- 1Department of Orthopedic Surgery, The Sixth Medical Center of PLA General Hospital, Beijing, China
- 2Department of Traumatology and Orthopaedics, Beijing Jishuitan Hospital (The Fourth Medical College of Peking University), Beijing, China
Objective: This retrospective study aims to explore the effect of silver nanoparticles with thermoplastic polyurethane (TPU/NS) on the rehabilitation of diabetic patients with open fracture of lower extremities.
Methods: Diabetic patients (n = 98) with open fracture of lower extremities treated in our hospital were analyzed retrospectively from June 2015 to December 2021. TPU/NS nanocomposites were prepared for postoperative treatment of diabetic patients with open fracture of lower extremities. First, the cultured Staphylococcus aureus and Escherichia coli were used to test the antibacterial effect of TPU/NS dressing in vitro. After using TPU/NS dressing (observation group) and traditional dressing (control group), the inflammatory reaction, clinical treatment, functional rehabilitation, and adverse reactions in patients were compared.
Results: TPU/NS dressing effectively inhibited the growth of bacteria with a minimum inhibitory concentration of 2 μg/mL. The usage of TPU/NS dressing reduced the inflammatory reaction by reducing positive rate of bacteria after the dressing on the seventh day postoperatively. Besides, the times of dressing, stopping time of wound exudation, wound healing time, length of hospital stay, and VAS score in the observation group were lower than those in the control group; the incidence of adverse reactions after treatment was lower in the observation group as compared with the control group (17.07% vs. 35.09%). Meanwhile, the functional rehabilitation and life quality of patients in the observation group were better TPU/NS dressing treatment.
Conclusion: TPU/NS dressing has the function of promoting the postoperative recovery of patients by inhibiting the bacterial infection of the wound, thus improving the limb function and life quality. As a result, there was a tremendous potential to apply the constructed TPU/NS membrane to diabetic patients with open fractures, especially those with soft tissue injury.
Introduction
Poorly controlled diabetes mellitus (DM), a common metabolic disease, negatively affects outcomes associated with several lower extremity orthopedic conditions and complications including fractures (1, 2). Moreover, higher baseline fracture risks in both type I (T1MD) and type II diabetes (T2MD) were reported to result in a higher overall incidence of fractures in women (3). There was an expected rise to 366 million individuals with DM by 2030 worldwide; therefore, the incidence of open fractures of the lower limb is also on the rise (3, 4). The wounds have the characteristics of foreign body pollution, serious tissue defects, and poor blood supply, which are easily associated with infection, especially in patients with diabetes (5). If wound infection occurs after fixation surgery, it may lead to delayed healing, multiple debridements, bone and soft tissue defects, and joint stiffness, resulting in permanent loss of function and even amputation (6, 7).
It is generally believed that most of the pathogens causing the infection are introduced during injury or surgery (8). Infection means that there are sufficient numbers of toxic microorganisms, which can destroy the local defense mechanism of patients (9). Once the bacteria invade, any given systemic antibiotics are difficult to play the effective roles (10). Therefore, we are looking for a postfracture wound dressing to effectively prevent infection of fracture wounds.
Nanosilver (NS) as one type of excellent antibacterial agent has strong and broad-spectrum antiviral activity against both Gram-positive and -negative bacteria, including multiple drug tolerance bacteria, for example, methicillin-resistant Staphylococcus aureus (MRSA) (11). What is more, there is some proposal that NS suppressed bacteria via various mechanisms, such as cell membrane destruction, DNA replication interference, and respiratory function inhibition without causing drug resistance (12). Therefore, NS may be an appropriate antibacterial agent contained in biological materials. However, choosing a suitable wound dressing is still a question that needs to be explored (13). Thermoplastic polyurethane (TPU) is a type of biocompatible and biodegradable elastomer that has been approved by the Food and Drug Administration (FDA) and has been widely used in biomedical sciences (14), which does not only display significant chemical stability but also good mechanical behavior (15). These excellent properties indicate that TPU has the potential to become a kind of wound dressing (16).
In this study, prepared TPU/NS antibacterial wound dressings were applied to the postoperative wounds of open fracture patients with DM to observe and analyze its effect on rehabilitation and pain improvement.
