
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 11 October 2022
Sec. Genitourinary Surgery and Interventions
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.955932
 Vincenzo Vento1
Vincenzo Vento1 Andrea Benedetto Galosi2,3
Andrea Benedetto Galosi2,3 Andrea Ranghino4
Andrea Ranghino4 Luca Montecchiani1
Luca Montecchiani1 Luca Felici1
Luca Felici1 Silvia Loggi5
Silvia Loggi5 Elisabetta Cerutti5
Elisabetta Cerutti5 Giulio Milanese2,3
Giulio Milanese2,3 Carmine Franzese2,3
Carmine Franzese2,3 Daniele Castellani2,3*
Daniele Castellani2,3* Emanuele Gatta1
Emanuele Gatta1
Background: Giant angiomyolipoma is usually associated with genetic syndromes and complications (spontaneous rupture and bleeding, hematuria, hypertension) and mass-related symptoms (flank and abdominal pain).
Case presentation: We present a case of a 20-year-old woman suffering from tuberous sclerosis who was referred to our hospital with a giant angiomyolipoma causing abdominal pain. A contrast-enhanced computed tomography showed a left angiomyolipoma, measuring 28 cm × 17 cm × 27 cm. After a multidisciplinary team discussion, the patient was submitted for a nephrectomy. Percutaneous temporary occlusion of the main renal artery was achieved through an endovascular balloon catheter. Through the balloon catheter guidewire, 2,500 IU of heparin was infused to reduce the risk of tumor vein thrombosis and venous embolism. This allowed a safe kidney manipulation through a left thoracoabdominal approach. The postoperative course was uneventful. Pathology showed a 40 cm × 30 cm × 9 cm and 10 kg AML. One year after surgery, the patient is on follow-up, and her estimated glomerular filtration is 120.5 ml/min/1.73 m2.
Conclusion: The present case showed that the endovascular control of the main renal artery could be considered a useful approach to safely managing huge renal masses when renal hilar control is expected to be very difficult.
Renal angiomyolipoma (AML) is a benign and uncommon solid renal tumor. Two types of AMLs are described, including sporadic (80%) and those associated with genetic syndromes (20%) such as tuberous sclerosis complex (TSC), von Hippel–Lindau syndrome, and neurofibromatosis type 1 (1, 2). Small AMLs (<4 cm) can be managed conservatively and monitored with a yearly ultrasound or computed tomography (CT) scan (2, 3), while large and growing masses, with complications (spontaneous rupture and retroperitoneal bleeding, hematuria, hypertension) or with mass-related symptoms (flank pain, abdominal pain) usually require treatments (4). Patients affected by TSC with large AMLs experience spontaneous hemorrhages in 25%–50% of cases, leading to emergency treatments that can be associated with poor outcomes (4). Therefore, elective treatments are surgical resection or selective artery embolization. In huge lesions, surgery is the treatment of choice for symptoms or complications. Surgical management of huge lesions consists of nephrectomy with early arterial ligation. However, this can be hazardous when the giant renal mass shields the aorta and vena cava entirely. In addition, AMLs are composed of large and frail networks of veins that cover the squishy tumor, and therefore, mass handling and displacement must be performed carefully to avoid bleeding (5). In some cases, it is advisable for a preoperative artery embolization to be performed several days or hours before surgery as a separate procedure (6). However, this preoperative approach has the disadvantage of involving staged procedures and higher costs. We report a case of a giant AML associated with TSC treated using a novel one-stage hybrid approach.
A 20-year-old Caucasian woman was referred to our hospital with a rapidly growing abdominal mass associated with anemia and mild abdominal and left flank pain. Past medical history was positive for sporadic TSC diagnosed 9 years before. She was on mTOR inhibitor therapy for a cerebral subependymal giant cell astrocytoma. The patient reported a history of abdominal swelling in previous years and was on follow-up for bilateral angiomyolipoma since early infancy. The physical examination showed a palpable abdominal tender mass extending from the left flank to the right iliac fossa. She had no dermatologic lesions.
A contrast-enhanced CT scan showed a left renal tumor of 28 cm × 17 cm × 27 cm (Figure 1), suggestive of a giant angiomyolipoma, occupying the entire abdominal cavity and shifting abdominal organs. A 10 cm × 6.7 cm × 7.9 cm angiomyolipoma was also present in the right kidney (Figure 1A). The left renal artery and vein were dilated to 11 and 26 mm, respectively. The estimated glomerular filtration was 127 ml/min/1.73m2. She denied hematuria.
