- 1Department of General Surgery, Department of Gastrointestinal Surgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
- 2Department of General Surgery, Peking University Fifth School of Clinical Medicine, Beijing Hospital, Beijing, China
Cavernous lymphangioma often occurs in the head, neck, trunk, and extremities of infants and children, and it is rare to cause a small intestine intussusception in adults. In this case, a 32-year-old woman presented with abdominal pain, vomiting, and a 5 cm × 5 cm abdominal mass on the left side of the abdomen. Laboratory tests showed anemia and CT showed small intestinal intussusception. After conservative treatments, her symptoms disappeared. However, 18F-FDG PET/CT suggested malignancy and her symptoms reappeared after eating something. Segmental jejunal resection was performed and pathology showed submucosal cavernous lymphangioma. At the 1-year follow-up, the patient was asymptomatic. Then this paper reviewed the literature on small intestinal cavernous lymphangioma in adults and found that this is the first English case report of intussusception caused by a jejunal submucosal cavernous lymphangioma in an adult. Current problem is that adult intussusception and intestinal lymphangioma are difficult to diagnose preoperatively. Imaging techniques such as tomography and PET/CT aid in the diagnosis of these benign lesions. Surgical resection was considered to be the required treatment and seems to have had no recurrence in adults according to the literature.
Introduction
Intussusception caused by small intestinal cavernous lymphangioma in adults is rare. The etiology of intussusception in adults is often benign polyps, Meckel's diverticulum, malignant lesions, or idiopathic lesions, which account for approximately 1%–5% of mechanical intestinal obstruction and are malignant in up to 77% of cases (1, 2). Lymphangioma is a malformation of the lymphatic vessels and is a benign tumor that often occurs in the head, neck, extremities, and trunk region with an incidence of 1: 2,000–4,000, and most patients (80%–90%) are diagnosed before the age of two years (3). Lymphangiomas account for 6% of small intestinal tumors in children (4) and approximately 1.4%–2.4% in adults (5). Abdominal lymphangiomas account for about 5% of lymphangiomas, often in the mesentery and about 5% in the retroperitoneum (6, 7). The incidence of cavernous lymphangioma is not known. As small intestinal cavernous lymphangiomas are benign lesions and have various clinical presentations, it is difficult to preoperatively diagnose and be associated with intussusception. In addition, patients had a good prognosis after partial resection of the small intestine in the literature. To the best of our knowledge, this is the first English report of intussusception caused by jejunal submucosal lymphangioma in an adult. Our purpose is to report a case and highlight this tumor within variable symptoms, the differential diagnosis for jejunal intussusception, and to identify computed tomography (CT) may help with preoperative diagnosis.
Case report
A 32-year-old woman presented with abdominal pain and vomiting for 10 days, with a history of cesarean sections in 2015 and 2017, without family history of gastrointestinal tumors.
Vital signs remained stable and within normal ranges. Abdominal examination revealed tenderness and a 5 cm × 5 cm abdominal mass on the left side of the abdomen, without signs of peritoneal irritation.
Laboratory tests showed that White blood cell was 6.54 × 109/L, Neutrophil percentage was 87.4%, Hemoglobin was 10.3 g/dl, Amylase was 141 U/L, and D dimer was 630 ng/ml. Tumor biomarkers were not known. Contrast-enhanced CT of the abdomen showed possible small intestinal intussusception of the left-sided upper abdomen. There was the presence of fat within a loop of the jejunum which suggested intussusception with a jejunal lesion and a small amount of pelvic fluid (Figure 1). Magnetic Resonance Imaging (MRI) demonstrated thickening of the proximal jejunum intestinal wall (Figure 2). 18F-FDG positron emission tomography/computed tomography (18F-FDG PET/CT) showed that the distal intestinal metabolic activity increased unevenly, and multiple small lymph nodes were found around the intestine without abnormal metabolic activity (Figure 3).
