10.1017/s0022215100096870
32. Dennis DP, Kashima HK. Carbon dioxide laser posterior cordectomy for treatment of bilateral vocal cord paralysis. Ann Otol Rhinol Laryngol. (1989) 98:930–4. doi: 10.1177/000348948909801203
33. Dispenza F, Dispenza C, Marchese D, Kulamarva G, Saraniti C. Treatment of bilateral vocal cord paralysis following permanent recurrent laryngeal nerve injury. Am J Otolaryngol. (2012) 33:285–8. doi: 10.1016/j.amjoto.2011.07.009
34. Crumley RL. Phrenic nerve graft for bilateral vocal cord paralysis. Laryngoscope. (1983) 93:425–8. doi: 10.1002/lary.1983.93.4.425
35. Dursun G, Gökcan MK. Aerodynamic, acoustic and functional results of posterior transverse laser cordotomy for bilateral abductor vocal fold paralysis. J Laryngol Otol. (2006) 120:282–8. doi: 10.1017/S0022215106000715
36. Eckel HE, Vössing M. Endolaryngeale Operationsverfahren zur Glottiserweiterung bei beidseitiger Rekurrenslahmung. Laryngorhinootologie. (1996) 75:215–22. doi: 10.1055/s-2007-997565
37. Eckel HE, Thumfart M, Vössing M, Wassermann K, Thumfart WF. Cordectomy versus arytenoidectomy in the management of bilateral vocal cord paralysis. Ann Otol Rhinol Laryngol. (1994) 103:852–7. doi: 10.1177/000348949410301105
38. Eckel HE. Die laserchirurgische mikrolaryngoskopische Glottiserweiterung zur Behandlung der beidseitigen Rekurrensparese. Laryngorhinootologie. (1991) 70:17–20. doi: 10.1055/s-2007-997976
39. Ejnell H, Mansson I, Hallén O, Bake B, Stenborg R, Lindström J. A simple operation for bilateral vocal cord paralysis. Laryngoscope. (1984) 94:954–8. doi: 10.1288/00005537-198407000-00018
40. Ejnell H, Tisell L-E. Acute temporary laterofixation for treatment of bilateral vocal cord paralyses after surgery for advanced thyroid carcinoma. World J Surg. (1993) 17:277–81. doi: 10.1007/BF01658947
41. Ezzat WF, Shehata M, Kamal I, Riad MA. Adjustable laterofixation of the vocal fold in bilateral vocal fold paralysis. Laryngoscope. (2010) 120:731–3. doi: 10.1002/lary.20826
42. Gammert C. Eine vereinfachte methode der endolaryngealen glottiserweiterung. Arch Otorhinolaryngol. (1977) 216:593–4. doi: 10.1007/BF00458999
43. Geterud A, Ejnell H, Stenborg R, Bake B. Long-term results with a simple surgical treatment of bilateral vocal cord paralysis. Laryngoscope. (1990) 100:1005–8. doi: 10.1288/00005537-199009000-00016
44. Gorphe P, Hartl D, Primov-Fever A, Hans S, Crevier-Buchman L, Brasnu D. Endoscopic laser medial arytenoidectomy for treatment of bilateral vocal fold paralysis. Eur Arch Oto-Rhino-Laryngol. (2013) 270:1701–5. doi: 10.1007/s00405-013-2414-3
45. Gupta AK, Mann SB, Nagarkar N. Surgical management of bilateral immobile vocal folds and long-term follow-up. J Laryngol Otol. (1997) 111:474–7. doi: 10.1017/s0022215100137685
46. Hans S. Aerodynamic and acoustic parameters in CO2 laser posterior transverse cordotomy for bilateral vocal fold paralysis. Acta Otolaryngol. (2000) 120:330–5. doi: 10.1080/000164800750001206
47. Harnisch W, Brosch S, Schmidt M, Hagen R. Breathing and voice quality after surgical treatment for bilateral vocal cord paralysis. Archives of otolaryngology -head & neck surgery. (2008) 134:278–84. doi: 10.1001/archoto.2007.44
48. Havens FZ. Bilateral paralysis of the vocal cords: treatment by transoral arytenoidectomy. Laryngoscope. (1953) 63:475–84. doi: 10.1288/00005537-195306000-00003
49. Helmus C. Microsurgical thyrotomy and arytenoidectomy for bilateral recurrent laryngeal nerve paralysis. Laryngoscope. (1972) 82:491–503. doi: 10.1288/00005537-197203000-00018
50. Herberhold C, Hück P. Posterior cordotomy by CO2 laser surgery for bilateral vocal cord paralysis: Kashima’s technique and modified technique. Adv Oto-Rhino-Laryngol. (1995) 49:174–5. doi: 10.1159/000424366