Materials and Methods
Clinical Information
The clinical data of patients with open fractures of lower limbs treated in our hospital from June 2015 to December 2021 were retrospectively analyzed. Inclusion criteria are as follows: (1) the clinical symptoms and imaging examination met the diagnostic criteria in Guidelines on Diagnosis and Treatment of Open Fractures in China (2019 Edition) (17); (2) DM was confirmed in line with the Diagnosis and Classification of Diabetes Mellitus published by American Diabetes Association (18); (3) all patients had new lower limb fractures and met the surgical treatment standards; (4) Gustilo grade: type II and type IIIA; (5) age > 18 years. Exclusion criteria are as follows: (1) patients had malignant tumors and pathological fracture; (2) wound debridement of patients were in grade C; (3) patients had coagulation dysfunction, vascular diseases, and autoimmune diseases; (4) patients had diabetic ulcers; (5) patients had mental disorders, cognitive dysfunction, or Alzheimer’s disease; (6) patients had renal or severe cardiopulmonary dysfunction; (7) patients were allergic to nanosilver; (8) patients had a preoperative infection; and (9) patients whose soft tissues hardly be second-stage sutured even if skin- muscle flap transfer, free skin grafting nor vacuum suction device. A total of 98 patients were included, including 59 males and 39 females. Their average age was 56.22 ± 19.53 years. The patients were divided into an observation group (using TPU/NS dressing, 41 cases) and a control group (using traditional dressing coated with benzalkonium chloride or iodophor, 57 cases) according to propensity score matching (PSM). All patients agreed to participate in the experiment and signed informed consent. This study has been approved by the hospital ethics committee, and it complies with the Declaration of Helsinki.
Preparation of TPU/NS
TPU membrane (Lubrizol, USA) placed into dopamine (DA) (Sangon, Shanghai, China) solution was soaked at 25°C for 20 h. During this period, membrane color turned from light white into dark brown. Then, the DA-coated film was placed into a 5 mM AgNO3 solution at 25°C for avoiding light for 6 h. At last, ion water was used to wash the sample twice followed by stoving at a temperature of 45°C lasting 4 h to obtain TPU/NS dressings. Scanning electron microscopy (SEM, Crossbeam 340, Zeiss, Germany) was adopted to observe the morphology of original TPU and TPU/NS films and energy-dispersive X-ray spectroscopy (EDS) was used to conduct compositional analysis. ImageJ software was adopted to measure silver nanoparticles' average pore size, porosity, and diameter, and two independent researchers read the data.
Antibacterial Properties of TPU/NS In Vitro
S. aureus (ATCC6538, Shanghai Huzheng Biotechnology Co., Ltd, China) and Escherichia coli (CICC10662, Shanghai Huzheng Biotechnology Co., Ltd) are common Gram-positive and Gram-negative bacteria in nosocomial infection, respectively, which are used to detect antibacterial properties of TPU/NS dressing. S. aureus and E. coli were cultured in a 24-well plate with bacterial liquid media containing tryptic soy broth (BD Bacto, Becton, Dickinson and Company, USA; pH 7.3) in a microorganism incubator (51028133, Thermo Fisher, USA) at 37°C and 5% CO2 according to a previous study (19). The cultured bacteria were adjusted to a concentration of 1 × 106 CFU/ml. The diluents of S. aureus and E. coli were divided into a blank control group and a TPU/NS group, in which a small number of TPU/NS nanocomposites were added to the TPU/NS group. At the same time, the synthetic TPU/NS solution was diluted into different concentration gradients by the double dilution method, which were added to the Petri dish with bacteria (1 × 106 CFU) for detecting the minimum inhibitory concentration. The activation states of S. aureus and E. coli were tested by a live/dead bacterial viability kit (AAT-B22411, AAT Bioquest, USA). In this kit, MycoLight 520 solution and propidium iodide (PI) solution were used for viable (green) and nonviable (red) bacteria, respectively.
Therapeutic Method
After fully wound debridement, appropriate fixation methods were adopted for the fracture, and the wound shall be sutured and disinfected. For the wound that could not be sutured in the first stage, the suture or skin grafting shall be performed after the infection was controlled in the second stage. Antibiotics were given half an hour before the operation, which were used three consecutive days after the operation. The additional usage of antibiotics was based on the wound infection. In addition, all patients were given health education about DM, as well as the customize diabetes diet program by the nutrition department. Meanwhile, daily blood glucose monitoring and medication guidance should be implemented. In terms of postoperative wound dressing, the patients were sutured and disinfected routinely on the open wounds. The patients in the control group were treated with traditional dressings coated with benzalkonium chloride or iodophor, while those in the observation group with TPU/NS dressings. During the dressing on the seventh day postoperatively, the exudates from the wounds of the patients should be extracted for bacterial culture, then the wound shall be disinfected with iodophor, and then, the corresponding dressings were used. The outcome indicators were evaluated 2 months after the operation.