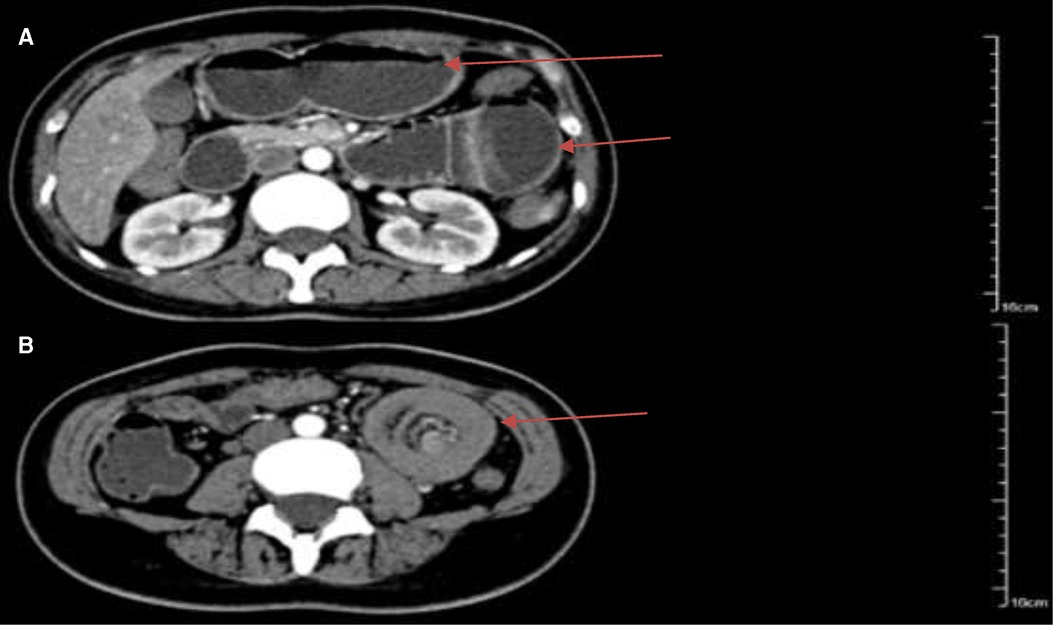
Figure 1. Arterial phase of abdominal contrast-enhanced computed tomography showed jejunal intussusception of the left-sided upper abdomen with concentric circle-like changes. (A) The intestinal canal of the upstream small intestine was dilated, and the outer intestinal wall was edematous and thickened (arrows); (B) there was the presence of the blood vessels and part of the mesenteric fat within a loop of jejunum which suggested intussusception with the jejunal lesion (arrow).
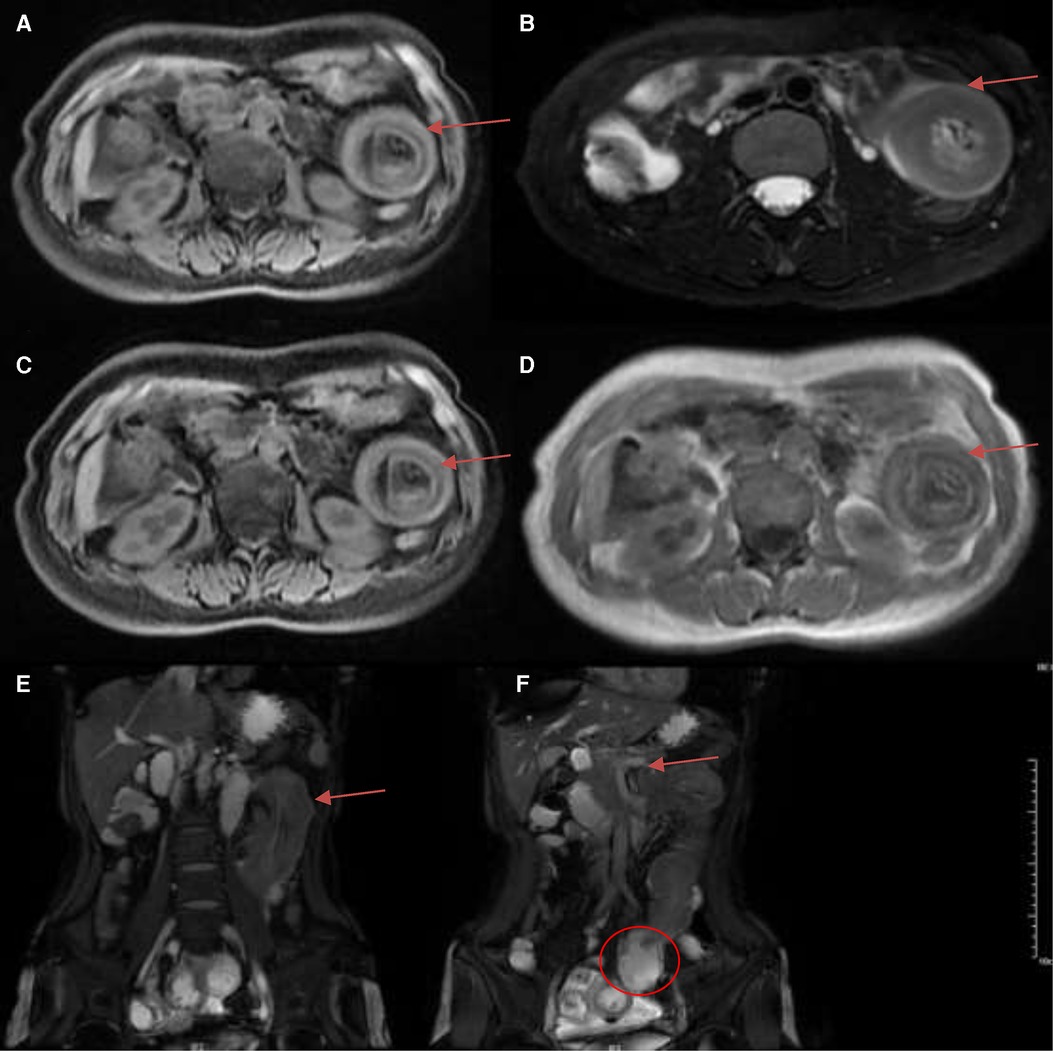
Figure 2. Magnetic resonance imaging (MRI) demonstrated jejunal intussusception of the left-sided upper abdomen with concentric circle-like changes. (A) T1 FS (arrow); (B) RTr OAx T2 fs (arrow); (C) WATER: Ax LAVA-Flex (arrow); (D) InPhase: