
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 31 October 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.955655
This article is part of the Research TopicCase Reports in Surgical Oncology: 2022View all 56 articles
Aggressive angiomyxoma (AAM) is an uncommon locally infiltrative tumor that frequently occurs in the pelvic soft tissues of female patients; it has a high rate of local recurrence. However, AAM is extremely rare in males. Herein, we present the case of a 70-year-old man with a gradually enlarging painless mass in the scrotum. The patient underwent local excision of the scrotal AAM, with no local relapse after 17 months of follow-up. In addition to the present case, the clinicopathological features of males with AAM reported in literature (to the best of our knowledge) are discussed in this report. The literature review revealed that the gross morphology, clinical process, and histopathology of AAM in males resemble those of AAM in females. In particular, estrogen receptor/progesterone receptor has been shown to be expressed in male patients, which may provide an option for hormone therapy. Moreover, in males, a lower recurrence rate has been observed after surgery to remove the tumor. However, more data are needed to validate this observation. This report emphasizes the importance of considering AAM as the differential diagnosis of myxoid neoplasms in male genital areas.
Aggressive angiomyxoma (AAM), a rare deep soft tissue tumor, occurs predominantly in the pelvis and perineum of women (1). The age of AAM onset in women has previously been reported to range from 6 to 77 years, with the peak incidence during the childbearing years (2). AAM is defined as a benign tumor with no malignant potential, however, AAM has a high probability of local recurrence in the form of local infiltration (3). At present, extensive surgical excision with tumor-free margins is the most commonly available treatment for AAM (2). The occurrence of this type of tumor is very rare in men. To the best of our knowledge, only 85 male AAM cases (including the present one) have been reported in literature (Table 1) since the disease was first described in 1983. In most of these cases, the tumors were detected in the perineal region, inguinal area, and scrotum. AAM is difficult to diagnose without pathological analysis, and misdiagnosis as prostate, testicular, or paratesticular cancer is common (4). Herein, we present the case of AAM arising from the scrotum in a 70-year-old man and describe the diagnosis and treatment procedure along with a literature review on previously reported AAM cases in males. This study was reported in agreement with principles of the CARE guidelines (5).
A 70-year-old man presented with a left scrotum mass that had been growing for the past 2 years. The mass was mobile, nonpainful, and had grown progressively larger over time. The patient was not febrile and did not present with frequent urination, urgency, pain, or hematuria. Magnetic resonance imaging (MRI) at another hospital revealed the shadow of a mass in the prostate and left scrotum. Considering the possibility of a malignant lesion, the patient visited our hospital for further diagnosis and treatment. During physical examination, a large, perineal mass approximately 15 cm in size with no obvious blood vessels on the surface was detected. MRI at our hospital revealed a well-capsulated mass protruding into the left scrotum in the left pelvis with a size of 13.2 × 10.0 × 4.3 cm (Figure 1). In addition, an ill-defined mass was detected in the prostate, with a maximum cross-sectional area of 6.6 × 5.5 cm. The patient's total prostate-specific antigen level was 7.572 ng/ml (reference value = 0–4 ng/ml). Excisional biopsy of the scrotal mass was performed after needle biopsy revealed the mass as AAM. As the needle biopsy of the prostate revealed no malignant histological appearance, the patient was not initially treated with surgery.
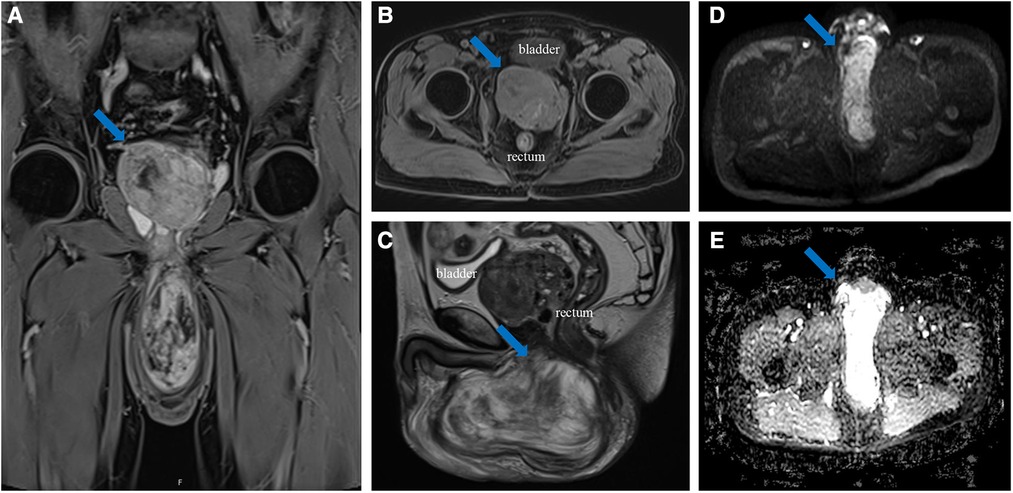
Figure 1. (A) coronal (weighted-sequence T1), (B) axial (weighted-sequence T1), (C) sagittal (weighted-sequence T2), (D) axial (DWI), and (E) axial (ADC) MRI reveal a well-capsulated mass protruding into the left scrotum in the left pelvis (arrows). ADCmean value = 2.16 × 10−3 mm2/s. MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient.