51. Holm AF, Wouters B, Van Overbeek JJM. CO2 laser cordectomy for bilateral vocal-cord paralysis. Lasers Med Sci. (1989) 4:93–6. doi: 10.1007/BF02032604
52. Hoover WB. Bilateral abductor paralysis: operative treatment by submucous resection of the vocal cords. Arch Otolaryngol Head aNeck Surg. (1932) 15:339–55. doi: 10.1001/archotol.1932.03570030357001
53. Issac ME. Effect of kashima’s surgery in bilateral abductor vocal cord palsy. Int J Phonosurg Laryngol. (2017) 7:10–2. doi: 10.5005/jp-journals-10023-1133
54. Jóri J, Rovó L, Czigner J. Vocal cord laterofixation as early treatment for acute bilateral abductor paralysis after thyroid surgery. Eur Arch Oto-Rhino-Laryngol. (1998) 255:375–8. doi: 10.1007/s004050050081
55. Joshua B, Feinmesser R, Zohar L, Shvero J. Endoscopic laser-assisted posterior ventriculocordectomy without tracheostomy for bilateral vocal cord immobility. Isr Med Assoc J. (2004) 6:336–8. PMID: 15214459
56. Kelly JD. Surgical treatment of bilateral paralysis of the abductor muscles. Arch Otolaryngol Head Neck Surg. (1941) 33:293–304. doi: 10.1001/archotol.1941.00660030296010
57. Khalifa MC. Simultaneous bilateral posterior cordectomy in bilateral vocal fold paralysis. Otolaryngology. (2005) 132:249–50. doi: 10.1016/j.otohns.2004.09.063
58. Khalil MA, Abdel Tawab HM. Laser posterior cordotomy: is it a good choice in treating bilateral vocal fold abductor paralysis? Clin Med Insights Ear Nose Throat. (2014) 7:13–7. doi: 10.4137/CMENT.S15888
59. King BT. New and function-restoring operation for bilateral abductor cord paralysis. J Am Med Assoc. (1939) 112:814. doi: 10.1001/jama.1939.02800090024005
60. Kleinsasser O, Nolte E. Endolaryngeale Arytaenoidektomie und submuköse partielle Chordektomie bei bilateralen Stimmlippenlähmungen. Laryngol Rhinol Otol (Stuttg). (1981) 60:397–401. doi: 10.1055/s-2007-1008753
61. Korkmaz MH, Bayır Ö, Tatar EÇ, Saylam G, Öcal B, Keseroğlu K, et al. Glottic airway gain after “suture arytenoid laterofixation” in bilateral vocal cord paralysis. Acta Otolaryngol. (2015) 135:931–6. doi: 10.3109/00016489.2015.1042554
62. Kressner A. Über zwei neue operative Verfahren bei bilateraler Posticusparalyse. Archiv für Ohren-, Nasen- und Kehlkopfheilkunde. (1949) 155:459–82. doi: 10.1007/BF02156795
63. Laccourreye O, Escovar M-IP, Gerhardt J, Hans S, Biacabe B, Brasnu D. CO2 Laser endoscopic posterior partial transverse cordotomy for bilateral paralysis of the vocal fold. Laryngoscope. (1999) 109:415–8. doi: 10.1097/00005537-199903000-00014
64. Lagier A, Nicollas R, Sanjuan M, Benoit L, Triglia J-M. Laser cordotomy for the treatment of bilateral vocal cord paralysis in infants. Int J Pediatr Otorhinolaryngol. (2009) 73:9–13. doi: 10.1016/j.ijporl.2008.09.009
65. Lawson G, Remacle M, Hamoir M, Jamart J. Posterior cordectomy and subtotal arytenoidectomy for the treatment of bilateral vocal fold immobility: functional results. J Voice. (1996) 10:314–9. doi: 10.1016/s0892-1997(96)80013-0
66. León X, Venegas MP, Orús C, Quer M, Maranillo E, Sañudo JR. Inmovilidad glótica: estudio retrospectivo de 229 casos. Acta Otorrinolaringol Esp. (2001) 52:486–92. doi: 10.1016/s0001-6519(01)78240-4
67. Lidia Z-G, Magdalena F, Mieczyslaw C. Endoscopic laterofixation in bilateral vocal cords paralysis in children. Int J Pediatr Otorhinolaryngol. (2010) 74:601–3. doi: 10.1016/j.ijporl.2010.02.025
68. Lim RY. Laser arytenoidectomy. Arch Otolaryngol. (1985) 111:262–3. doi: 10.1001/archotol.1985.00800060086013
69. Luczaj J, Koszytyla-Hojna B, Rutkowski R, Rogowski M. Operacyjne poszerzenie szpary głośni z zastosowaniem lasera CO2 w dysfonii porażennej. Pol Merkur Lekarski. (2008) 24:385–91.
