
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 06 January 2023
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.951472
This article is part of the Research Topic Management of Borderline Ovarian Tumor: The best treatment is a real challenge in the era of precision medicine View all 6 articles
 Lina Niu1,†
Lina Niu1,† Weibin Wang1,†
Weibin Wang1,† Yongjun Xu2
Yongjun Xu2 Tao Xu3
Tao Xu3 Jiali Sun4
Jiali Sun4 Weiqin Lv1
Weiqin Lv1 Junli Zhang1
Junli Zhang1 Lirong Qiu1
Lirong Qiu1 XuFeng Dong5
XuFeng Dong5 Yun Shang1
Yun Shang1 Lizhen Zhang1
Lizhen Zhang1 Junxia Wang1*
Junxia Wang1*
Objective: This study aimed to investigate the clinical value of ultrasonography combined with tumor markers in the diagnosis and prediction of recurrence of borderline ovarian tumors (BOTs) and analyze the value of the combination of two different auxiliary examinations in the diagnosis and prediction of recurrence of BOTs.
Methods: Here, 221 patients with BOTs confirmed by postoperative pathology were enrolled. Their clinical data, including the ultrasonography features, tumor markers, and clinicopathological data, were retrospectively analyzed.
Results: The statistical data of the 221 cases with BOTs were as follows: 94 (42.5%) with left-sided lesions, 102 (46.2%) with right-sided lesions, and 25 (11.3%) with bilateral lesions. Moreover, 93 cases (42.1%) had a borderline serous tumor, 110 (49.8%) had a borderline mucinous tumor, 12 (5.4%) had a borderline serous mucinous tumor, 2 (0.9%) had a borderline endometrioid tumor, 1 (0.5%) had a borderline Brenner tumor, and 2 (0.9%) had a clear cell BOT. There were 104 cases (47.1%) with a tumor diameter of ≤10 cm and 117 cases (52.9%) with a tumor diameter of >10 cm as suggested by ultrasonography. There were 89 cases (40.3%) with septation, 44 (19.9%) with papilla, and 97 (43.9%) with blood flow as demonstrated by ultrasonography. Carbohydrate antigen 125 (CA 125) was elevated in 132 cases (59.7%), and CA 19-9 was elevated in 52 cases (23.5%).
Conclusion: In general, BOTs are difficult to diagnose preoperatively and have a certain recurrence rate. Ultrasonography combined with CA 125 and CA 19-9 is significant for the preoperative diagnosis and selection of surgical modality for BOTs and could be used as a guideline to achieve good preoperative preparation and avoid secondary surgery.
The pathological types of borderline ovarian tumors (BOTs) vary greatly (1). They have certain recurrence and mortality rates, and there are difficulties in differentiating BOTs from benign ovarian and malignant tumors, difficulties in the preoperative and intraoperative diagnosis, and the risk of excessive trauma, secondary surgery, and postoperative recurrence in patients. The ultrasound is certainly a cost-effective exam and the role of ultrasound is well defined in primary diagnosis of ovarian tumors. Although it is still controversial during follow-up of surgically treated ovarian tumors, the ultrasound is an efficient tool in the early detection of ovarian tumors (2, 3).
In the present study, the general clinical data of 221 patients were retrospectively analyzed, and the ultrasonography characteristics of the BOTs, the CA 125 and CA 19-9 values for the preoperative diagnosis of the disease, and the prediction of the recurrence were analyzed in depth in combination with a literature analysis (4, 5). The value of the combined application of ultrasonography and CA 125/CA 19-9 detection in the diagnosis and prediction of the disease's recurrence was also discussed.
A total of 221 patients with ovarian tumors admitted to our hospital from January 2010 to December 2018 were selected for the retrospective analysis. All patients were diagnosed with BOTs by postoperative pathological examination with complete ultrasonography and clinicopathological data. They had a minimum age of 13 years and a maximum age of 84 years, with a mean age of 43.20 ± 14.86 years. All patients were treated surgically, and relevant pathological histological findings were obtained from the postoperative specimens. This study was approved by the Ethics Committee of Yuncheng Central Hospital (No. YXLL 2022011-1).
