- 1Department of Thoracic Surgery, Shenyang Chest Hospital & Tenth People's Hospital, Shenyang, China
- 2Department of Health Management, Shengjing Hospital of China Medical University, Shenyang, China
Background: Prognostic factors in a pneumonectomy (PN) are not yet fully defined. This study sought to analyze and evaluate long-term survival after pneumonectomies (PNs) for patients with non-small cell lung cancer (NSCLC).
Methods: We obtained data from the Surveillance, Epidemiology, and End Results (SEER) database for patients who underwent PNs between 2004 and 2015. Propensity score matching (PSM) analysis and Kaplan–Meier curves were used to estimate overall survival (OS), while univariate and multivariable Cox proportional hazards regression analyses were applied to create a forest plot.
Results: In total, 1,376 patients were grouped according to right/left PNs. Before matching, OS was worse after a right PN [hazard ratio (HR): 1.459; 95% CI 1.254–1.697; P < 0.001] and after matching, survival differences between groups were not significant (HR: 1.060; 95% CI 0.906–1.240; P = 0.465). Regression analysis revealed that age, gender, grade, lymph node dissection, N-stage, and chemotherapy were independent predictors of OS (P < 0.05). Chemotherapy was associated with improved OS (P < 0.001).
Conclusions: Laterality was not a significant prognostic factor for long-term survival after a PN for NSCLC. Chemotherapy was a significant independent predictor of improved OS. Long-term survival and outcomes analyses should be conducted on larger numbers of patients.
Introduction
The incidence of lung cancer and associated mortality rates are among the highest of all malignant tumors in China and the world. The most recent estimate predicts 236,740 new cases and 130,180 deaths in 2022, emphasizing the serious worldwide effects of this disease, which has a 5-year survival rate of approximately 22 percent (1). About 80% of patients with lung cancer have NSCLC. Radical resection remains the preferred treatment for NSCLC and current widely-accepted surgical techniques include lobectomy, segmentectomy, PN, and pulmonary sleeve with pulmonary artery reconstruction (2–4).
Central lung tumors are relatively common in clinical practice. Some can be treated with lobectomy or pulmonary sleeve resection, but when these methods cannot remove the tumor completely, a PN is required, including the dissection of half of the lung tissue and the pulmonary arteries, veins, and main bronchi, accompanied by systematic lymph node dissection. The prognostic factors for left and right PNs remain under investigation. Dr. Graham reported the world's first PN for lung cancer in 1933 (5). However, a PN remains challenging with high complications, such as bronchopleural fistula, progressive pulmonary hypertension, respiratory failure, etc. (6, 7). Depending on the surgeon's experience, and the histological and anatomical characteristics of the lung and tumor, survival may be better for a left PN than the right (8). The reason may be that the contribution of the right lung to the whole is larger than that of the left lung (9).
It is controversial whether the treatment of lung cancer patients with left or right PN is beneficial to long-term survival. And survival data is lacking in the existing literature (10). This study aimed to analyze and evaluate long-term survival after a PN in patients with NSCLC. We used a population-based national registry, the SEER database, to analyze clinical characteristics and prognosis for unilateral and bilateral PNs. We analyzed the factors affecting PNs and applied the Cox proportional hazards model to create a forest plot of individual hazard ratios for the overall survival of patients who underwent left vs. right PNs.