The gross examination of the excisional biopsy revealed a grey–pink or grey–yellow mass measuring 13.0 × 7.0 × 5.5 cm in volume, with a completely smooth capsule on the surface and soft and translucent texture in tissue sections (Figure 2A). Histologically, the tumor comprised spindle cells, myxoid matrix containing thick-walled vessels of varying sizes, and cordlike collagen fibers. The spindle cells were arranged in a wavy or parallel pattern, with no atypia or nuclear division (Figures 2B–D). Immunohistochemical staining revealed that the tumor cells were positive for vimentin, CD34 (Figure 3A), desmin (Figure 3B), estrogen receptor (ER) (Figure 3C), and progesterone receptor (PR) (Figure 3D) but negative for CK, SMA, and S-100. The tumor had a very low Ki-67 proliferation index of 1%. Based on the morphology and the immunological phenotype, the patient was diagnosed with AAM.
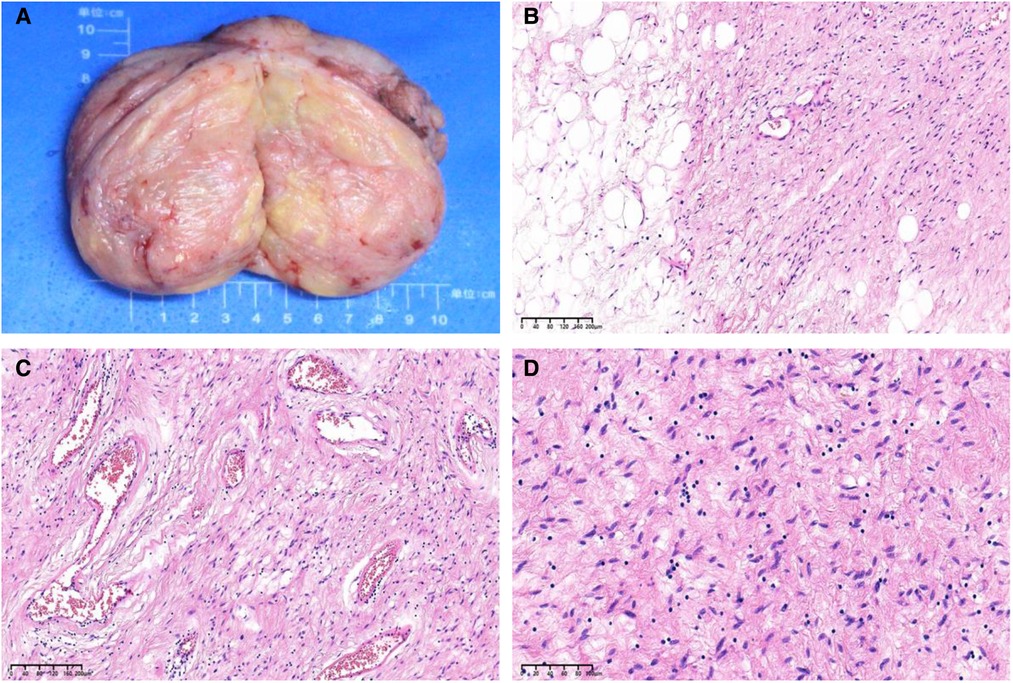
Figure 2. (A) A grey–pink or grey–yellow, well-circumscribed, solid mass measuring 13.0 × 7.0 × 5.5 cm. (B) Microscopic sections demonstrate a spindle cell tumor entrapping the adipose tissue (hematoxylin and eosin [H&E] stain, ×100 magnification). (C) The tumor comprises spindle cells in a loose myxoid matrix containing irregular, variably sized blood vessels and cordlike collagen fibers (H&E stain, ×100 magnification). (D) The tumor cells are eosinophilic, small, and spindle shaped, with slightly deep staining and stellate nuclei (H&E stain, ×200 magnification).