Inflammatory Response Test
Before treatment and 1, 3, 7, and 10 days after treatment, two tubes of 5 ml of venous blood were taken from each patient, and one tube was centrifuged at 3,000 rpm to obtain serum. Levels of white blood cell (WBC), neutrophils (NEU%), and erythrocyte sedimentation rate (ESR) were measured by an automatic blood cell analyzer (XN-2000, XISEN Meikang), C-reactive protein (CRP) by the automatic biochemical analyzer (AU-680, Beckman), as well as concentrations of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were detected by an automatic chemiluminescence immunoanalyzer (DXI-800, Beckman). If the above indicators had a downward trend, it was considered an “improved inflammation status.” During the dressing on the seventh day postoperatively, the wound secretion was taken for bacterial culture, and the positive rates of bacterial between the two groups were compared.
Evaluation of Clinical Treatment and Adverse Reactions
The clinical recovery of patients is evaluated through the times of dressing, stopping time of wound exudation, wound healing time, and length of hospital stay. The visual analog scale (VAS) (20) score is used to evaluate the pain degree of patients’ wounds. The lower the score, the lower the pain. The adverse reactions of the two groups were recorded, including wound infection, delayed healing, scar hyperplasia, soft tissue necrosis, and fracture pain.
Comparison of Functional Rehabilitation
The functional assessment scale (FIM) (21) was used to evaluate the functional rehabilitation of the two groups of patients, which was divided into self-care function, action, and cognitive function. The higher the score, the better the lower limb function recovery. In terms of life quality, the Orthopedic Quality of Life (SF-36) questionnaire (22) was used to evaluate patients’ quality of life. The questionnaire contained some items such as physical function, emotional function, role function, social function, and other dimensions. Each dimension was expressed by 0–100 points; the higher the score, the better the patients’ quality of life.
Statistical Methods
SPSS 20.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, California, USA) were used to analyze data and draw the statistical picture. Measurement data presented as mean ± standard deviation (SD) were compared using a t-test between two groups, and enumeration data were compared using a χ2 test. A value of P < 0.05 indicated that this difference showed statistical significance.
Results
Comparison of General Information of Both Group Patients
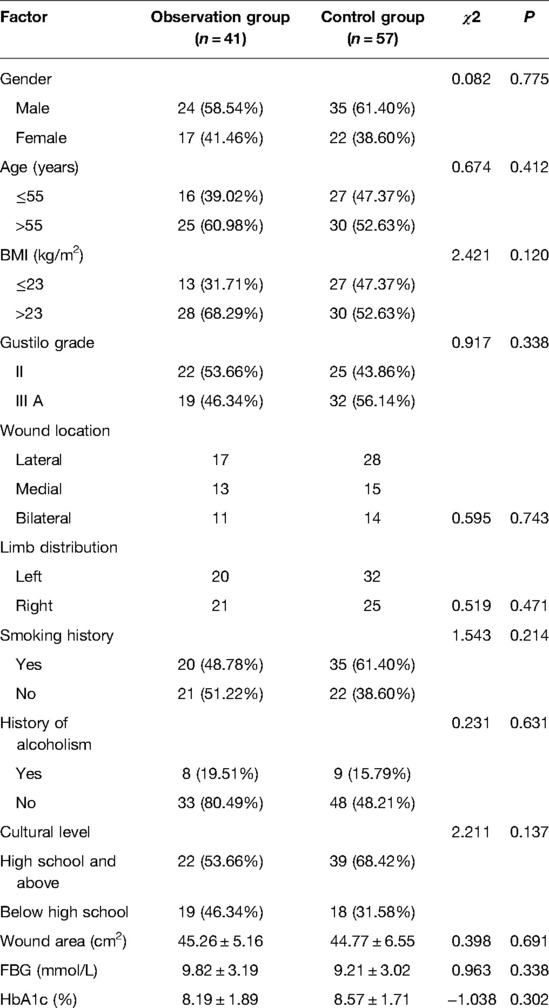
The baseline information of the patients from the observation group (n = 41) and the control group (n = 57) is shown in Table 1. There were no remarkable differences in gender, age, BMI, Gustilo grade, wound location, limb distribution, wound area, FBG, HbA1c, smoking history, history of alcoholism, and cultural level between the two groups (all P > 0.05)
Evaluation of Bacteriostasis of TPU/NS Dressing
Due to the exposure of soft tissue and bone, the wound of open fracture is very easy to be complicated with infection. Effectively killing bacteria and reducing viable bacteria were important methods to promote rehabilitation of patients and prevent related complications. In this study, S. aureus and E. coli were selected to study the antibacterial effect of TPU/NS dressing. We observed the characteristics of TPU/NS by SEM. The results showed that TPU/NS nanoparticles were evenly distributed and well dispersed, with a spherical shape of 95 nm (Figure 1A). Meanwhile, TPU/NS dressing could reduce viable (green) bacteria with increased nonviable (red) bacteria (Figures 1B–I), and the minimum inhibitory concentration of TPU/NS was 2 μg/ml. The above results show that TPU/NS dressing can effectively use silver ions to achieve excellent antibacterial properties.