Ax LAVA-Flex (arrow); (E) BH Cor 2D Fiesta Shim showed the intussusception of the long segment of jejunum from the pelvis to the left-sided upper abdomen, with concentric circle-like changes about 14.2 cm (arrows); (F) BH Cor 2D Fiesta Shim showed the thickening of the proximal jejunum intestinal wall with the thickest part about 2.6 cm, and the lumen was narrowed with peripheral exudation (arrow). The distal segment of the jejunum and ileum were dilated, with fluid accumulation and slightly thickened walls (circle).
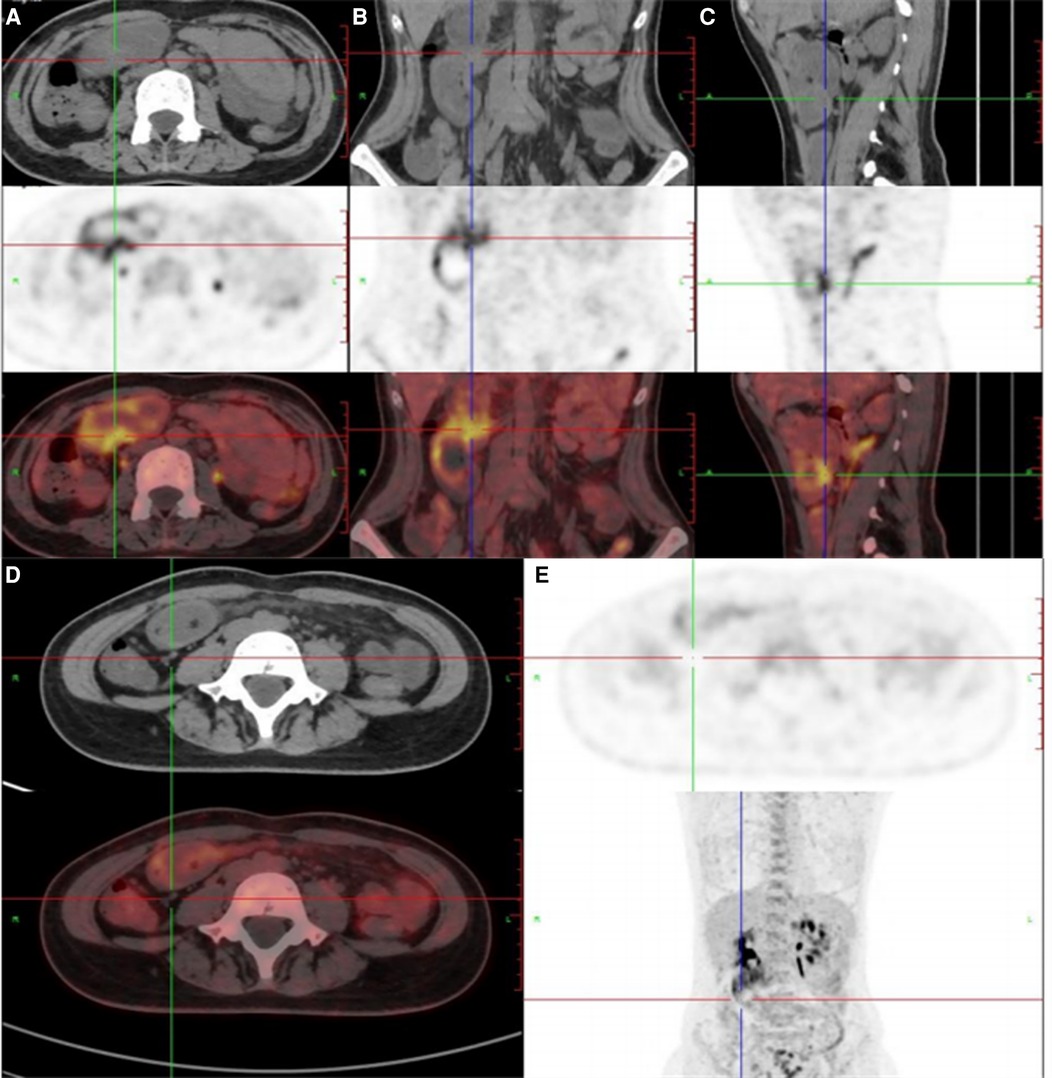
Figure 3. (A–C) 18F-FDG positron emission tomography/computed tomography showed that the distal intestinal metabolic activity increased unevenly; (D,E) multiple small lymph nodes were around the intestine without abnormal metabolic activity.