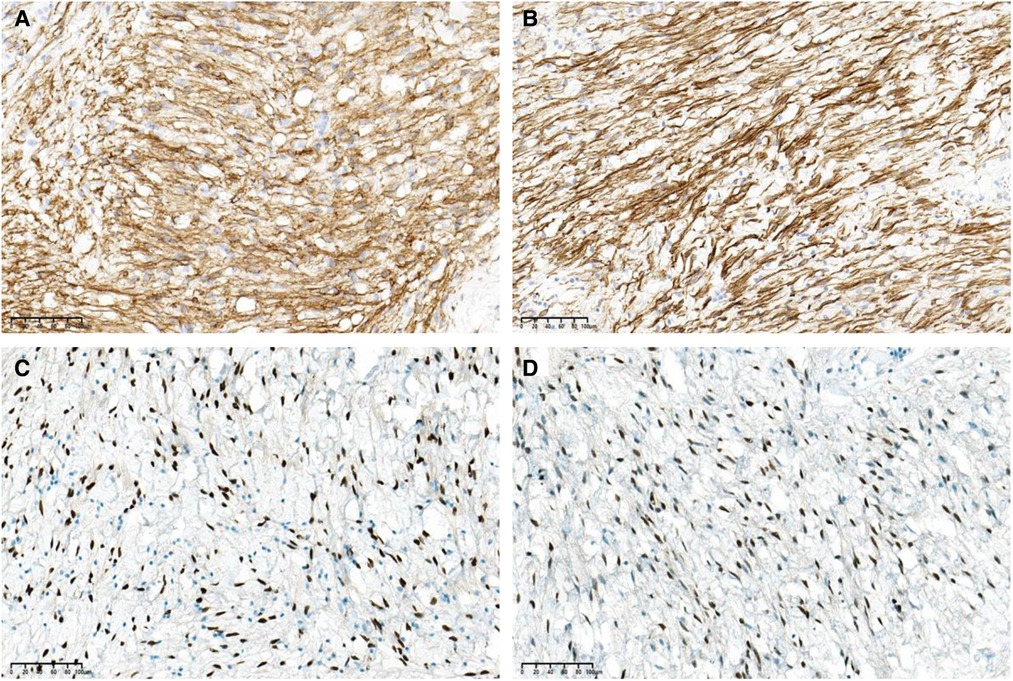
Figure 3. The tumor cells are positive for (A) CD34, (B) desmin, (C) estrogen receptor (ER), and (D) progesterone receptor (PR) (immunohistochemical stain, ×200 magnification).
The hematoxylin and eosin staining of the prostate needle biopsy did not reveal prostate cancer (Supplementary Figure S1A-B), and immunohistochemical staining showed that the tumor was positive for P63 (Supplementary Figure S1C) and negative for P504S (Supplementary Figure S1D).
The patient was treated surgically. First, left arc-shaped incision of the perineum was performed, the perineal skin and muscles were dissected according to the anatomical level and the superficial perineal fascia was dissected. The scrotal mass was located below the urogenital diaphragm (The superior fascia of the urogenital diaphragm is adjacent to the prostate, and the inferior fascia is adjacent to the bulbar corpus cavernosum) and clearly demarcated from the surrounding tissue. The scrotal mass was then surgically removed with clear margins. A needle biopsy of the prostate was also performed intraoperatively. The patient was postoperatively followed-up for 17 months; no recurrence or metastasis has been detected.
AAM was first described as a separate histopathological entity by Steeper and Rosai in 1983. Considering its benign nature, the term “aggressive” was modified to “deep” in the fourth edition of the World Health Organization Classification of Soft Tissue Tumors in 2013 (4). In men, only case reports or case series have been reported in literature. The available cases reported in males are described in Table 1 (4, 6–45). These men aged from 9 months to 82 years, with an average age of 49 years. The most common sites were the pelvis and genital areas, particularly the scrotum. Six tumors have been reported in the head and neck area, four in the engrafted kidney or urinary tract, three in the prostate, two in the alimentary tract, two in the lower limbs, one in the axillary region, one in the buttock, and one in the claviculate. Most of these patients were usually asymptomatic, whereas a small number of patients presented with inguinal hernia and testicular tumors. In the present case, the tumor developed in the scrotum and presented as a gradually increasing mass with no typical clinical symptoms.