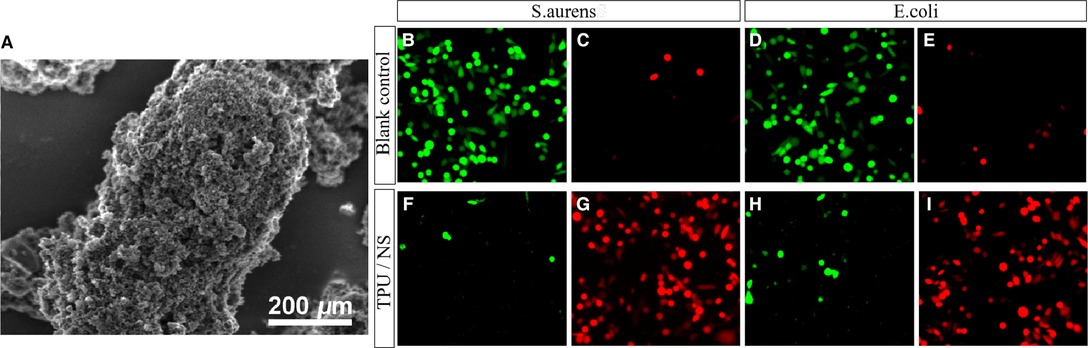
Figure 1. Characterization and antibacterial properties of TPU/NS. (A) Scanning electron microscopy image of TPU/NS. (B,C) Viable and nonviable staining of cultured S. aureus (B,C) and E. coli (D,E) 12 h in the blank control group. (F–I) Viable and nonviable staining of cultured S. aureus (F,G) and E. coli (H,I) 12 h after adding TPU/NS nanocomposites.
Comparison of Serum Inflammatory Reaction and Positive Detection Rate of Wound Bacteria
Inflammatory factors can promote the occurrence of the inflammatory cascade, which is an important indicator of the degree of inflammation. It was found that the TPU/NS dressing could reduce WBC, NEU%, CRP, IL-6, and TNF-α in serum as compared to the control dressing without affecting ESR between both groups (Figures 2A–F). In addition, TPU/NS dressing suppressed the positive detection rate of bacteria (4.88% vs. 12.28%) (Figure 2G). The above results suggested that TPU/NS dressing can better reduce the level of inflammatory factors to prevent the occurrence of infection.
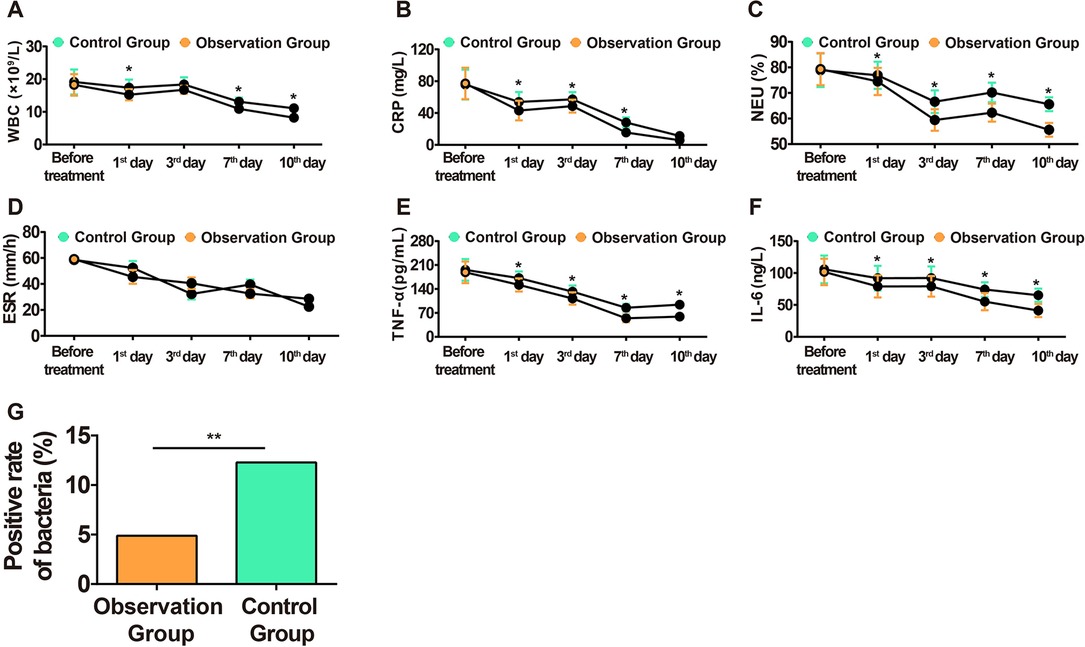
Figure 2. Comparison of inflammatory reaction in both groups. (A) Changes in the WBC level in blood. (B) Changes in the CRP level in serum. (C) Changes in the NEU% level in blood. (D) Changes in the ESR level in blood. (E) Changes in the TNF-α level in serum. (F) Changes in the IL-6 level in serum. (G) Bacterial positive detection rate on the 7th day after the operation. *P < 0.05, **P < 0.01.