Transabdominal ultrasonography, transvaginal ultrasonography, a combination of transabdominal and transvaginal ultrasonography, and other gynecologic ultrasonography examinations were conducted in all patients. The carbohydrate antigen 125 (CA 125) and CA 19-9 detections were conducted in the hospital. The patients’ sonographic characteristics were analyzed, including the number of masses (unilateral and bilateral), location (left-sided, right-sided, and pelvis), size, morphology (especially the internal translucency of the masses), and the presence or absence of septation and papillae. The tumor markers, postoperative pathological examination results, and recurrence were also examined.
Among the 221 enrolled cases, there were 94 (42.5%) with left-sided lesions, 102 (46.2%) with right-sided lesions, and 25 (11.3%) with bilateral lesions. The detailed pathological results and tumor type distribution are demonstrated in Table 1. Compared with other pathological types, borderline serous tumors were more likely to be bilaterally involved (i.e., 60.0% of the serous tumors, 36.0% of the mucinous tumors, and 4.0% of the serous mucinous tumors). There was no statistically significant difference in the tumor type distribution (P > 0.05). There were 89 cases (40.3%) with septation, 44 cases (19.9%) with papilla, and 97 cases (43.9%) with blood flow as demonstrated by ultrasonography.
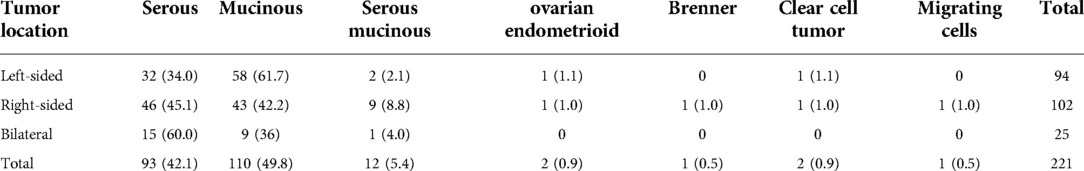
Table 1. The pathological data of 221 patients with borderline ovarian tumors (cases and percentages).
The ultrasonography features comprised cystic solid masses (i.e., regular morphology), clear cystic walls, and cystic textures. Several BOTs had visible septation, most with visible blood flow, and mainly manifested as multifocal masses. The ultrasound sonogram results, tumor markers, and pathological features are shown in Table 2.
Among the 221 patients, 132 (59.7%) had elevated CA 125 levels, and 89 (40.3%) had normal CA 125 levels; the CA 19-9 level was elevated in 52 cases (23.5%), while 169 (76.5%) had normal CA 19-9 levels. The details are illustrated in Table 3.
In 1973, the World Health Organization (WHO) officially termed ovarian tumors as borderline malignant tumors (i.e., carcinoma with low malignant potential) or low-grade potentially malignant tumors in the histological classification of ovarian tumors (6). The term borderline/atypical proliferative tumor was adopted to designate this tumor type, which was previously referred to as low malignant potential tumor (7) in the 2014 edition of WHO Classification of Tumors of the Female Genital Organs (8). Currently, it is difficult to confirm the preoperative diagnosis of BOTs, and the confirmation of a specific diagnosis needs to be combined with clinical manifestations, imaging examinations, tumor marker detection, etc. An intraoperative frozen section can assist in the diagnosis, but the final confirmation still relies on pathological examination.
In many studies, BOTs are assigned to a special manifestation of malignant tumors. According to epidemiological research, the clinical incidence of BOTs is increasing yearly, as is the proportion of malignant tumors, which is directly related to improvements in pathology and ultrasonography diagnosis technology and the application of tumor markers to a certain extent (9). In the present study, approximately 62.9% of patients had no obvious symptoms of discomfort and requested surgery due to pelvic masses found by physical examination.