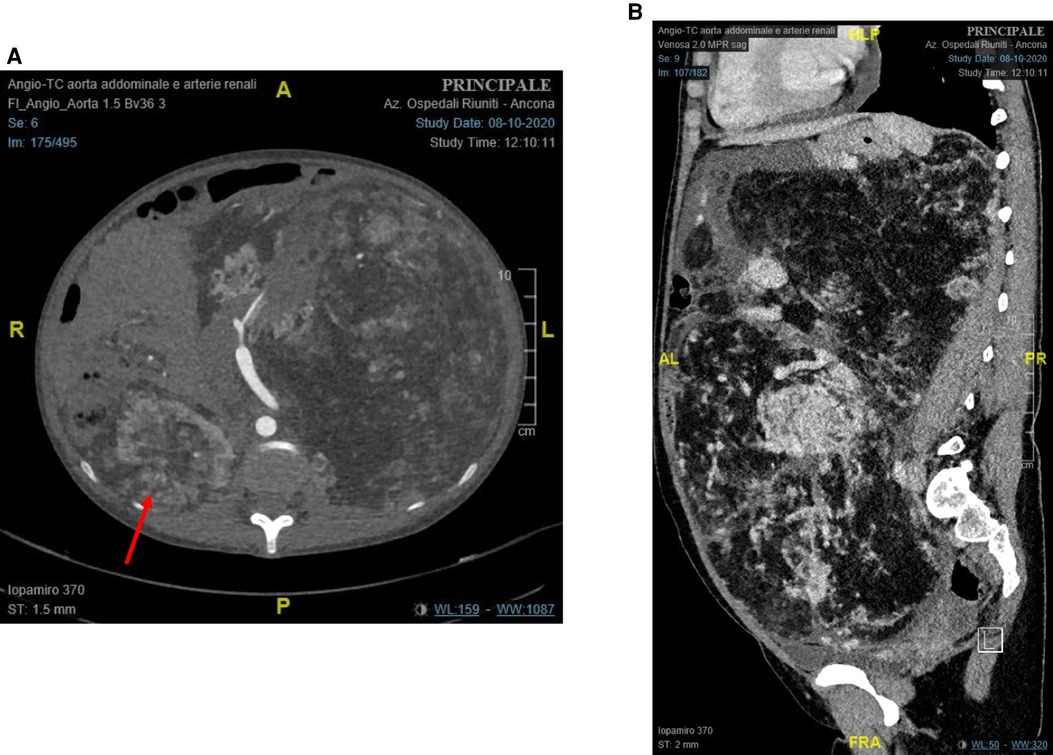
Figure 1. Preoperative contrast-enhanced computed tomography of the abdomen: (A) axial plane (red arrow: right renal angiomyolipoma); and (B) sagittal plane.
A careful multidisciplinary assessment involving urologists, vascular surgeons, anesthesiologists, and nephrologists was carried out. m-TOR treatment was considered ineffective, while it could be considered an option in renal cell carcinoma to shrink the tumor (7). Due to the kidney size, location, and bleeding risk, nephrectomy with a hybrid approach was suggested.
Percutaneous temporary occlusion of the main renal artery was performed in the same session of nephrectomy. Surgery began with a preliminary angiography through a left femoral artery percutaneous access to evaluate the left renal artery (Figure 2A). The renal artery was end-clamped using a 12 mm × 40 mm angioplasty balloon (Cordis Powerflex Pro, Cordis, Miami Lakes, FL, USA) (Figure 2B). Through the balloon catheter guidewire, 2,500 IU of heparin was infused to reduce the risk of tumor vein thrombosis and venous embolism. By a left thoracic-phreno-laparotomy at the level of the ninth intercostal space, a retroperitoneal kidney dissection was performed (Figure 3A). After careful isolation of the kidney, the renal artery was progressively accessed and exposed after kidney displacement. Then, the endovascular balloon was removed, and the main renal artery was ligated and sectioned. Nephrectomy was completed after isolation, ligation, and section of the renal vein. Surgical time was 234 min. The estimated blood loss was 300 ml. A left thoracic drain was left in place for 72 h. The postoperative course was uneventful, and the patient required no blood transfusion. The patient was discharged on the sixth postoperative day. A postoperative CT scan showed a complete resection (Figure 4). Pathological examination revealed a tumor measuring 40 cm × 30 cm × 9 cm and 10 kg of weight and confirmed the presence of AML (Figure 3B).