Comparison of Clinical Treatment
The clinical treatment of patients was evaluated from the aspects of the times of dressing, stopping time of wound exudation, wound healing time, hospital stay, and average VAS score. The study showed that the above indexes in the observation group were significantly lower than those in the control group (Figure 3), suggesting that the use of TPU/NS dressing effectively promoted patients’ rehabilitation and reduced the pain of wounds.
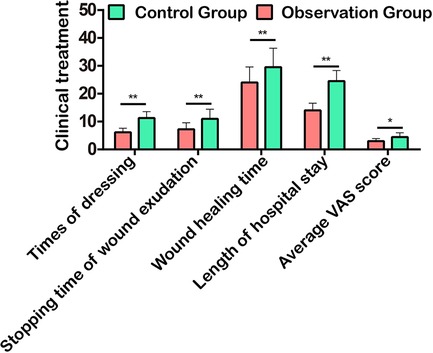
Figure 3. Comparison of clinical treatment in terms of times of dressing, stopping time of wound exudation, wound healing time, hospital stay, and average VAS score. *P < 0.05, **P < 0.01.
Comparison of Adverse Reactions After Treatment
The incidence of adverse reactions after treatment was evaluated in terms of wound infection, delayed healing, scar hyperplasia, soft tissue necrosis, and fracture pain. The study found that the total incidence of the above adverse reactions in the observation group was significantly lower than that in the control group (17.07% vs. 35.09%, Table 2), suggesting that the use of TPU/NS dressing can better inhibit the proliferation of bacteria and promote wound recovery.
Comparison of Functional Rehabilitation and Life Quality
After treatment, the recovery indexes of low limb function in self-care function, cognitive function, and action of patients in the observation group were significantly better than those in the control group (Figure 4A), and the physical function, emotional function, role function, social function, and other dimensions of their life quality were remarkably higher than the other (Figure 4B), suggesting that TPU/NS dressing is more conducive to wound recovery to better promote the rehabilitation of patients.
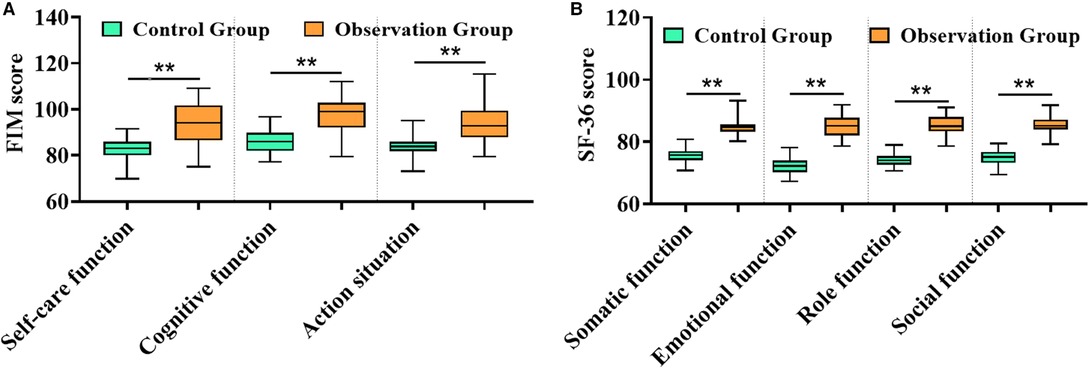
Figure 4. Comparison of rehabilitation between both groups. (A) FIM score. (B) SF-36 score. **P < 0.01.
Discussion
During the treatment of open fractures, patients often had seriously wound due to high-violence injury, and tension blisters and wound infection are easy to appear in the later stage, resulting in the adverse impact on the recovery of postoperative wounds, the long treatment cycle, as well as poor prognosis (23). In addition, during the treatment, the degree of pain in the wound is also a key factor affecting the rehabilitation effect. Although the pain can promote blood circulation in patients, excessive pain was reported to have an adverse impact on postoperative rehabilitation (24). Therefore, taking effective prevention and treatment for infection after the operation has important clinical significance for postoperative recovery and suppression of complications (25).