Here, 93 cases (42.1%) had a borderline serous tumor, 110 (49.8%) had a borderline mucinous tumor, 12 (5.4%) had a borderline serous mucinous tumor, 2 (0.9%) had a borderline endometrioid tumor, 1 (0.5%) had a borderline Brenner tumor, and 2 (0.9%) had a clear cell BOT. The ultrasonography results demonstrated that 104 cases (47.1%) had a tumor diameter of ≤10 cm and that 117 (52.9%) had a tumor diameter of >10 cm. There were 89 cases (40.3%) with septation, 44 (19.9%) with papilla, and 97 (43.9%) with blood flow as demonstrated by ultrasonography. In the laboratory examination, CA 125 was elevated in 132 cases (59.7%), and 89 cases (40.3%) had normal CA 125 levels. In general, BOTs have greatly varied biological behavior and diverse clinical manifestations that are difficult to accurately diagnose preoperatively, and their prognosis is significantly correlated with the surgical approach taken. A previous study suggested that 65% of patients are <40 years old (10), that the tumors are increasing in the young population, and that the patients are mainly women of reproductive age. Most patients with BOTs may achieve a good prognostic outcome with a high five-year survival rate, and the risk of recurrence and metastasis exists in only a few cases (4). In clinical treatment, it is important to reduce the recurrence rate and maximize the preservation of patients’ reproductive function, and unnecessary surgical trauma should be avoided, which is the key to the diagnosis and treatment of BOTs. Impairing autophagic flux could be a novel strategy of cancer therapy (11). However, controversies still exist.
The preoperative identification of pelvic masses mainly relies on gynecologic ultrasonography and the detection of tumor markers, and intraoperative diagnosis mainly relies on intraoperative frozen section examination. Previous study has shown that the ovarian cancer has some specific ultrasound characteristics (12). However, BOTs and ovarian tumors have similar ultrasonographic features, it is difficult to accurately distinguish BOTs from other ovarian tumors (13). Papillary protrusion is a characteristic manifestation of BOTs. In ovarian tumors, whether benign or malignant, the possibility of papilla is high, and as the risk of malignancy increases, the possibility of irregular papilla and papilla with an increased volume increases accordingly. In several cases with borderline serous tumors, septation occurs, and small papillae may appear on the septation. In contrast, borderline mucinous tumors are more likely to have septal-like structures, and the tumor size is significantly larger (13). Once a typical papillary ultrasound presentation is found, the possibility that the patient has a borderline tumor cannot be ruled out. A serous cystic adenoma is more likely to have a similar ultrasound presentation, making it difficult to accurately diagnose BOTs and leading to false positivity. However, for women of childbearing age, considering the reproductive function and avoiding secondary surgery, there is a need for active early diagnosis and treatment. Moreover, the risks of preoperative and intraoperative misdiagnosis and missed diagnosis should be minimized. Additionally, the surgical staging is mainly pursued via a minimally invasive approach in case of BOTs or apparently early stage ovarian cancer. Minimally invasive approach is associated with reduced hospitalization, fewer post-operative complications and better cosmetic results (14, 15).
In a previous study concerning the ultrasound presentation and CA 125 level in BOTs and invasive tumors, univariate and multivariate logistic regression analyses were used to investigate the independent risk factors for BOTs, mainly including the tumor size, solid tumor component, septation, and CA 125 level (16). It was found that the specificity of CA 125 was not high but was able to sensitively determine the extent of disease involvement. Based on this, a scientific assessment of disease progression and an accurate prediction of tumor recurrence could be achieved. Ultrasonography diagnosis of an ovarian tumor requires continuous and dynamic observation of the tumor as a whole. It has been shown that BOTs’ histological type, size, and location (unilateral or bilateral) correlate with the accuracy of intraoperative frozen sections (17). The present study also had to provide the pathologist with a detailed medical history, ultrasonography findings, tumor markers, and intraoperative findings when sending the frozen sections. Upon retrospectively analyzing the ultrasonography images of the 221 patients included here, most sonographers made descriptive diagnoses, such as “cystic mass” and “multifocal cystic mass,” except for a few, who suggested the diagnosis of BOTs. Therefore, it could be inferred that the diagnosis of BOTs is difficult for most ultrasonographers. Transabdominal ultrasound often has difficulty in timely detecting micro-papillary structures in the wall, whereas transvaginal ultrasound can effectively identify and diagnose BOTs. The results of the multivariate analysis revealed that the tumor size and cystic solidity and the CA 125 level are clinically significant in predicting tumor recurrence, suggesting that ovarian tumors with a diameter >10 cm, cystic solidity, or solidity on ultrasonography and elevated CA 125 levels have a high risk of postoperative recurrence. The details are demonstrated in Tables 4, 5. For patients with these characteristics, it is recommended to undergo comprehensive staged surgery and enhanced follow-up. The CA 125 level can be adopted as a screening and postoperative review monitoring index for patients with BOTs, and great importance should be attached to patients with elevated CA 125 levels (18, 19).