70. Manolopoulos L, Stavroulaki P, Yiotakis J, Segas J, Adamopoulos G. CO2 And KTP-532 laser cordectomy for bilateral vocal fold paralysis. J Laryngol Otol. (1999) 113:637–41. doi: 10.1017/s002221510014472x
71. Maurizi M, Paludetti G, Galli J, Cosenza A, Di Girolamo S, Ottaviani F. CO2 laser subtotal arytenoidectomy and posterior true and false cordotomy in the treatment of post-thyroidectomy bilateral laryngeal fixation in adduction. Eur Arch Oto-Rhino-Laryngol. (1999) 256:291–5. doi: 10.1007/s004050050248
72. Meurman Y. Laterofixation der Stimmlippe bei doppelseitiger Posticuslähmung. Archiv für Ohren-, Nasen- und Kehlkopfheilkunde. (1943) 153:163–71. doi: 10.1007/BF01973613
73. Misiołek M, Kłębukowski L, Lisowska G, Czecior E, Ścierski W, Orecka B, et al. Przydatność arytenoidektomii laserowej i laterofiksacji w leczeniu obustronnego porażenia fałdów głosowych. Otolaryngologia polska. (2012) 66:109–16. doi: 10.1016/S0030-6657(12)70757-6
74. Misiolek M, Ziora D, Namyslowski G, Misiolek H, Kucia J, Scierski W, et al. Long-term results in patients after combined laser total arytenoidectomy with posterior cordectomy for bilateral vocal cord paralysis. Eur Arch Oto-Rhino-Laryngol. (2007) 264:895–900. doi: 10.1007/s00405-007-0288-y
75. Mohamed NN, Sorour SS, El-Anwar MW, Quriba AS, Mahdy MA. Comparison between laser- and diathermy-assisted posterior cordotomy for bilateral vocal cord abductor paralysis. JAMA Otolaryngol. (2013) 139:923–30. doi: 10.1001/jamaoto.2013.3554
76. Mondal PK, Pal I, Bera SP, Mondal AR, Biswas S. Surgical management of bilateral abductor paralysis by extralaryngeal approach. Indian J Otolaryngol Head Neck Surg. (2005) 57:75–7. doi: 10.1007/BF02907639
77. Motta S, Moscillo L, Imperiali M, Motta G. CO2 laser treatment of bilateral vocal cord paralysis in adduction. ORL. (2003) 65:359–65. doi: 10.1159/000076055
78. Moustafa H, El-Guindy A, El-Sherief S, Targam A. The role of endoscopic laterofixation of the vocal cord in the treatment of bilateral abductor paralysis. J Laryngol Otol. (1992) 106:31–4. doi: 10.1017/s0022215100118523
79. Nawka T, Sittel C, Gugatschka M, Arens C, Lang-Roth R, Wittekindt C, et al. Permanent transoral surgery of bilateral vocal fold paralysis: a prospective multi-center trial. Laryngoscope. (2015) 125:1401–8. doi: 10.1002/lary.25137
80. Newman MH, Work WP. Arytenoidectomy revisited. Laryngoscope. 1976;86:840–9. doi: 10.1288/00005537-197606000-00010
81. Olthoff A, Zeiss D, Laskawi R, Kruse E, Steiner W. Laser microsurgical bilateral posterior cordectomy for the treatment of bilateral vocal fold paralysis. Ann Otol Rhinol Laryngol. (2005) 114:599–604. doi: 10.1177/000348940511400804
82. Ossoff RH, Sisson GA, Duncavage JA, Moselle HI, Andrews PE, McMillan WG. Endoscopic laser arytenoidectomy for the treatment of bilateral vocal cord paralysis. Laryngoscope. (1984) 94:1293–7. doi: 10.1288/00005537-198410000-00006
83. Özdemir S, Tuncer Ü, Tarkan Ö, Kara K, Sürmelioğlu Ö. Carbon dioxide laser endoscopic posterior cordotomy technique for bilateral abductor vocal cord paralysis: a 15-year experience. JAMA Otolaryngol. (2013) 139:401–4. doi: 10.1001/jamaoto.2013.41