With the continuous development of nanomedical technology in the past decade, nanodressings have gradually been applied in clinical practice (26). Bacterial infections have long been a thorny issue in clinical anti-infective treatment, and NS as a new type of antibacterial agent has the advantages of high antibacterial activity and a broad antibacterial spectrum without drug resistance (27). Due to the exposure of soft tissue and bone in the wound of open fracture, it is very easy to be complicated with infection. Effectively killing bacteria and reducing viable bacteria are important methods to promote patients’ rehabilitation and prevent related complications. Therefore, NS dressing brings hope to the clinical postoperative anti-infection. The NS dressing using a TPU membrane as a carrier processes pure silver into elemental silver particles with a particle size of about 10 nm and loads them on the TPU membrane. In the antibacterial property experiments, it was found that TPU/NS dressing had a significant antibacterial effect in patients accompanied by the reduced inflammatory reaction, the improved functional rehabilitation, and the alleviated postoperative wound pain. Subsequently, we also tested the positive rate of bacteria in the two groups during the dressing on the seventh day postoperatively. The results showed that the positive rate in the observation group was significantly lower. This also suggests that the application of the nanosilver dressing in open fracture postoperative patients can effectively reduce wound bacteria.
A previous study has pointed out that, unlike the single target effect of antibiotics, NS has a variety of ways to inhibit bacteria (28). For example, it can cause obvious morphological changes in the bacteria by attaching to the surface of bacteria, destroying its cell wall and cell membrane, causing a large number of substances required for maintaining bacterial metabolism to leak, and even causing bacteria to lyse and die. In addition, it has been pointed out in other literature studies that nanosilver can react with enzymes or proteins containing sulfur groups, thus affecting the metabolism of bacteria and at the same time collapsing proton power, leading to the death of bacteria (29).
We compared the times of dressing, stopping time of wound exudation, length of hospital stay, and wound healing time between the two groups, and the results showed that the clinical treatment of the observation group was better with a lower incidence of complications than that of the control group, suggesting that TPU/NS can effectively promote patients' recovery due to the reduction of the positive rate of bacteria. Recently, studies have pointed out that one of the reasons for the outstanding antibacterial properties of nanosilver is that it has a quantum size effect, meaning that the antibacterial effect of nanosilver is related to its particle size. The smaller the particle diameter, the stronger the antibacterial effect. This is the reason why the antibacterial effect of nanosilver in the clinic is stronger than that of ordinary anionic antibacterial agents (30, 31). Finally, we compared both groups on the quality of life, and the results showed that the observation group was under a remarkably higher quality of life, indicating that optimized TPU/NS dressing after the operation can reduce the psychological and physiological trauma stress response of patients to reduce complications, shorten hospital stay, reduce the risk of readmission, and reoperation rate, thus promoting the recovery of patients as soon as possible.
However, in this study, we did not set up a blank control group using the TPU membrane alone, and prospective observation was lacking, which may offset the results. In addition, the safety of TPU/NS dressings should be evaluated by determining the liver and kidney function in diabetic patients with open fractures of lower extremities, as well as serum deposition because hepatocellular damage and nephropathy were associated with diabetes (32). Furthermore, the wound-healing rate would be assessed in the future to validate our clinical results. The above shortcomings should be improved in the follow-up study.
Conclusion