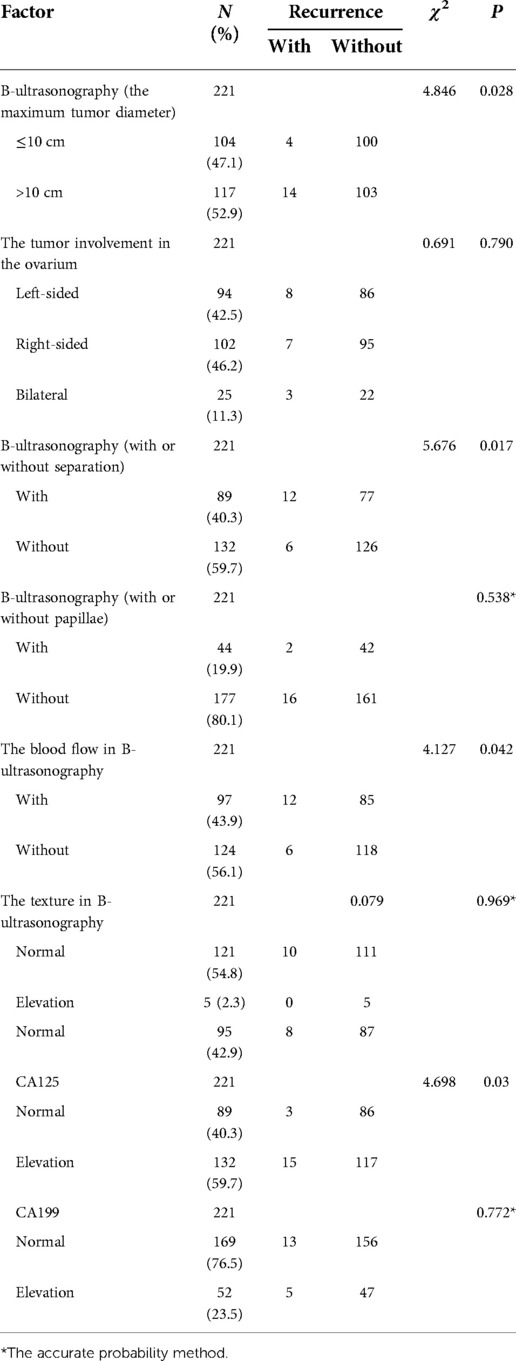
Table 4. The chi-square analysis of the ultrasonography characteristics, levels of CA125 and CA199 and recurrence in patients.
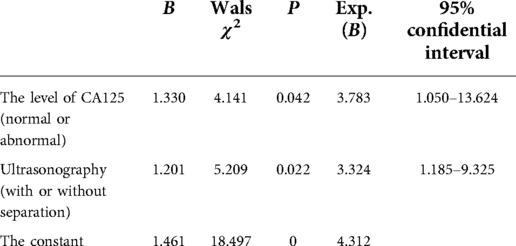
Table 5. The logistic regression analysis of the influencing factors for the recurrence of borderline ovarian tumors.
In the present study, the clinical characteristics, tumor markers, ultrasonography features, and recurrence rates of BOTs were analyzed, but the recurrence of different pathological tumor types was not studied in detail. This was a retrospective study with some missing data, a small sample size, and a short follow-up duration for some of the patients, so long-term follow-up is needed for good clinical analysis in future studies. In future work, in-depth, careful analysis and discussion of the ultrasonography features, levels of CA 125 and CA 19-9, and prognostic features of BOTs with different pathological subtypes should also be conducted.