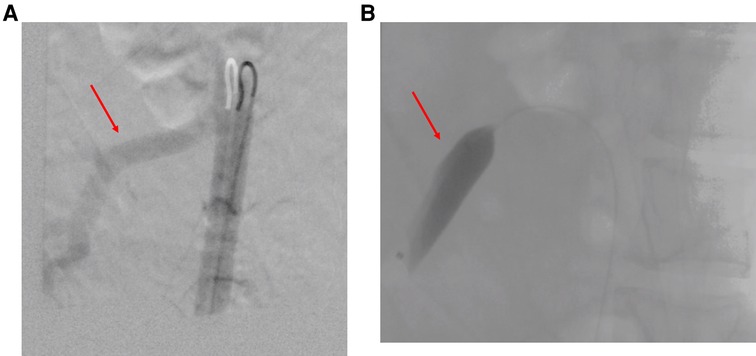
Figure 2. (A) Intraoperative left renal artery angiography (red arrow: main renal artery). (B) Endovascular clamping of the left renal artery (endovascular balloon occluding the main renal artery).
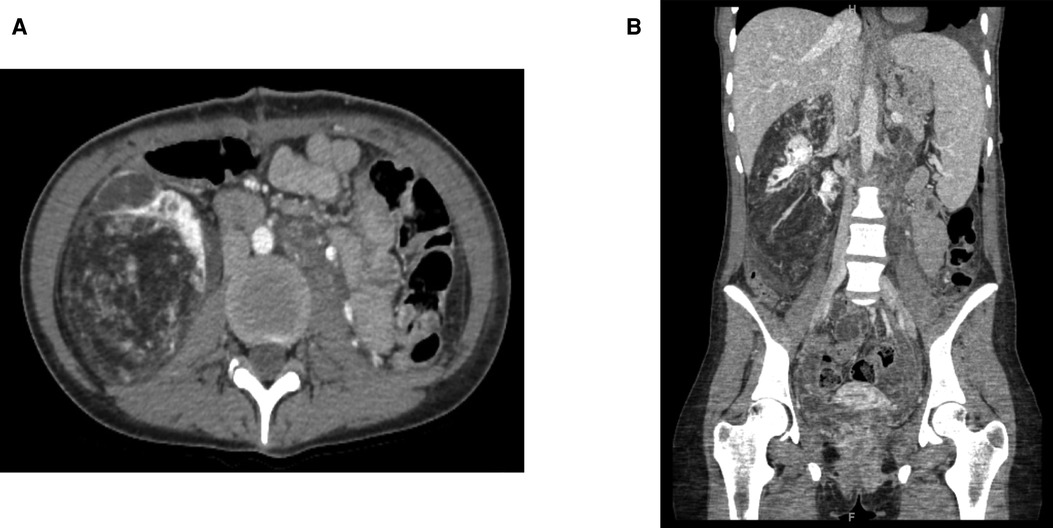
Figure 4. Postoperative contrast-enhanced computed tomography of the abdomen: (A) axial plane; (B) sagittal plane.
According to recent recommendations (8), the patient has started treatment with everolimus to reduce the growth of the right AML and cerebral subependymal giant cell astrocytoma. One year after surgery, the patient is still under everolimus treatment and in follow-up. Her estimated glomerular filtration is 120.5 ml/min/1.73m2, with no proteinuria or hematuria. The right angiomyolipoma grew up to 11.1 cm × 6.9 cm × 8.1 cm, but the patient refused partial nephrectomy and is currently asymptomatic.
This case report has described the largest renal AML ever reported, outlining how it was safely treated with a hybrid surgical technique. Usually, surgical treatment is indicated in AML when facing lesions over 4 cm or complications (spontaneous rupture, tumor hemorrhage, hematuria) and/or mass-related symptoms (flank pain, abdominal pain) appear (2). AMLs associated with TSC usually present with multiple, large, and symptomatic tumors. In a low percentage of large AML (3%), the smooth muscle may develop a proliferation of epithelioid cells that have a potential malignant behavior, known as epithelioid angiomyolipoma (5). It is not possible to differentiate the latter with AML by CT scanning or magnetic resonance imaging. Therefore, the progressively growing mass must be removed. However, every surgery should rely on a nephron-sparing approach whenever possible to preserve renal function (9). Conversely, nephrectomy is indicated only when a renal AML is very large, in case of suspicion of malignancy or when other treatment options cannot be performed (10). In these cases, an elective treatment is indicated. In huge AML, surgery is the treatment of choice to relieve symptoms. In large masses, artery embolization has been used several days or hours before surgery as a separate preoperative procedure (6). Indeed, one of the largest renal AMLs published in the literature was reported by Taneja et al. (39 cm × 25 cm × 9 cm, weight 7.5 kg) (11). The authors performed an open nephrectomy several days after renal artery embolization. This preoperative approach has various disadvantages in giant tumors, such as pain, hemodynamic instability, fever-induced necrosis, renal vein thrombosis, and embolism, as well as high costs related to embolization, anesthesia, and staged procedures.