Materials and methods
Patients
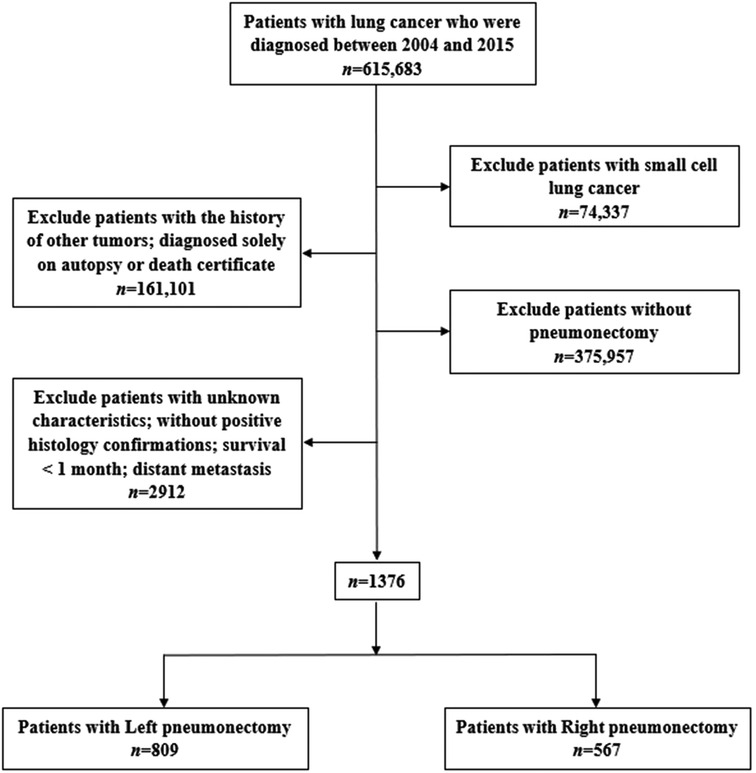
We extracted data from the SEER database (https://seer.cancer.gov/) through SEER*Stat software (v8.3.6, https://seer.cancer.gov/seerstat/) to identify patients with a confirmed diagnosis of NSCLC between 2004 and 2015 and those who underwent a PN (SEER Surgery Codes: 55, 56, 65, 66, 70) were included in our study. The inclusion criteria were: (1) diagnosis between 2004 and 2015 (615,683), (2) diagnosis of NSCLC confirmed microscopically, (3) only one primary tumor and available clinical information, (4) survival for at least 1 month and active follow-up. The exclusion criteria were: (1) incomplete survival or clinical data, including unknown race, grade, etc.; distant metastasis; survival for <1 month (2864), (2) small cell lung cancer (74,337), (3) without pneumonectomy (375,957), (4) with the history of other tumors and diagnosed solely on autopsy or death certificate (161,101; Figure 1).
Variables
The covariates included age, gender, race, marriage, primary site, summary stage, grade, lymph node dissection, tumor stage, T-stage, N-stage, radiotherapy, and chemotherapy. We classified age into four groups: ≤50, 51–60, 61–70, and 71 and older. The grade was classified as well-differentiated (I), moderately differentiated (II), poorly differentiated (III), and undifferentiated (IV). The lymph node dissections included 1–3 removed and ≥4 removed. We followed the eighth edition American Joint Committee on Cancer (AJCC) lung cancer staging system and updated the T-stage (T1, T2, T3, and T4), N-stage (N0, N1, N2–N3), and tumor stage (I, II, III) for all patients in all periods. Overall survival (OS) was defined as the time from diagnosis to death from any cause. To avoid bias between left and right PN groups, we applied 1: 1 PSM (11) for age, gender, race, marriage, primary site, grade, summary stage, lymph node dissection, tumor stage, T-stage, N-stage, radiotherapy, and chemotherapy.
Statistical analysis