In summary, although it is difficult to accurately diagnose BOTs preoperatively, ultrasonography can provide an accurate and detailed description of the characteristics of the masses, and combined with the levels of tumor markers, it can indicate benignity or malignancy. Therefore, it is easier and more accurate to judge by the intraoperative frozen sections according to the preoperative indications. Patients’ general condition and ultrasonography features are also valuable in predicting the recurrence rate and can aid in the surgical approach selection, postoperative monitoring, and follow-up (20), minimizing the trauma caused by secondary surgery and reducing unnecessary recurrence and death caused by recurrence.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of Yuncheng Central Hospital (YXLL2022011-1). The patients/participants provided their written informed consent to participate in this study.
Conception and design of the research: LN. Acquisition of data: TX, JS, LQ, WL, JZ, YS, LZ. Analysis and interpretation of the data: LN, WW, YX. Statistical analysis: XD. Writing of the manuscript: LN. Critical revision of the manuscript for intellectual content: WW, JW. All authors contributed to the article and approved the submitted version.
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hauptmann S, Friedrich K, Redline R, Avril S. Ovarian borderline tumors in the 2014 WHO classification: evolving concepts and diagnostic criteria. Virchows Arch. (2017) 470(2):125–42. doi: 10.1007/s00428-016-2040-8
2. Rosati A, Gueli Alletti S, Capozzi VA, Mirandola M, Vargiu V, Fedele C, et al. Role of ultrasound in the detection of recurrent ovarian cancer: a review of the literature. Gland Surg. (2020) 9(4):1092–101. doi: 10.21037/gs-20-357
3. Capozzi VA, Rosati A, Rumolo V, Ferrari F, Gullo G, Karaman E, et al. Novelties of ultrasound imaging for endometrial cancer preoperative workup. Minerva Med. (2021) 112(1):3–11. doi: 10.23736/S0026-4806.20.07125-6
4. Nyangoh-Timoh K, Bendifallah S, Dion L, Ouldamer L, Levêque J. Tumeurs frontières de l’ovaire. Recommandations pour la pratique clinique du CNGOF – pertinence des marqueurs tumoraux [borderline ovarian tumours: CNGOF guidelines for clinical practice - value of tumor markers]. Gynecol Obstet Fertil Senol. (2020) 48(3):277–86. doi: 10.1016/j.gofs.2020.01.015
5. Skírnisdóttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960-2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. (2008) 123(8):1897–901. doi: 10.1002/ijc.23724
6. Dai Q. Problems and research progress in ultrasonic diagnosis of borderline ovarian tumors and malignant tumors. Chin J Med Ultrasound. (2017) 14(2):81–4. CNKI:SUN:ZHCD.0.2017-02-001
7. Kumari S, Kumar S, Bhatla N, Mathur S, Thulkar S, Kumar L. Oncologic and reproductive outcomes of borderline ovarian tumors in Indian population. Gynecol Oncol Rep. (2021) 36:100756. doi: 10.1016/j.gore.2021.100756
8. Shen DH, Chen DB. Interpretation of changes in WHO classification of female genital tumors, 4th edition. Chin J Obstet Gynecol. (2014) 49(9):717–20. doi: 10.3760/cma.j.issn.0529-567x.2014.09.020
9. Song T, Lee DH, Jung YW, Yun BS, Seong SJ, Choi CH, et al. Elevated preoperative CA125 or CA19-9 in borderline ovarian tumors: could it be suggestive of advanced stage or a poor prognosis? Gynecol Obstet Invest. (2018) 83(1):45–51. doi: 10.1159/000475817
10. Gui Y, Dai Q. The ultrasound features of borderline ovarian tumors. Oncoradiology. (2016) 25(1):33–7. CNKI:SUN:YXYX.0.2016-01-008