We would like to use laparoscopy, but we did not choose minimally invasive surgery because of the relatively limited abdominal space caused by intestinal obstruction. Jejuno-jejunal intussusception was identified and reduced, about 15 cm distal from the Treitz ligament. Segmental jejunal resection and ligation of mesenteric vessels from the distal 5 cm to the proximal 5 cm of the intussusception was performed, followed by side-to-side anastomosis. A soft, pedunculated polyp was found inside the wall of the jejunum. The stalk of the polyp and the entire length of the intestine was examined without any additional lesions found. The gross examination of this resected segment showed a 9 cm × 5 cm × 2 cm gray and red soft polyp confined to the submucosa. Pathology showed cavernous lymphangioma of the small intestine with secondary intussusception. Necrosis, hemorrhage, ulcer formation, and acute suppurative inflammation were in the intestinal wall. There was no tumor seen in lymph nodes (0/39). Microscopically, cysts were identified in mucosal lamina propria and submucosa layers (hematoxylin-eosin staining). The endothelial cells were partially positive for D2-40, CD31, CD34 (Figure 4), desmin, and elastin in immunohistochemical staining.
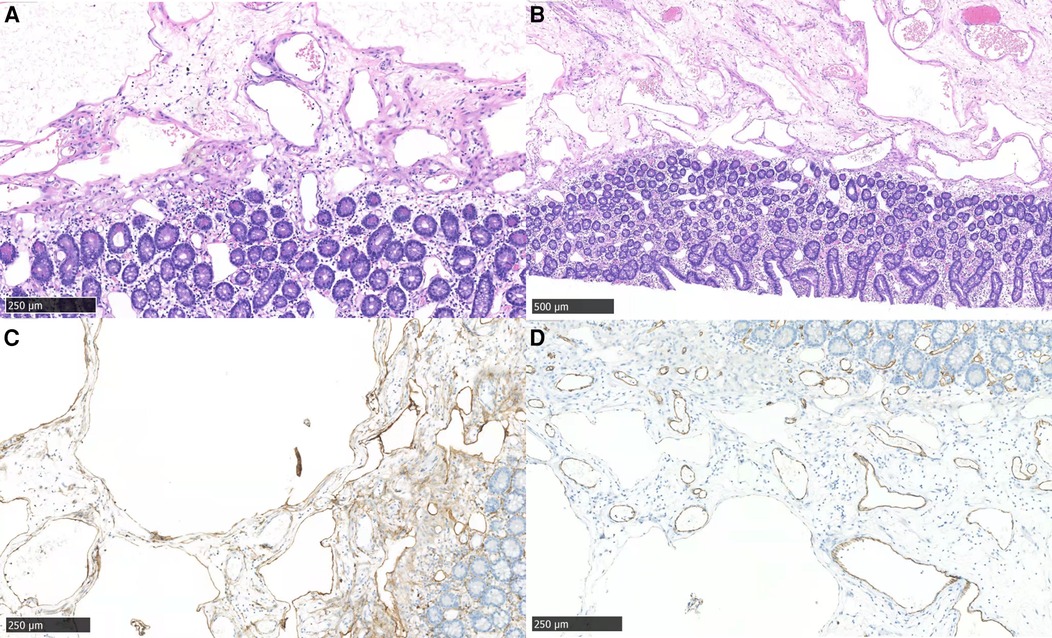
Figure 4. Microscopic features of the resected tumor. (A) morphology showed cysts in mucosal lamina propria and submucosa (hematoxylin-eosin staining, ×40); (B) mucosal lamina propria and submucosa of the jejunum (hematoxylin-eosin staining, ×20); (C,D) the endothelial cells were partially positive for D2-40 (C) and CD31 (D) (×40).