84. Pearlman SJ, Killian EW. Thyrotomy approach for arytenoidectomy in bilateral abductor paralysis of the vocal cords. Ann Otol Rhinol Laryngol. (1953) 62:207–12. doi: 10.1177/000348945306200126
85. Pinto JA, Godoy L, Marquis VWPB, Sonego TB, Leal C. Bilateral vocal fold immobility: diagnosis and treatment. Braz J Otorhinolaryngol. (2011) 77:594–9. doi: 10.1590/S1808-86942011000500010
86. Plouin-Gaudon I, Lawson G, Jamart J, Remacle M. Subtotal carbon dioxide laser arytenoidectomy for the treatment of bilateral vocal fold immobility: long-term results. Ann Otol Rhinol Laryngol. (2005) 114:115–21. doi: 10.1177/000348940511400206
87. Prasad U. CO2 Surgical laser in the management of bilateral vocal cord paralysis. J Laryngol Otol. (1985) 99:891–4. doi: 10.1017/s0022215100097863
88. Rao R, Shenoy V, Prasad V, Kamath P, Hazarika P, Rao KS. A comparative study of laser posterior cordotomy and vocal cord lateralization. Egypt J Ear Nose Throat Allied Sci. (2015) 16:255–8. doi: 10.1016/j.ejenta.2015.08.005
89. Remacle M, Lawson G, Mayné A, Jamart J. Subtotal carbon dioxide laser arytenoidectomy by endoscopic approach for treatment of bilateral cord immobility in adduction. Ann Otol Rhinol Laryngol. (1996) 105:438–45. doi: 10.1177/000348949610500604
90. Rinne J. Late results of laterofixation in the treatment of bilateral abductor paralysis of the vocal cords: a clinical study with long-term follow-up. Clin Otolaryngol Allied Sci. (1991) 16:436–41. doi: 10.1111/j.1365-2273.1991.tb01035.x
91. Rontal M, Rontal E. Use of laryngeal muscular tenotomy for bilateral midline vocal cord fixation. Ann Otol Rhinol Laryngol. (1994) 103:583–9. doi: 10.1177/000348949410300801
92. Rovó L, Jóri J, Iván L, Brzózka M, Czigner J. “Early” vocal cord laterofixation for the treatment of bilateral vocal cord immobility. Eur Arch Oto-Rhino-Laryngol. (2001) 258:509–13. doi: 10.1007/s004050100378
93. Sato K, Umeno H, Nakashima T. Laser arytenoidectomy for bilateral median vocal fold fixation. Laryngoscope. (2001) 111:168–71. doi: 10.1097/00005537-200101000-00029
94. Scheer AA. Laryngofissure approach in surgical treatment of bilateral abductor paralysis. AMA Arch Otolaryngol. (1953) 57:173–81. doi: 10.1001/archotol.1953.00710030192006
95. Segas J, Stavroulakis P, Manolopoulos L, Yiotakis J, Adamopoulos G. Management of bilateral vocal fold paralysis: experience at the university of Athens. Otolaryngology. (2001) 124:68–71. doi: 10.1067/mhn.2001.111599
96. Sessions DG, Ogura JH, Heeneman H. Surgical management of bilateral vocal cord paralysis. Laryngoscope. (1976) 86:559–66. doi: 10.1288/00005537-197604000-00012
97. Sethi A, Anand V, Das A, Sethi D. Surgical management of bilateral abductor vocal cord paralysis using Coblation technology. J Laryngol Voice. (2016) 6:44. doi: 10.4103/jlv.JLV_11_16
98. Shvero J, Koren R, Stern Y, Segal K, Feinmesser R, Hadar T. Laser posterior ventriculocordectomy with partial arytenoidectomy for the treatment of bilateral vocal fold immobility. J Laryngol Otol. (2003) 117:540–3. doi: 10.1258/002221503322112969