11. Xu XH, Chen YC, Xu YL, et al. Garcinone E blocks autophagy through lysosomal functional destruction in ovarian cancer cells. World J Tradit Chin Med. (2021) 2:209–16. doi: 10.4103/wjtcm.wjtcm_83_20
12. Ghirardi V, De Felice F, Rosati A, Ergasti R, Gueli Alletti S, Mascilini F, et al. A laparoscopic adjusted model able to predict the risk of intraoperative capsule rupture in early-stage ovarian cancer: laparoscopic ovarian cancer spillage score (LOChneSS study). J Minim Invasive Gynecol. (2022) 29(8):961–7. doi: 10.1016/j.jmig.2022.04.014
13. Franchi D, Boveri S, Radice D, Portuesi R, Zanagnolo V, Colombo N, et al. Ultrasonographic diagnosis and longitudinal follow-up of recurrences after conservative surgery for borderline ovarian tumors. Am J Obstet Gynecol. (2016) 215(6):756.e1–e9. doi: 10.1016/j.ajog.2016.07.024
14. Cianci S, Capozzi VA, Rosati A, Rumolo V, Corrado G, Uccella S, et al. Different surgical approaches for early-stage ovarian cancer staging. A large monocentric experience. Front Med. (2022) 25(9):880681. doi: 10.3389/fmed.2022.880681
15. Capozzi VA, Armano G, Rosati A, Tropea A, Biondi A. The robotic single-port platform for gynecologic surgery: a systematic review of the literature and meta-analysis. Updates Surg. (2021) 73(3):1155–67. doi: 10.1007/s13304-020-00812-8
16. Geomini P, Bremer G, Kruitwagen R, Mol BW. Diagnostic accuracy of frozen section diagnosis of the adnexal mass: a metaanalysis. Gynecol Oncol. (2005) 96(1):1–9. doi: 10.1016/j.ygyno.2004.09.042
17. Song T, Choi CH, Kim HJ, Kim MK, Kim TJ, Lee JW, et al. Accuracy of frozen section di-agnosis of borderline ovarian tumors. Gynecol Oncol. (2011) 122(1):127–31. doi: 10.1016/j.ygyno.2011.03.021
18. Plett H, Ricciardi E, Harter P, Ataseven B, Heitz F, Prader S, et al. Dataset on patients with recurrent borderline ovarian tumors and table with review of literature on fertility and oncologic outcomes of patients with borderline ovarian tumors. Data Brief. (2020) 30:105653. doi: 10.1016/j.dib.2020.105653
19. Niu L, Tian H, Xu Y, Cao J, Zhang X, Zhang J, et al. Recurrence characteristics and clinicopathological results of borderline ovarian tumors. BMC Womens Health. (2021) 21(1):134. doi: 10.1186/s12905-021-01263-y
20. De Decker K, Jaroch KH, Edens MA, Bart J, Kooreman LFS, Kruitwagen RFPM, et al. Frozen section diagnosis of borderline ovarian tumors with suspicious features of invasive cancer is a devil's dilemma for the surgeon: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2021) 100(8):1369–76. doi: 10.1111/aogs.14105
Keywords: CA 125, CA 19-9, borderline ovarian tumors, recurrence, differential diagnosis, ultrasonography
Citation: Niu L, Wang W, Xu Y, Xu T, Sun J, Lv W, Zhang J, Qiu L, Dong X, Shang Y, Zhang L and Wang J (2023) The value of ultrasonography combined with carbohydrate antigen 125 and 19-9 detection in the diagnosis of borderline ovarian tumors and prediction of recurrence. Front. Surg. 9:951472. doi: 10.3389/fsurg.2022.951472
Received: 24 May 2022; Accepted: 10 October 2022;
Published: 6 January 2023.
Edited by:
Andrea Rosati, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Nguyen Minh Duc, Pham Ngoc Thach University of Medicine, Vietnam© 2023 Niu, Wang, Xu, Xu, Sun, Lv, Zhang, Qiu, Dong, Shang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junxia Wang d2FuZ2p1bnhpYXdqeDAzMUAxMjYuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.