Most tumors in the reviewed cases were >10 cm in size as AAMs are not easily detected early. The largest tumors found in women and men, respectively, were 60 and 28 cm in size (42, 43). Most tumors are ill defined, making complete resection difficult and resulting in frequent local recurrence. However, few tumors demonstrate partial or complete encapsulation. In most cases, the cut surface was smooth, homogeneous, soft, and gray, with few firm and cystic types (6). In the present case, the tumor was completely encapsulated, with a maximum diameter of 13 cm, and had a smooth, gray, soft cut surface.
Misdiagnosis in AAM is common because it can mimic other diseases, including hydrocele, inguinal hernia, or paratesticular neoplasia (4). Preoperative diagnosis is often difficult and challenging because of the rarity of these tumors and lack of specific imaging features (44). AAM is diagnosed based on the histopathological examination of postoperative specimens. The histopathological features of males with AAM reported in literature are similar to those reported in classical female cases. Microscopically, AAM comprises small-sized spindle cells or stellate cells embedded in a loose myxoid matrix with abundant collagen fibers and variably sized vessels. Blood vessels ranging from capillary-like to thick-walled vessels, which are the most prominent feature of AAM, can be observed (46). In the present case, no evidence of atypical mitotic activity or nuclear atypia was noted. While hypercellularity, cytological atypia, abundant fibrosclerotic stroma, and increased vascularity have been reported in recurrent cases (2), in the present case, the classical morphology was found. Immunohistochemical staining plays a crucial role in the diagnosis of AAM, although there is no specific immunohistochemical marker of AAM. The neoplastic cells of AAM are generally positive for desmin, vimentin, SMA, CD34, ER, and PR but negative for S-100 and CK in female patients (47). Previous studies have shown that ER and PR stains are generally negative in males with AAM compared with females (37). However, in some cases (including the present case), ER and PR may be expressed on AAM tumor, which may provide a hormonal therapeutic option (6). It is worth noting that the relationship of AAM with hormone receptor expression in males has not been well described, and more research is needed in the future. More recently, high mobility group A protein 2 (HMGA2) was revealed as a sensitive but not specific novel marker for AAM diagnosis (48). While nuclear staining can be useful in cases where cytoplasmic staining is nonspecific and of no diagnostic importance (49), the present case was diagnosed without HMGA2 staining considering the typical morphology of the tumor.
AAM should be distinguished from angiomyofibroblastoma, myxoid liposarcoma, myxoma, superficial angiomyxoma, and myxoid neurofibroma. Angiomyofibroblastoma is a benign tumor that has recently been described as histologically similar to AAM, with myofibroblastic cells clustered in abundant myxoid stroma. This tumor contains several areas of hypo- and hypercellular cells, often clustered around blood vessels. Another key histological feature of angiomyofibroblastoma is the presence of multinucleated giant cells (50). Myxoid liposarcoma must be considered as a differential diagnosis when tumor cells infiltrate adipose tissues. It can be easily distinguished from AAM as myxoid liposarcoma is marked with adipocytes set in abundant thin-walled vessels. Myxoma, a benign tumor of the extremities, is characterized by an abundance of myxoid stroma and benign spindle and stellate cells surrounded by small blood vessels (and not blood vessels of different sizes). Superficial angiomyxoma, also known as cutaneous myxoma, usually occurs in the skin of nongenital areas. Histologically, the tumor lacks thick-walled vessels and the myxoid areas can form pools. With respect to the immunophenotype, ER and PR are generally not expressed but S-100 may be expressed in superficial angiomyxoma (51). Myxoid neurofibroma is another AAM-resembling myxoid tumor that commonly occurs in the extremities; however, the clusters of wavy nerve tumor cells are usually strongly S-100 positive.