99. Songu M, Aslan H, Denizoglu I, Ozkul Y, Basoglu S, Ates D, et al. Vocal and ventricular fold lateralization using crossing sutures with the thyroplasty window technique for bilateral vocal fold immobility: long-term results. Acta Otolaryngol. (2013) 133:1201–6. doi: 10.3109/00016489.2013.815363
100. Su W-F, Liu S-C, Tang W-S, Yang M-C, Lin Y-Y, Huang T-T. Suture lateralization in patients with bilateral vocal fold paralysis. J Voice. (2014) 28:644–51. doi: 10.1016/j.jvoice.2013.12.012
101. Thornell WC. Intralaryngeal approach for arytenoidectomy in bilateral abductor paralysis of the vocal cords; a preliminary report. Arch Otolaryngol. (1948) 47:505–8. doi: 10.1001/archotol.1948.00690030527016
102. Tucker HM. Long-term results of nerve-muscle pedicle reinnervation for laryngeal paralysis. Ann Otol Rhinol Laryngol (1989) 98:674–6. doi: 10.1177/000348948909800903
103. Virmani N, Dabholkar J. Laser-assisted posterior cordotomy for bilateral vocal fold paralysis: our experience. J Head Neck Phys Surg. (2016) 4:23. doi: 10.4103/2347-8128.182852
104. Werner JA, Lippert BM. Laterofixation der Stimmlippe statt Tracheotomie bei akuter beidseitiger Stimmlippenparese. Deutsche medizinische Wochenschrift. (2002) 127:917–22. doi: 10.1055/s-2002-25381
105. Whicker JH, Devine KD. Long-term results of Thornell arytenoidectomy in the surgical treatment of bilateral vocal cord paralysis. Laryngoscope. (1972) 82:1331–6. doi: 10.1288/00005537-197207000-00023
106. Wigand ME, Naumann C, Hölldobler G. Versuche zur Reinnervation des Abduktormuskels nach Recurrenslähmung durch Einpflanzen freier Nerventransplantate zum Nervus phrenicus. Arch Klin Exp Oh-, Nas- U Kehlk Heilk. (1969) 194:372–7. doi: 10.1007/BF02594494
107. Woodman d. A modification of the extralaryngeal approach to arytenoidectomy for bilateral abductor paralysis. Arch Otolaryngol. (1946) 43:63–5. doi: 10.1001/archotol.1946.00680050073011
108. Woodman d. Rehabilitation of the larynx in cases of bilateral abductor paralysis; open approach to arytenoidectomy, with report of the past 4 years’ experience. Arch Otolaryngol. (1949) 50:91–6. doi: 10.1001/archotol.1949.00700010098008
109. Woodson G, Weiss T. Arytenoid abduction for dynamic rehabilitation of bilateral laryngeal paralysis. Ann Otol Rhinol Laryngol. (2007) 116:483–90. doi: 10.1177/000348940711600702
110. Yılmaz T, Süslü N, Atay G, Özer S, Günaydın RÖ, Bajin MD. Comparison of voice and swallowing parameters after endoscopic total and partial arytenoidectomy for bilateral abductor vocal fold paralysis: a randomized trial. JAMA Otolaryngol. (2013) 139:712–8. doi: 10.1001/jamaoto.2013.3395
111. Zenev I, Sapundzhiev N. Arytenoidcordectomy for bilateral vocal cord paralysis: primary and revision procedure. SANAMED. (2015) 10:23–9. doi: 10.5937/sanamed1501023Z
112. Sittel C, Wassermann K, Mathen F, Eckel HE. Die uni- und bilaterale Lähmung des Nervus laryngeus inferior (recurrens). Pneumologie. (2001) 55:568–78. doi: 10.1055/s-2001-19004
113. Lee JW, Bon-Mardion N, Smith ME, Marie JP. Bilateral selective laryngeal reinnervation for bilateral vocal fold paralysis in children. JAMA Otolaryngol Head Neck Surg. (2020) 146:401–7. doi: 10.1001/jamaoto.2019.4863