The patient was hospitalized for 11 days after surgery. At the 3-month follow-up, the abdominal CT looked the same as the one after partial bowel resection. At the 1-year follow-up, the patient was asymptomatic, her hemoglobin was normal and the gastrointestinal endoscopy showed smooth duodenal mucosa.
Discussion
From 1940 to 1961, Viar reviewed 9 lymphangiomas in 52 cases of mesenteric cysts, mostly in the ileum and mesentery (8). Hanagiri reviewed 34 cases of small intestinal lymphangioma in the Japanese language literature from 1967 to 1990 (5). Gareth Morris-Stiff reviewed 19 case reports, 8 of which were Asian (9). Šileikis A et al. suggested that abdominal lymphangiomas occurred more frequently in young men (7). There were 4 jejuno-jejunal and 2 ileo-ileal intussusception cases secondary to small intestinal lymphangioma in adults but were not cavernous lymphangiomas (10–15). Wan YL et al. reported the first case of cystic lymphangioma of the cecal intussusception in an adult (16).
The authors searched Pubmed, Clinical Key, and Web of Science for English literature and CNKI, WAN FANG, and CQVIP for Chinese literature from inception up to January 1, 2022, for “lymphangioma” and “small intestine” as keywords. Patients under 18 years old were excluded, and those whose pathology showed “cavernous lymphangioma” were included. There were 23 cases in English literature from 1961 to 2021 (5, 8, 9, 17–33). Women account for 56.5% (13/23) of all cases, young people who were under 50 years old made up 56.5% (13/23) of them, there were 52.2% (12/23) cases whose lesions were located in the jejunum and there were 47.8% (11/23) Asian patients (Supplementary Table S1). There were 36 Chinese patients from 1951 to 2020 (Supplementary Table S2). 66.7% (24/36) of them were males, 77.8% (28/36) were under 50 years old, and 69.4% (25/36) were located in the jejunum. Only 3 of them had English abstracts, so most Chinese cases could not be reviewed in English.
The etiology of lymphangioma is unclear. Congenital lymphangiomas arise from abnormal embryonic development of the lymphatic system with gradual accumulation of lymph. In adults, lymphangiomas may develop secondary to lymphatic duct injury caused by infection, tumor, surgery, trauma, or radiation (34). We reported one case and collected three cases with a history of surgery (19, 27, 33), and two patients had tumor history (25, 32). Lymphangioma may be associated with PI3K/AKT/MTOR Signaling Pathway, Wnt/β-Catenin Signaling Pathways, and VEGF-C (35). Histopathologically, Wegner classified three types of cavernous lymphangiomas: simple, cavernous, and cystic lymphangiomas (36). Cavernous lymphangiomas have dilated and enlarged lymphatic lumens that are often connected to adjacent normal lymphatic spaces (23). Immunohistochemical markers help with diagnosis, D2-40 is a lymphatic endothelial cell marker, while CD31 and CD34 represent vascular endothelial cells.
Clinical presentation is nonspecific. It may be asymptomatic. Sometimes the mass grows slowly and aggressively, encasing and compressing the surrounding organs causing symptoms, and sometimes complications cause acute abdominal disease: torsion, intussusception, or rupture of cystic structures leading to acute peritonitis or intestinal obstruction. Invasion of the intestinal wall can cause gastrointestinal bleeding (8).
Preoperative diagnosis is difficult. However, endoscopy, ultrasound, CT, MRI, and PET/CT can help us. Patients with chronic black stools or abdominal masses can have lesions detected by endoscopy, and the development of small intestinal endoscopy is very helpful, while for patients with acute abdomen are difficult to use endoscopy. The septa in a cystic mass are usually more accurately delineated by ultrasound than by other techniques. But the accuracy of preoperative ultrasound localization and diagnosis is low (16). On CT scan, intussusception presents a typical intestinal-within-intestinal appearance with a pooled accuracy of 77.8% (37). And lymphangiomas present as unilocular or multilocular lesions with contrast enhancement of the cyst walls and septa, whose density is close to water (38). Cystic hypodense lesions with thin cyst wall, homogeneous density, long T1T2 signal, and compression lipid high signal are often present on MRI scans. PET/CT can detect hypermetabolic tumor cells to identify malignant tumors (25). According to our patient's PET/CT, we consider jejunal malignant lesions, infectious lesions, Kaposiform Hemangioendothelioma, and malignancies with mesenteric panniculitis as the differential diagnosis (39). So the resection margin should be from the distal 10 cm–15 cm to the proximal 10 cm–15 cm of the intestinal segment including the tumor, with ligation of mesenteric vessels and removal of the associated mesentery, regional lymph nodes, and adjacent organs the lesion involved. Nuclear imaging of lymphatic vessels and lymphangiography have a direct diagnostic role in defining the lesion, and detecting lymphatic patency and can be used to assess the function of the lymphatic system (40).