We mixed NS into porous TPU membranes by adopting biomimetic polydopamine and then prepared a kind of wound dressing that has biocompatibility, flexibility, and antibiosis. The whole production process is simple, gentle, and environmentally friendly. TPU/NS dressing has powerful mechanical strength and great flexibility, exhibiting acceptable antibacterial activity against a variety of bacteria. We applied it to the antibacterial treatment of postoperative wounds of DM patients with joint fractures. TPU/NS dressings can lighten the postoperative pain of patients by inhibiting bacterial infection of wounds and promoting postoperative recovery of patients. Therefore, there was a tremendous potential to apply the constructed TPU/NS membrane to open fracture diabetic patients, especially those with soft tissue injury.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Sixth Medical Center of PLA General Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DZ and DCY contributed to the conception and design of the study, and manuscript writing. RFM collected clinical data of patients and prepared software for data analysis. SKN performed data analysis and interpretation. YL made contributions to figures and tables. YZ assisted in the design of the study and participated in manuscript revision. SWZ reviewed and revised the manuscript. All authors read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu GT, Sanders DT, Raspovic KM, Wukich DK. Trauma in the diabetic limb. Clin Podiatr Med Surg. (2019) 36:499–523. doi: 10.1016/j.cpm.2019.02.012
2. Gehling DJ, Lecka-Czernik B, Ebraheim NA. Orthopedic complications in diabetes. Bone. (2016) 82:79–92. doi: 10.1016/j.bone.2015.07.029
3. Gortler H, Rusyn J, Godbout C, Chahal J, Schemitsch EH, Nauth A. Diabetes and healing outcomes in lower extremity fractures: a systematic review. Injury. (2018) 49:177–83. doi: 10.1016/j.injury.2017.11.006
4. Bohme B, d'Otreppe V, Ponthot JP, Balligand M. Intraosseous stress distribution and bone interaction during load application across the canine elbow joint: a preliminary finite element analysis for determination of condylar fracture pathogenesis in immature and mature dogs. Res Vet Sci. (2016) 106:143–8. doi: 10.1016/j.rvsc.2016.03.014
5. Milner C, Samson D, Tan S. Unstable dorsal fracture-dislocations of the proximal interphalangeal joint: volar plate fixation with or without bone graft. J Hand Surg Asian Pac. (2019) 24:50–4. doi: 10.1142/S2424835519500097
6. Goetzen M, Hofmann-Fliri L, Arens D, Zeiter S, Stadelmann V, Nehrbass D, et al. Does metaphyseal cement augmentation in fracture management influence the adjacent subchondral bone and joint cartilage? An in vivo study in sheep stifle joints. Medicine (Baltimore). (2015) 94:e414. doi: 10.1097/MD.0000000000000414
7. Mozaffarian K, Bayatpour A, Vosoughi AR. Simultaneous volar dislocation of distal interphalangeal joint and volar fracture-subluxation of proximal interphalangeal joint of little finger: a new mechanism of injury. J Hand Surg Asian Pac. (2016) 21:422–4. doi: 10.1142/S2424835516720206
8. Han K, Lee JM, Achanta A, Kongkaewpaisan N, Kongwibulwut M, Eid AI, et al. Emergency surgery score accurately predicts the risk of post-operative infection in emergency general surgery. Surg Infect (Larchmt). (2019) 20:4–9. doi: 10.1089/sur.2018.101
9. Nicholson JA, Tsang STJ, MacGillivray TJ, Perks F, Simpson A. What is the role of ultrasound in fracture management? Diagnosis and therapeutic potential for fractures, delayed unions, and fracture-related infection. Bone Joint Res. (2019) 8:304–12. doi: 10.1302/2046-3758.87.BJR-2018-0215.R2
10. Depypere M, Morgenstern M, Kuehl R, Senneville E, Moriarty TF, Obremskey WT, et al. Pathogenesis and management of fracture-related infection. Clin Microbiol Infect. (2020) 26:572–8. doi: 10.1016/j.cmi.2019.08.006
11. Nergiz Adiguzel E, Esen E, Aylaz G, Keskinkilic Yagiz B, Kiyan M, Dogan A, et al. Do nano-crystalline silver-coated hernia grafts reduce infection? World J Surg. (2018) 42:3537–42. doi: 10.1007/s00268-018-4661-3
12. Anuj SA, Gajera HP, Hirpara DG, Golakiya BA. Bactericidal assessment of nano-silver on emerging and re-emerging human pathogens. J Trace Elem Med Biol. (2019) 51:219–25. doi: 10.1016/j.jtemb.2018.04.028
13. Obagi Z, Damiani G, Grada A, Falanga V. Principles of wound dressings: a review. Surg Technol Int. (2019) 35:50–7.
14. Kose N, Caylak R, Peksen C, Kiremitci A, Burukoglu D, Koparal S, et al. Silver ion doped ceramic nano-powder coated nails prevent infection in open fractures: in vivo study. Injury. (2016) 47:320–4. doi: 10.1016/j.injury.2015.10.006
15. Wang H, Liu X, Christiansen DE, Fattahpour S, Wang K, Song H, et al. Thermoplastic polyurethane with controllable degradation and critical anti-fouling properties. Biomater Sci. (2021) 9:1381–96. doi: 10.1039/D0BM01967D
16. Liu M, Liu T, Chen X, Yang J, Deng J, He W, et al. Nano-silver-incorporated biomimetic polydopamine coating on a thermoplastic polyurethane porous nanocomposite as an efficient antibacterial wound dressing. J Nanobiotechnology. (2018) 16:89. doi: 10.1186/s12951-018-0416-4