The surgical removal of AAM with clear margins is the traditional treatment to prevent local recurrence. However, it is not clear whether the recurrence rate is associated with the surgical margin status (52). In women, the recurrence rates of 71%, 85%, and 94% have been observed within the first 3, 5, and 7 years of local excision, respectively (53). As shown in Table 1, 79 of the 85 male patients were followed-up after surgery, and the data revealed only 4 recurrences (4.7%), excluding 2 deaths (one died intraoperatively and one died because of pulmonary tuberculosis). The patients with recurrence were treated with wide excision, and the procedures were performed 9 months to 7 years after first surgery. Possible reasons for the lower local recurrence rate in male patients are sample limitations or lower hormone expression. It is generally known that AAM has no metastatic tendency; however, two female patients (aged 63 and 27 years) showed metastasis to the lung (54, 55). By contrast, as expected, no metastatic cases have been reported in males. Moreover, owing to the low proliferative activity of AAM, the role of radiotherapy and chemotherapy is unclear and limited. In recent years, hormonal therapy has been considered an adjunctive treatment for ER and/or PR positive female patients with primary large mass or local relapse that is not amenable to surgery (56). Unfortunately, it is not clear whether the relapse rate is higher when hormone therapy is discontinued (57). In the present case, only local resection was performed, and although the patient was both ER and PR positive, hormonal therapy was not advised as few data are available on hormonal therapy in male patients with AAM. Long-term follow-up surveillance is required because of the aggression and relapse characteristics of this tumor. At present, the patient has been followed-up for 17 months without any recurrence or metastasis.
In summary, a rare case of scrotal AAM was reported and previously reported male cases with AAM were summarized. In particular, the review revealed that the clinicopathological characteristics of AAM in men are similar to those of AAM in women, including the expression of ER and PR. This provides an opportunity to treat such male patients with hormone therapy. Moreover, the literature review revealed a low recurrence rate (4.7%) in males after the surgical excision of the tumor; however, more data are needed to confirm this observation. Finally, AAM should be distinguished from myxoid neoplasms in male genital areas.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
C-KY: conceptualization. Y-PW and HC: data curation. YC: investigation, validation, and writing of the original draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.955655/full#supplementary-material.
1. Altinmakas E, Dogan H, Temur M, Guneyli S. Incidentally detected perineal aggressive angiomyxoma in an asymptomatic patient with uterine leiomyomas. J Obstet Gynaecol. (2021) 41(7):1178–9. doi: 10.1080/01443615.2020.1849070
2. Magro G, Angelico G, Michal M, Broggi G, Zannoni GF, Covello R, et al. The wide morphological spectrum of deep (aggressive) angiomyxoma of the vulvo-vaginal region: a clinicopathologic study of 36 cases, including recurrent tumors. Diagnostics (Basel). (2021) 11(8):1360. doi: 10.3390/diagnostics11081360
3. Kanao H, Aoki Y, Tanigawa T, Matoda M, Okamoto S, Nomura H, et al. En bloc resection of an aggressive angiomyxoma by a novel combination laparoscopic and open perineal approach. J Minim Invasive Gynecol. (2019) 26(4):598–9. doi: 10.1016/j.jmig.2018.07.008