Most patients undergo surgical resection of the lesion and part of the small intestine, and pancreaticoduodenectomy is performed for lesions involving the head of the pancreas (28, 29). In this case, when the patient came to the emergency room, she was not allowed to eat or drink and was instead given intravenous fluids for hydration. After using omeprazole sodium for injection, lactulose, and glycerin enema, her symptoms disappeared and started to pass gas. Her family chose conservative treatments then they went to the department of gastroenterology for MR and PET/CT. Until she ate something and then presented with abdominal pain and vomiting again, they agreed to surgery. With the popularity of endoscopy, successful microscopic resection of the lesion has also been performed instead of surgery (17, 33). Surgical resection of part of the small intestinal after endoscopic labeling was successful in some cases (9, 26). Atsushi Kohga et al. used single-incision laparoscopic-assisted surgery (SILS) for a small intestinal intussusception caused by a cystic lymphangioma (14). The prognosis is good, without a case of recurrence reported in the literature.
Conclusion
In summary, we report a rare adult case with jejuno-jejunal intussusception caused by a small intestine cavernous lymphangioma. Most of the cases are under 50 years old, men, Asian, and located in jejunum. Preoperative diagnosis is difficult, but CT, ultrasound, and PET/CT are helpful. Surgical resection is a good curative method without a case of recurrence reported in the literature. The final diagnosis of cavernous lymphangioma will be made by histological examination.
Patient perspective
The patient was uninterested in follow-up or learning about the condition. She had the same abdominal pain after exercising when she was 14 years old. But there was no abnormality on gastroscopy and the pain disappeared soon. However, the pain existed for 10 days without eating anything. She was particularly impressed that she had many CT scans, but the diagnosis was not verified. She hoped the doctor would confirm the diagnosis as soon as possible so she could feel easy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Beijing Hospital, Beijing Hospital, Chinese Academy of Medical Sciences, Beijing 100730, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZN: Conceptualization, Methodology, Software, Writing – Original draft preparation. AQ: Supervision. FY and WZ: Investigation, resources. JW: Writing – Reviewing and Editing. All authors contributed to the article and approved the submitted version.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgments
Thanks for Dr. Du Jun's contributions to the pathology of lymphangioma.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.953840/full#supplementary-material.
References
1. Marinis A, Yiallourou A, Samanides L, Dafnios N, Anastasopoulos G, Vassiliou I, et al. Intussusception of the bowel in adults: a review. World J Gastroenterol. (2009) 15(4):407–11. doi: 10.3748/wjg.15.407
2. Honjo H, Mike M, Kusanagi H, Kano N. Adult intussusception: a retrospective review. World J Surg. (2015) 39(1):134–8. doi: 10.1007/s00268-014-2759-9
3. Perkins JA, Manning SC, Tempero RM, Cunningham MJ, Edmonds JL Jr., Hoffer FA, et al. Lymphatic malformations: review of current treatment. Otolaryngol Head Neck Surg. (2010) 142(6):795–803.e1. doi: 10.1016/j.otohns.2010.02.026
4. Fonkalsrud EW. Congenital malformations of the lymphatic system. Semin Pediatr Surg. (1994) 3(2):62–9. doi: 10.1111/j.1440-1681.1985.tb00893.x