114. Reker U, Rudert H. Die modifizierte posteriore Chordektomie nach Dennis und Kashima bei der Behandlung beidseitiger Rekurrensparesen. Laryngorhinootologie. (1998) 77:213–8. doi: 10.1055/s-2007-996963
115. Thorpe RK, Kanotra SP. Surgical management of bilateral vocal fold paralysis in children: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. (Feb 2021) 164(2):255–63. doi: 10.1177/0194599820944892
116. Chen X, Wan P, Yu Y, Li M, Xu Y, Huang P, et al. Types and timing of therapy for vocal fold paresis/paralysis after thyroidectomy: a systematic review and meta-analysis. J Voice. (2014) 28:799–808. doi: 10.1016/j.jvoice.2014.02.003
117. Li Y, Garrett G, Zealear D. Current treatment options for bilateral vocal fold paralysis: a state-of-the-art review. Clin Exp Otorhinolaryngol. (2017) 10:203–12. doi: 10.21053/ceo.2017.00199
118. Doyle PJ, Brummett RE, Everts EC. Results of surgical section and repair of the recurrent laryngeal nerve. Laryngoscope. (1967) 77:1245–54. doi: 10.1288/00005537-196708000-00003
119. Müller AH, Hagen R, Pototschnig C, Foerster G, Grossmann W, Baumbusch K, et al. Laryngeal pacing for bilateral vocal fold paralysis: voice and respiratory aspects. Laryngoscope. (2017) 127:1838–44. doi: 10.1002/lary.26428
120. Zealear DL, Billante CR, Courey MS, Sant’Anna GD, Netterville JL. Electrically stimulated glottal opening combined with adductor muscle botox blockade restores both ventilation and voice in a patient with bilateral laryngeal paralysis. Ann Otol Rhinol Laryngol. (2002) 111:500–6. doi: 10.1177/000348940211100605
121. Billante CR, Zealear DL, Courey MS, Netterville JL. Effect of chronic electrical stimulation of laryngeal muscle on voice. Ann Otol Rhinol Laryngol. (2002) 111:328–32. doi: 10.1177/000348940211100408
122. Mau T, Pan H-M, Childs LF. The natural history of recoverable vocal fold paralysis: implications for kinetics of reinnervation. Laryngoscope. (2017) 127:2585–90. doi: 10.1002/lary.26734
123. Husain S, Sadoughi B, Mor N, Sulica L. Time course of recovery of iatrogenic vocal fold paralysis. Laryngoscope. (2019) 129:1159–63. doi: 10.1002/lary.27572
Keywords: bilateral vocal fold paralysis, treatment outcome, meta-analysis, surgery, decannulation
Citation: Titulaer K, Schlattmann P and Guntinas-Lichius O (2022) Surgery for bilateral vocal fold paralysis: Systematic review and meta-analysis. Front. Surg. 9:956338. doi: 10.3389/fsurg.2022.956338
Received: 30 May 2022; Accepted: 8 July 2022;
Published: 22 July 2022.
Edited by:
Małgorzata Wierzbicka, Poznan University of Medical Sciences, PolandReviewed by:
Marc Joseph Remacle, Centre Hospitalier de Luxembourg, LuxembourgPaul Bryson, Cleveland Clinic, United States
Joanna Jackowska, Poznan University of Medical Sciences, Poland
© 2022 Titulaer, Schlattmann and Guntinas-Lichius. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orlando Guntinas-Lichius b3JsYW5kby5ndW50aW5hc0BtZWQudW5pLWplbmEuZGU=
†ORCID Orlando Guntinas-Lichius orcid.org/0000-0001-9671-0784
Specialty Section: This article was submitted to Otorhinolaryngology - Head and Neck Surgery, a section of the journal Frontiers in Surgery
 Kai Titulaer
Kai Titulaer Peter Schlattmann2
Peter Schlattmann2 Orlando Guntinas-Lichius
Orlando Guntinas-Lichius