4. Chen CF, Wang TY, Chen M, Lin YC. Rare paratesticular aggressive angiomyxoma mimicking an epididymal tumor in an 82-year-old man: case report. Open Med (Wars). (2021) 16(1):973–7. doi: 10.1515/med-2021-0317
5. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. Care guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
6. Idrees MT, Hoch BL, Wang BY, Unger PD. Aggressive angiomyxoma of male genital region. Report of 4 cases with immunohistochemical evaluation including hormone receptor status. Ann Diagn Pathol. (2006) 10(4):197–204. doi: 10.1016/j.anndiagpath.2005.09.002
7. Hatano K, Tsujimoto Y, Ichimaru N, Miyagawa Y, Nonomura N, Okuyama A. Rare case of aggressive angiomyxoma presenting as a retrovesical tumor. Int J Urol. (2006) 13(7):1012–4. doi: 10.1016/j.urology.2020.05.028
8. Wu CC, Yang SS, Chin DT, Hsieh CH, Hsueh YM, Tsai YC. Scrotal aggressive angiomyxoma mimicking inguinal hernia. Asian J Androl. (2007) 9(5):723–5. doi: 10.1111/j.1745-7262.2007.00286.x
9. Bothig R, Ahyai S, Kuhn K, Pramono S. Aggressive angiomyxoma in a male patient: a case report. Aktuelle Urol. (2008) 39(1):64–7. doi: 10.1055/s-2007-959216
10. Pai CY, Nieh S, Lee JC, Lo CP, Lee HS. Aggressive angiomyxoma of supraclavicular fossa: a case report. Head Neck. (2008) 30(6):821–4. doi: 10.1002/hed.20747
11. Heffernan EJ, Hayes MM, Alkubaidan FO, Clarkson PW, Munk PL. Aggressive angiomyxoma of the thigh. Skeletal Radiol. (2008) 37(7):673–8. doi: 10.1007/s00256-008-0465-0
12. Minagawa T, Matsushita K, Shimada R, Takayama H, Hiraga R, Uehara T, et al. Aggressive angiomyxoma mimicking inguinal hernia in a man. Int J Clin Oncol. (2009) 14(4):365–8. doi: 10.1007/s10147-008-0850-7
13. Sylvester DC, Kortequee S, Moor JW, Woodhead CJ, Maclennan KA. Aggressive angiomyxoma of larynx: case report and literature review. J Laryngol Otol. (2010) 124(7):793–5. doi: 10.1017/S0022215109992350
14. Morag R, Fridman E, Mor Y. Aggressive angiomyxoma of the scrotum mimicking huge hydrocele: case report and literature review. Case Rep Med. (2009) 2009:157624. doi: 10.1155/2009/157624
15. Plumb A, Shanks JH, Kochhar R. Development of aggressive angiomyxoma like tumour in a renal transplant. Clin Radiol. (2010) 65(5):423–6. doi: 10.1016/j.crad.2010.01.012
16. Sawada Y, Ito F, Nakazawa H, Tsushima N, Tomoe H, Aiba M. A rare benign genitourinary tumor in a Japanese male: urinary retention owing to aggressive angiomyxoma of the prostate. Rare Tumors. (2010) 2(1):e15. doi: 10.4081/rt.2010.e15
17. Bajaj MS, Mehta M, Kashyap S, Pushker N, Lohia P, Chawla B, et al. Clinical and pathologic profile of angiomyxomas of the orbit. Ophthalmic Plast Reconstr Surg. (2011) 27(2):76–80. doi: 10.1097/IOP.0b013e3181c53d53
18. Rocco F, Cozzi G, Spinelli MG, Rocco BM, Albo G, Finkelberg E, et al. Massive recurring angiomyxoma of the scrotum in a obese man. Rare Tumors. (2011) 3(3):e31. doi: 10.4081/rt.2011.e31
19. Mishulin A, Lever JF, Porter W, Servat JJ, Gladstone G, Black E. Aggressive glabellar angiomyxoma with orbital extension. Orbit. (2012) 31(5):361–3. doi: 10.3109/01676830.2012.710925
20. Nayal B, Rao L, Rao AC, Sharma S, Shenoy R. Extragenital aggressive angiomyxoma of the axilla and the chest wall. J Clin Diagn Res. (2013) 7(4):718–20. doi: 10.7860/JCDR/2013/5458.2891
21. Gaunay GS, Barazani Y, Kagen AC, Stember DS. Aggressive angiomyxoma of the scrotum. Clin Imaging. (2013) 37(6):1122–4. doi: 10.1016/j.clinimag.2013.06.007
22. Karwacki GM, Stockli M, Kettelhack C, Mengiardi B, Studler U. Radiographic diagnosis and differentiation of an aggressive angiomyxoma in a male patient. J Radiol Case Rep. (2013) 7(7):1–6. doi: 10.3941/jrcr.v7i7.1154
23. Saha K, Sarkar S, Jash D, Chatterjee S, Saha AK. Aggressive angiomyxoma of greater omentum with pleural effusion in a young male. J Cancer Res Ther. (2014) 10(2):371–3. doi: 10.4103/0973-1482.136661