5. Hanagiri T, Baba M, Shimabukuro T, Hashimoto M, Takemoto H, Inoue A, et al. Lymphangioma in the small intestine: report of a case and review of the Japanese literature. Surg Today. (1992) 22(4):363–7. doi: 10.1007/BF00308747
6. Breidahl WH, Mendelson RM. Retroperitoneal lymphangioma. Australas Radiol. (1995) 39(2):187–91. doi: 10.1111/j.1440-1673.1995.tb00270.x
7. Šileikis A, Danys D, Žaldokas G, Strupas K. Abdominal lymphangiomas in adults: case report and literature review. Lietuvos Chirurgija. (2015) 14:111–5. doi: 10.15388/LietChirur.2015.2.8250
8. Viar WN, Scott WF Jr., Donald JM. Mesenteric cavernous lymphangiomata: brief review and report of two cases. Ann Surg. (1961) 153:157–60. doi: 10.1097/00000658-196101000-00019
9. Morris-Stiff G, Falk GA, El-Hayek K, Vargo J, Bronner M, Vogt DP. Jejunal cavernous lymphangioma. BMJ Case Rep. (2011) 2011. 2–4. doi: 10.1136/bcr.03.2011.4022
10. Iwaz S, Aardsma N, Emmadi R. Jejunal lymphangioma and recurrent intussusception in an adult patient; a case report and review of literature. Hum Pathol: Case Rep. (2018) 12:22–3. doi: 10.1016/j.ehpc.2017.12.003
11. Limaiem F, Khalfallah T, Marsaoui L, Bouraoui S, Lahmar A, Mzabi S. Jejunal lymphangioma: an unusual cause of intussusception in an adult patient. Pathologica. (2015) 107(1):19–21. doi: 10.1016/S0735-1097(14)60605-7
12. Akashige T, Sato K, Odajima H, Yamazaki S. A case report of recurrent intussusception caused by small bowel lymphangioma in an adult. Int J Surg Case Rep. (2020) 75:126–30. doi: 10.1016/j.ijscr.2020.09.030
13. Khan K, Kleess L, Ganga R, DePaz H, Santopietro R. Small bowel lymphangioma causing ileo-ileal intussusception in adults. Int J Surg Case Rep. (2017) 41:469–72. doi: 10.1016/j.ijscr.2017.11.033
14. Kohga A, Kawabe A, Hasegawa Y, Yajima K, Okumura T, Yamashita K, et al. Ileo-ileal intussusception caused by lymphangioma of the small bowel treated by single-incision laparoscopic-assisted ileal resection. World J Gastroenterol. (2017) 23(1):167–72. doi: 10.3748/wjg.v23.i1.167
15. Samuelson H, Giannotti G, Guralnick A. Jejunal lymphangioma causing intussusception in an adult: an unusual case with review of the literature. Ann Med Surg (Lond). (2018) 34:39–42. doi: 10.1016/j.amsu.2018.08.020
16. Wan YL, Lee TY, Hung CF, Ng KK. Ultrasound and CT findings of a cecal lymphangioma presenting with intussusception. Eur J Radiol. (1998) 27(1):77–9. doi: 10.1016/S0720-048X(97)00061-2
17. Joao M, Gravito-Soares E, Lopes S, Amaro P. Jejunal cavernous lymphangioma: successful endoscopic treatment of a rare cause of small bowel bleeding. Ann Gastroenterol. (2021) 34(6):891. doi: 10.20524/aog.2021.0662
18. Tan B, Zhang SY, Wang YN, Li Y, Shi XH, Qian JM. Jejunal cavernous lymphangioma manifested as gastrointestinal bleeding with hypogammaglobulinemia in adult: a case report and literature review. World J Clin Cases. (2020) 8(1):140–8. doi: 10.12998/wjcc.v8.i1.140
19. Rojas CL, Molina GA. Lymphangioma cavernous of the small bowel mesentery, an infrequent cause of acute abdomen in adult. J Surg Case Rep. (2018) 2018(2):rjy018. doi: 10.1093/jscr/rjy018
20. Hong IT, Cha JM, Lee JI, Joo KR, Baek IH, Shin HP, et al. A case of cavernous lymphangioma of the small bowel mesentery. Korean J Gastroenterol. (2015) 66(3):172–5. doi: 10.4166/kjg.2015.66.3.172
21. Xu X, Liu W, Zheng C. A rare cause of repeated gastrointestinal bleeding. Mesenteric cavernous lymphangioma. Gastroenterology. (2014) 146(4):e11–3. doi: 10.1053/j.gastro.2013.11.040
22. Vennarecci G, Ceribelli C, Laurenzi A, Moroni E, Ettorre GM. Giant cavernous mesenteric lymphangioma in adult. Updates Surg. (2013) 65(4):317–9. doi: 10.1007/s13304-012-0157-0
23. Tang S-J, Bhaijee F. Small bowel lymphangioma. Video J Encycl GI Endosc. (2014) 1(3):663–5. doi: 10.1016/j.vjgien.2013.03.002