17. Yu B, Wu X, Tang P, Zhang Y. Guidelines on diagnosis and treatment of open fractures in China (2019). Chin J Orthop Trauma. (2019) 21:921–8. doi: 10.3760/cma.j.issn.1671-7600.2019.11.001
18. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2011) 34(Suppl 1):S62–69. doi: 10.2337/dc11-S062
19. Hu W, Murata K, Zhang D. Applicability of LIVE/DEAD BacLight stain with glutaraldehyde fixation for the measurement of bacterial abundance and viability in rainwater. J Environ Sci (China). (2017) 51:202–13. doi: 10.1016/j.jes.2016.05.030
20. Turnbull A, Sculley D, Escalona-Marfil C, Riu-Gispert L, Ruiz-Moreno J, Girones X, et al. Comparison of a Mobile health electronic visual analog scale app with a traditional paper visual analog scale for pain evaluation: cross-sectional observational study. J Med Internet Res. (2020) 22:e18284. doi: 10.2196/18284
21. Colorado B, Del Toro D, Tarima S. Impact of contact isolation on FIM score change, FIM efficiency score, and length of stay in patients in acute inpatient rehabilitation facility. PM R. (2014) 6:988–91. doi: 10.1016/j.pmrj.2014.05.017
22. Somuncu E, Kara Y. The effect of parathyroidectomy on quality of life in primary hyperparathyroidism: evaluation with using sf-36 and phpqol questionnaire. Endocr J. (2021) 68:87–93. doi: 10.1507/endocrj.EJ20-0417
23. Miller EA, Friedrich JB. Management of finger joint dislocation and fracture-dislocations in athletes. Clin Sports Med. (2020) 39:423–42. doi: 10.1016/j.csm.2019.10.006
24. Kokubo T, Hashimoto T, Suda Y, Waseda A, Ikezawa H. Radiographic shape of foot with second metatarsophalangeal joint dislocation associated with hallux Valgus. Foot Ankle Int. (2017) 38:1374–9. doi: 10.1177/1071100717728579
25. Wang HY, Wang BB, Huang M, Wu XT. Treatment of closed subtalar joint dislocation: a case report and literature review. Chin J Traumatol. (2020) 23:367–71. doi: 10.1016/j.cjtee.2020.08.008
26. Kumar PT, Lakshmanan VK, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG, et al. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. (2012) 4:2618–29. doi: 10.1021/am300292v
27. Anuj SA, Gajera HP, Hirpara DG, Golakiya BA. Bacterial membrane destabilization with cationic particles of nano-silver to combat efflux-mediated antibiotic resistance in Gram-negative bacteria. Life Sci. (2019) 230:178–87. doi: 10.1016/j.lfs.2019.05.072
28. Joshi N, Kocher GS, Kalia A, Banga HS. Development of nano-silver alkaline protease bio-conjugate depilating eco-benign formulation by utilizing potato peel based medium. Int J Biol Macromol. (2020) 152:261–71. doi: 10.1016/j.ijbiomac.2020.02.251
29. Tareq FK, Fayzunnesa M, Kabir MS, Nuzat M. Evaluation of dose dependent antimicrobial activity of self-assembled chitosan, nano silver and chitosan-nano silver composite against several pathogens. Microb Pathog. (2018) 114:333–9. doi: 10.1016/j.micpath.2017.12.010
30. Mahadevan S, Vijayakumar S, Arulmozhi P. Green synthesis of silver nano particles from Atalantia monophylla (L) Correa leaf extract, their antimicrobial activity and sensing capability of H2O2. Microb Pathog. (2017) 113:445–50. doi: 10.1016/j.micpath.2017.11.029
31. Davoodbasha M, Kim SC, Lee SY, Kim JW. The facile synthesis of chitosan-based silver nano-biocomposites via a solution plasma process and their potential antimicrobial efficacy. Arch Biochem Biophys. (2016) 605:49–58. doi: 10.1016/j.abb.2016.01.013
32. El-Sherbiny M, El-Shafey M, Said E, Shaker GA, El-Dosoky M, Ebrahim HA, et al. Dapagliflozin, liraglutide, and their combination attenuate diabetes mellitus-associated hepato-renal injury-insight into oxidative injury/inflammation/apoptosis modulation. Life (Basel). (2022) 12:764. doi: 10.3390/life12050764
Keywords: nano-silver, open fracture, diabetes mellitus, rapid rehabilitation, postoperative recovery
Citation: Zhang D, Yao D, Ma R, Nan S, Lv Y, Zhu Y and Zhu S (2022) Effect of Silver Nanoparticles With Thermoplastic Polyurethane on Postoperative Rehabilitation of Diabetic Patients With Open Fracture of Lower Extremities. Front. Surg. 9:954155. doi: 10.3389/fsurg.2022.954155
Received: 27 May 2022; Accepted: 23 June 2022;
Published: 11 July 2022.
Edited by:
Songwen Tan, Central South University, ChinaReviewed by:
Hanrui Wang, First Affiliated Hospital of Jiamusi University, ChinaYi Zhang, Chun’an County Traditional Chinese Medicine Hospital, China
Copyright © 2022 Zhang, Yao, Ma, Nan, Lv, Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiwen Zhu emh1c2hpd2VuZ3VrZUAxNjMuY29t Yue Zhu aWxvdmViamZzQDE2My5jb20=
†These authors have contributed equally to this work.
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Dong Zhang
Dong Zhang Dongchen Yao
Dongchen Yao Ruofei Ma
Ruofei Ma Shaokui Nan1
Shaokui Nan1 Shiwen Zhu
Shiwen Zhu