24. Wang Z, Liu Y, Yang L, Gu L, He Y, Huang D, et al. Maxillary aggressive angiomyxoma showing ineffective to radiotherapy: a rare case report and review of literature. Int J Clin Exp Pathol. (2015) 8(1):1063–7. PMID: 25755820
25. Caruso F, Terrier P, Bonvalot S. Lessons from an aggressive angiomyxoma unrecognized and treated as rectal prolapse. Int J Colorectal Dis. (2015) 30(7):993–4. doi: 10.1007/s00384-014-2084-7
26. Wang Z, Wei YB, Yin Z, Yan B, Li D, Zhou KQ, et al. Diagnosis and management of scrotal superficial angiomyxoma with the aid of a scrotoscope: case report and literature review. Clin Genitourin Cancer. (2015) 13(4):e311–e3. doi: 10.1016/j.clgc.2014.11.009
27. Smith HG, Thway K, Messiou C, Barton DP, Thomas JM, Hayes AJ, et al. Selective marginal resections in the management of aggressive angiomyxomas. J Surg Oncol. (2016) 114(7):828–32. doi: 10.1002/jso.24420
28. Draeger DL, Protzel C, Hakenberg OW. Aggressive angiomyxoma as a rare differential diagnosis of enlargement of the scrotum. Clin Genitourin Cancer. (2016) 14(2):e237–9. doi: 10.1016/j.clgc.2015.12.022
29. Ahmed MAM, Uehelie MA, Rage AMA, Mohey A, Noureldin YA. Aggressive angiomyxoma of the penis: the first case report in a 9-month-old infant. Urology. (2017) 104:187–90. doi: 10.1016/j.urology.2016.12.045
30. Sharma N, Tomar TS, Mathew AP, Chandramohan K, Preethi R, Mony RP. Aggressive angiomyxoma of inguinoscrotal region mimicking inguinal hernia: a case report. Indian J Surg. (2017) 79(6):571–3. doi: 10.1007/s12262-017-1659-2
31. Gorsi U, Naranje P, Rathi M, Nada R, Khandelwal N. Aggressive angiomyxoma of transplanted kidney mimicking posttransplant lymphoproliferative disorder. Saudi J Kidney Dis Transpl. (2017) 28(2):425–7. doi: 10.4103/1319-2442.202778
32. Damodaran S, Gengan D, Walling ST. Aggressive angiomyxoma involving penis and urethra - a case report. Urol Case Rep. (2017) 13:110–2. doi: 10.1016/j.eucr.2017.03.019
33. Ismail MI, Wong YP, Tan GH, Fam XI. Paratesticular aggressive angiomyxoma: a rare case. Urol Ann. (2017) 9(2):197–9. doi: 10.4103/UA.UA_168_16
34. Aydin AM, Katipoglu K, Baydar DE, Bilen CY. Long-standing aggressive angiomyxoma as a paratesticular mass: a case report and review of literature. SAGE Open Med Case Rep. (2017) 5:2050313X17712090. doi: 10.1177/2050313X17712090
35. Umranikar S, Ubee S, Williams G. Aggressive angiomyxoma of the perineum: a rare presentation in a male with 4 years follow up. J Surg Case Rep. (2017) 2017(8):rjx086. doi: 10.1093/jscr/rjx086
36. Hsieh F, Chuang KT, Wu YT, Lin CH. Aggressive angiomyxoma-report of a rare male buttock lesion. Plast Reconstr Surg Glob Open. (2018) 6(8):e1879. doi: 10.1097/GOX.0000000000001879
37. Neyaz A, Husain N, Anand N, Srivastava P. Rare paratesticular aggressive angiomyxoma with negative oestrogen and progesterone receptors in a male patient. BMJ Case Rep. (2018) 2018:bcr2017222164. doi: 10.1136/bcr-2017-222164
38. Serao A, Tiranti D, Ferraro M, Malinaric R, Re P, Calamaro P. Incidental finding of paratesticular aggressive angiomyxoma in a 72-year-old monorchid male. Urologia. (2020) 87(4):194–8. doi: 10.1177/0391560319881082
39. Addesso M, Caputo A, D'Antonio A, Napodano G, Sanseverino R. A large paraprostatic mass in a man: rare presentation of aggressive angiomyxoma. Urology. (2020) 143:e3–4. doi: 10.1016/j.urology.2020.06.014
40. Kirkilessis G, Kakavia K, Bougiouklis D, Papadopoulos A, Lampropoulos C, Kirkilessis I. Aggressive angiomyxoma to 57-year old man. J Surg Case Rep. (2020) 2020(9):rjaa313. doi: 10.1093/jscr/rjaa313