24. Moncao CR. Approach to upper digestive hemorrhage with diagnosis of cavernous lymphangioma. Arq Bras Cir Dig. (2013) 26(1):69–70. doi: 10.1590/S0102-67202013000100016
25. Hwang SS, Choi HJ, Park SY. Cavernous mesenteric lymphangiomatosis mimicking metastasis in a patient with rectal cancer: a case report. World J Gastroenterol. (2009) 15(31):3947–9. doi: 10.3748/wjg.15.3947
26. Huang Q, Minor MA, Weber HC. Clinical challenges and images in GI. (Page 1170) Diagnosis: cavernous lymphangioma of the jejunum. Gastroenterology. (2009) 136(4):1465. doi: 10.1053/j.gastro.2008.09.034
27. Christofi N, Hextall A. Cystic cavernous lymphangioma of the mesentery in a patient with cowden syndrome. J Obstet Gynaecol. (2007) 27(3):329–30. doi: 10.1080/01443610701269267
28. Fujishiro M, Kamoshida T, Hotra S, Hirai S, Oka Y, Sato M, et al. Retroperitoneal lymphangioma with a duodenal lesion in an adult. J Gastroenterol. (2002) 37(5):381–6. doi: 10.1007/s005350200053
29. Chung JH, Suh YL, Park IA, Jang JJ, Chi JG, Kim YI, et al. A pathologic study of abdominal lymphangiomas. J Korean Med Sci. (1999) 14(3):257–62. doi: 10.3346/jkms.1999.14.3.257
30. Seki H, Ueda T, Kasuya T, Kotanagi H, Tamura T. Lymphangioma of the jejunum and mesentery presenting with acute abdomen in an adult. J Gastroenterol. (1998) 33(1):107–11. doi: 10.1007/s005350050053
31. Barquist ES, Apple SK, Jensen DM, Ashley SW. Jejunal lymphangioma. An unusual cause of chronic gastrointestinal bleeding. Dig Dis Sci. (1997) 42(6):1179–83. doi: 10.1023/A:1018889620827
32. Ikura Y, Hashimoto T, Takamine Y, Tani T, Konishi Y, Uchida H, et al. Lymphangioma of the duodenum: report of a case. Surg Today. (1994) 24(2):160–3. doi: 10.1007/BF02473401
33. Davis M, Fenoglio-Preiser C, Haque AK. Cavernous lymphangioma of the duodenum: case report and review of the literature. Gastrointest Radiol. (1987) 12(1):10–2. doi: 10.1007/BF01885092
34. Ha J, Yu YC, Lannigan F. A review of the management of lymphangiomas. Curr Pediatr Rev. (2014) 10(3):238–48. doi: 10.2174/1573396309666131209210751
35. Liu X, Cheng C, Chen K, Wu Y, Wu Z. Recent progress in lymphangioma. Front Pediatr. (2021) 9:735832. doi: 10.3389/fped.2021.735832
37. Hong KD, Kim J, Ji W, Wexner SD. Adult intussusception: a systematic review and meta-analysis. Tech Coloproctol. (2019) 23(4):315–24. doi: 10.1007/s10151-019-01980-5
38. Sohn JH, Byun JH, Park SH, Yoon SE, Kim KW, Hong HS, et al. Abdominal cavernous iymphangiomas: CT findings. Abdom Imaging. (2005) 30(6):689–93. doi: 10.1007/s00261-005-0326-4
39. Dong A, Zhang L, Wang Y, He T, Zuo C. Abdominal kaposiform hemangioendothelioma associated with lymphangiomatosis involving mesentery and ileum: a case report of MRI, CT, and 18F-FDG PET/CT findings. Medicine (Baltimore). (2016) 95(6):e2806. doi: 10.1097/MD.0000000000002806
Keywords: cavernous lymphangioma, intussusception, jejunal tumor, case report, small intestine
Citation: Zhao N, Fu Y, Wang Z, An Q and Jia W (2022) Case report: Submucosal cavernous lymphangioma causing jejuno-jejunal intussusception in an adult. Front. Surg. 9:953840. doi: 10.3389/fsurg.2022.953840
Received: 26 May 2022; Accepted: 5 September 2022;
Published: 22 September 2022.
Edited by:
Hirotoshi Kikuchi, Hamamatsu University School of Medicine, JapanReviewed by:
Firdaus Hayati, Universiti Malaysia Sabah, MalaysiaIndraneil Mukherjee, Florida Hospital Tampa, United States
© 2022 Zhao, Fu, Wang, An and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Wenzhuo amlhd2Vuemh1bzA2MDFAMTYzLmNvbQ==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Ning Zhao
Ning Zhao Yuhang Fu2
Yuhang Fu2