The hybrid one-stage approach that we presented here was based on concomitant endovascular renal artery clamping followed by nephrectomy. This approach has two main advantages. First, surgical maneuvers to achieve renal artery ligation were performed safely, reducing the risk of massive bleeding arising from the breakdown of a large and subtle venous network surrounding the mass. The second advantage was that we controlled the hemodynamic impact of clamping on cardiac venous backflow as the AML weight was almost 18% of the patient's weight (57 kg). Furthermore, according to the anesthesiologist's monitoring feedback, artery clamping was progressively applied. Consequently, a moderate decrease in cardiac venous return was observed and managed accordingly. In addition, the risk of renal vein thrombosis and subsequent embolism was reduced by injecting heparin through the angiographic catheter.
The thoracic-phreno-laparotomy approach was chosen to allow safe manipulation and access to the left renal hilum, displacing the huge mass and making open surgery a viable option for these huge kidney tumors in the modern era of robotic-assisted surgery.
In conclusion, the hybrid one-stage approach we described in this case was based on intraoperative endovascular renal artery clamping and surgical resection by the thoracoabdominal approach. This novel technique offers two main advantages: a safer approach to reaching the renal hilum and a safer way to manage the decrease of cardiac venous backflow, as well as being easy and time-saving. This technique could be useful to safely manage huge renal masses when hilar control is expected to be very difficult, such as in our case.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval were not required for the study on human participants in accordance with local legislation and institutional requirements. The patients/participants provided written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
VV, AR, LM, LF, SL, EC, GM, and CF managed the patient. AG and EG were the chief operating surgeons. DC revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a past co-authorship with one of the authors (DC).
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. (2008) 372:657–68. doi: 10.1016/S0140-6736(08)61279-9
2. Restrepo JCÁ, Millan DAC, Sabogal CAR, Bernal AFP, Donoso WD. New trends and evidence for the management of renal angiomyolipoma: a comprehensive narrative review of the literature. J Kidney Cancer VHL. (2022) 9:33–41. doi: 10.15586/jkcvhl.v9i1.177
3. Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging. (2014) 39:588–604. doi: 10.1007/s00261-014-0083-3
4. Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol. (1993) 150:1782–6. doi: 10.1016/s0022-5347(17)35895-0
5. Mazzucchelli R, Galosi AB, Scarpelli M, Lopez-Beltran A, Cheng L, Montironi R. Contemporary update on pathology-related issues of adult renal neoplasms. Anal Quant Cytopathol Histopathol. (2014) 36:1–8. PMID: 24902365
6. Shikino K, Ikusaka M. Giant bilateral sporadic renal angiomyolipoma. CMAJ. (2016) 188:821. doi: 10.1503/cmaj.150915
7. Galosi AB, Papaveri A, Castellani D, Agostini E, Burattini L, Dell’Atti L. Level IV tumor thrombus in non-metastatic renal cell cancer? No, thanks. Level II is better. Lessons learned from a case report. Urol Case Rep. (2021) 37:101660. doi: 10.1016/j.eucr.2021.101660
8. Curatolo P, Bjørnvold M, Dill PE, Ferreira JC, Feucht M, Hertzberg C, et al. The role of mTOR inhibitors in the treatment of patients with tuberous sclerosis complex: evidence-based and expert opinions. Drugs. (2016) 76:551–65. doi: 10.1007/s40265-016-0552-9
9. Dell’Atti L, Scarcella S, Manno S, Polito M, Galosi AB. Approach for renal tumors with low nephrometry score through unclamped sutureless laparoscopic enucleation technique: functional and oncologic outcomes. Clin Genitourin Cancer. (2018) 16:e1251–6. doi: 10.1016/j.clgc.2018.07.020
10. Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. Update on the diagnosis and management of renal angiomyolipoma. J Urol. (2016) 195:834–46. doi: 10.1016/j.juro.2015.07.126
Keywords: angiomyolipoma, endovascular procedures, nephrectomy, tuberous sclerosis, rare diseases
Citation: Vento V, Galosi AB, Ranghino A, Montecchiani L, Felici L, Loggi S, Cerutti E, Milanese G, Franzese C, Castellani D and Gatta E (2022) Case report: A hybrid technique for a safe nephrectomy in a giant kidney angiomyolipoma. Front. Surg. 9:955932. doi: 10.3389/fsurg.2022.955932
Received: 29 May 2022; Accepted: 15 September 2022;
Published: 11 October 2022.
Edited by:
Emanuele Rubilotta, Integrated University Hospital Verona, ItalyReviewed by:
Pietro Pepe, Cannizzaro Hospital, Italy© 2022 Vento, Galosi, Ranghino, Montecchiani, Felici, Loggi, Cerutti, Milanese, Franzese, Castellani and Gatta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Castellani Y2FzdGVsbGFuaWRhbmllbGVAZ21haWwuY29t
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.