41. Liu M, Zhai TS, Zhao XF, Feng LJ, Lyu XS, Hu LT, et al. Incidental para-ureteral aggressive angiomyxoma: a rare case report and literature review. BMC Urol. (2020) 20(1):182. doi: 10.1186/s12894-020-00755-7
42. Majumdar SK, Hussain M, Raha A, Barman S. “Gigantic aggressive angiomyxoma” of the jaws: a rare case report. J Oral Maxillofac Pathol. (2021) 25(1):205. doi: 10.4103/jomfp.JOMFP_233_20
43. Celik SU, Hesimov I, Kutlu B, Erkek AB. Aggressive angiomyxoma: a rare tumor of male pelvic cavity. Acta Med Port. (2018) 31(11):693–6. doi: 10.20344/amp.9062
44. Zhu Z, Yan J, Tang G. Aggressive angiomyxoma of the prostate: a case report. Medicine (Baltimore). (2018) 97(51):e13716. doi: 10.1097/MD.0000000000013716
45. Korecka KK, Hyla-Klekot LE, Kudela GP, Palen PA, Kajor MW, Koszutski TK. Aggressive angiomyxoma in an 11-year-old boy - diagnostic and therapeutic dilemmas: an unusual case report and review of the literature. Urology. (2020) 144:205–7. doi: 10.1016/j.urology.2020.05.028
46. Xu H, Sun P, Xu R, Wang L, Shi Y. Aggressive angiomyxoma in pregnancy: a case report and literature review. J Int Med Res. (2020) 48(7):300060520936414. doi: 10.1177/0300060520936414
47. Xie Y, Qian Y, Zou B. A giant aggressive angiomyxoma of vulva in a young woman: a case report. Medicine (Baltimore). (2019) 98(2):e13860. doi: 10.1097/MD.0000000000013860
48. Harkness R, McCluggage WG. Hmga2 is a useful marker of vulvovaginal aggressive angiomyxoma but may be positive in other mesenchymal lesions at this site. Int J Gynecol Pathol. (2021) 40(2):185–9. doi: 10.1097/PGP.000000000000068
49. McCluggage WG, Connolly L, McBride HA. Hmga2 is a sensitive but not specific immunohistochemical marker of vulvovaginal aggressive angiomyxoma. Am J Surg Pathol. (2010) 34(7):1037–42. doi: 10.1097/PAS.0b013e3181e32a11
50. Sutton BJ, Laudadio J. Aggressive angiomyxoma. Arch Pathol Lab Med. (2012) 136(2):217–21. doi: 10.5858/arpa.2011-0056-RS
51. McCluggage WG. A review and update of morphologically bland vulvovaginal mesenchymal lesions. Int J Gynecol Pathol. (2005) 24(1):26–38. PMID: 15626915
52. Chan YM, Hon E, Ngai SW, Ng TY, Wong LC. Aggressive angiomyxoma in females: is radical resection the only option? Acta Obstet Gynecol Scand. (2000) 79(3):216–20. PMID: 10716303
53. Zou R, Xu H, Shi Y, Wang J, Wang S, Zhu L. Retrospective analysis of clinicopathological features and prognosis for aggressive angiomyxoma of 27 cases in a tertiary center: a 14-year survey and related literature review. Arch Gynecol Obstet. (2020) 302(1):219–29. doi: 10.1007/s00404-020-05592-5
54. Siassi RM, Papadopoulos T, Matzel KE. Metastasizing aggressive angiomyxoma. N Engl J Med. (1999) 341(23):1772. doi: 10.1056/NEJM199912023412315
55. Blandamura S, Cruz J, Vergara LF, Puerto IM, Ninfo V. Aggressive angiomyxoma: a second case of metastasis with patient's death. Hum Pathol. (2003) 34(10):1072–4. doi: 10.1016/S0046-8177(03)00419-2
56. Pannier D, Cordoba A, Ryckewaert T, Robin Y-M, Penel N. Hormonal therapies in uterine sarcomas, aggressive angiomyxoma, and desmoid-type fibromatosis. Crit Rev Oncol Hematol. (2019) 143:62–6. doi: 10.1016/j.critrevonc.2019.08.007
Keywords: aggressive angiomyxoma, scrotum, local recurrence, male, soft tissue tumor
Citation: Chen Y, Wei Y, Chang H and Yu C (2022) Case report and literature review: Rare male aggressive angiomyxoma of the scrotum. Front. Surg. 9:955655. doi: 10.3389/fsurg.2022.955655
Received: 29 May 2022; Accepted: 10 October 2022;
Published: 31 October 2022.
Edited by:
Zhaolun Cai, Sichuan University, ChinaReviewed by:
Nguyen Minh Duc, Pham Ngoc Thach University of Medicine, Vietnam© 2022 Chen, Wei, Chang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ChunKai Yu eXVjaHVua2FpMjk3NkBianNqdGguY24=
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.