Continuous variables are expressed as mean ± SD and categorical variables are expressed as percentages. Variables were conducted by Student's t test, Pearson's Chi-square test, and ANOVA. Kaplan–Meier method we used to create survival curves and the differences between the curves were analyzed using a log-rank test. The Cox proportional hazards model was used to verify independent prognostic factors and calculate the HR and corresponding 95% CI. Forest plots describes specific results. We used the Statistical Product and Service Solutions 25.0 software (SPSS, Inc., Chicago, IL, United States) to analyze data. P values <0.05 (two-sided) were considered statistically significant. The forest plot and survival curves were drawn with a GraphPad Prism (Version 8.3.1).
Results
Patient characteristics
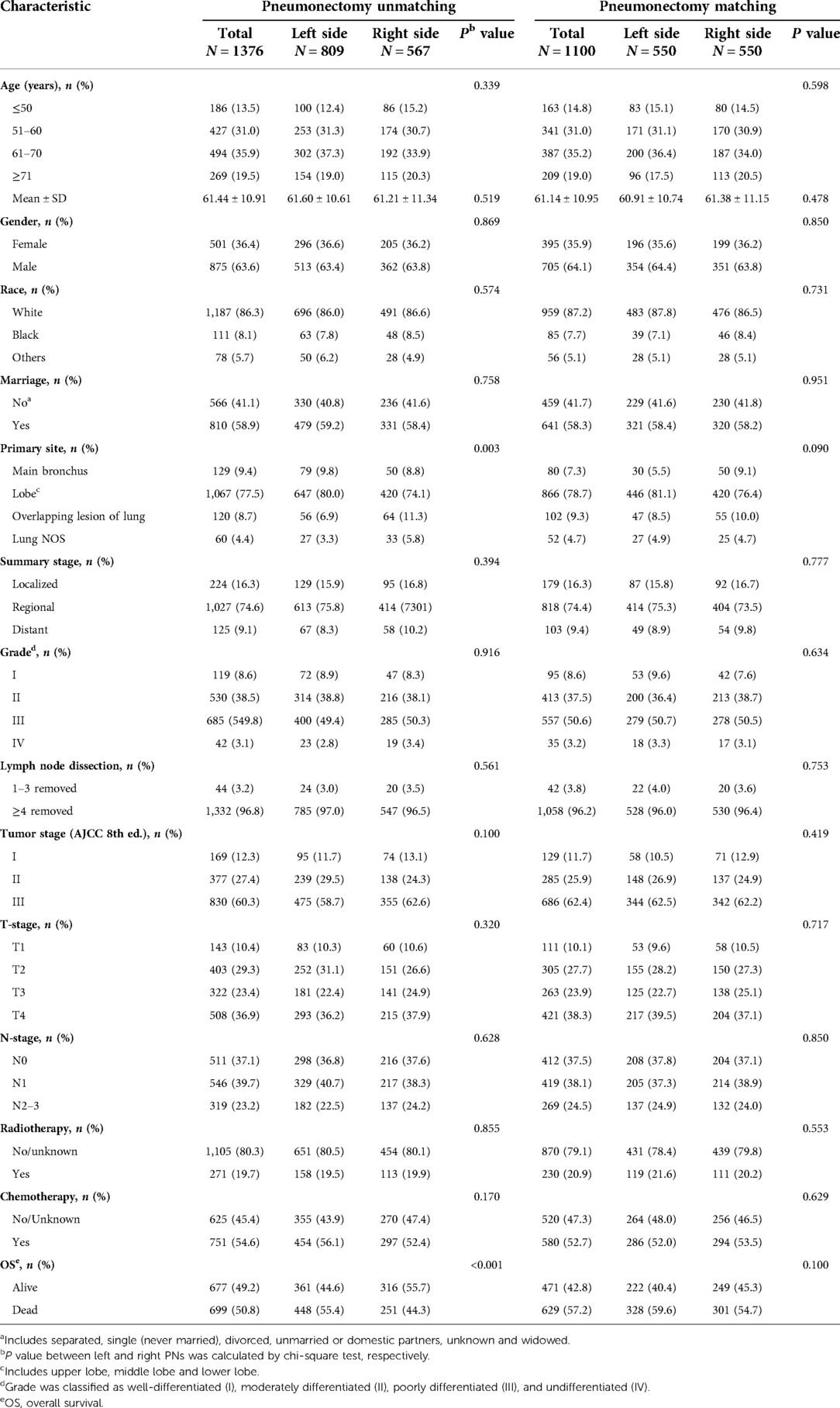
A total of 1,376 patients who underwent a PN between 2004 and 2015 were selected from the SEER database in this study. Of these, 809 (58.79%) had a left PN and 567 (41.21%) had a right PN. The primary tumor site was significantly different between the left- and right-sided PN groups (P < 0.05). The patient characteristics are shown in Table 1.
Survival analysis
The mean follow-up in all 1,376 patients was 31.26 ± 23.04 months (33.65 ± 23.01 months for a left PN and 28.85 ± 22.68 months for a right PN). Median OS was 54 (95% CI 41.62–66.38) months for a left PN vs. 29 (95% CI 22.01–36.00) months for a right PN. One-, three-, and five-year overall survival rates for all patients, left and right PN patients were 74.6%, 42.8%, and 22.3%; 82.0%, 58.4%, and 49.0%; and 67.6%, 46.3%, and 39.3%, respectively. Kaplan–Meier survival analysis suggested that OS was significantly worse for patients who had a right PN (HR: 1.459; 95% CI 1.254–1.697; P < 0.001) compared with a left PN (Figure 2A).
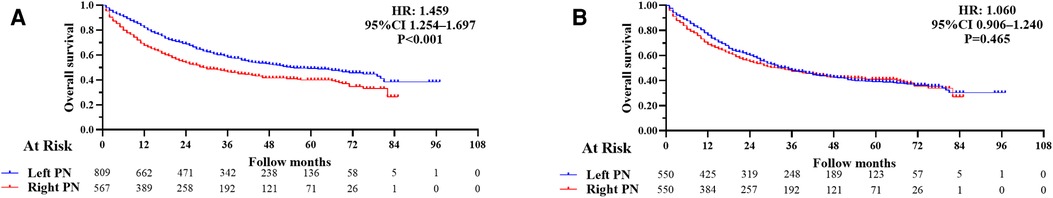
Figure 2. (A) Kaplan–Meier survival curves for overall survival following a left and right pneumonectomy (PN) before propensity score matching (overall survival, hazard ratio (HR): 1.459; 95% CI 1.254–1.697; P < 0.001). (B) Kaplan–Meier survival curves for overall survival in left and right PN after propensity score matching (overall survival, HR: 1.060; 95% CI 0.906–1.240; P = 0.465).
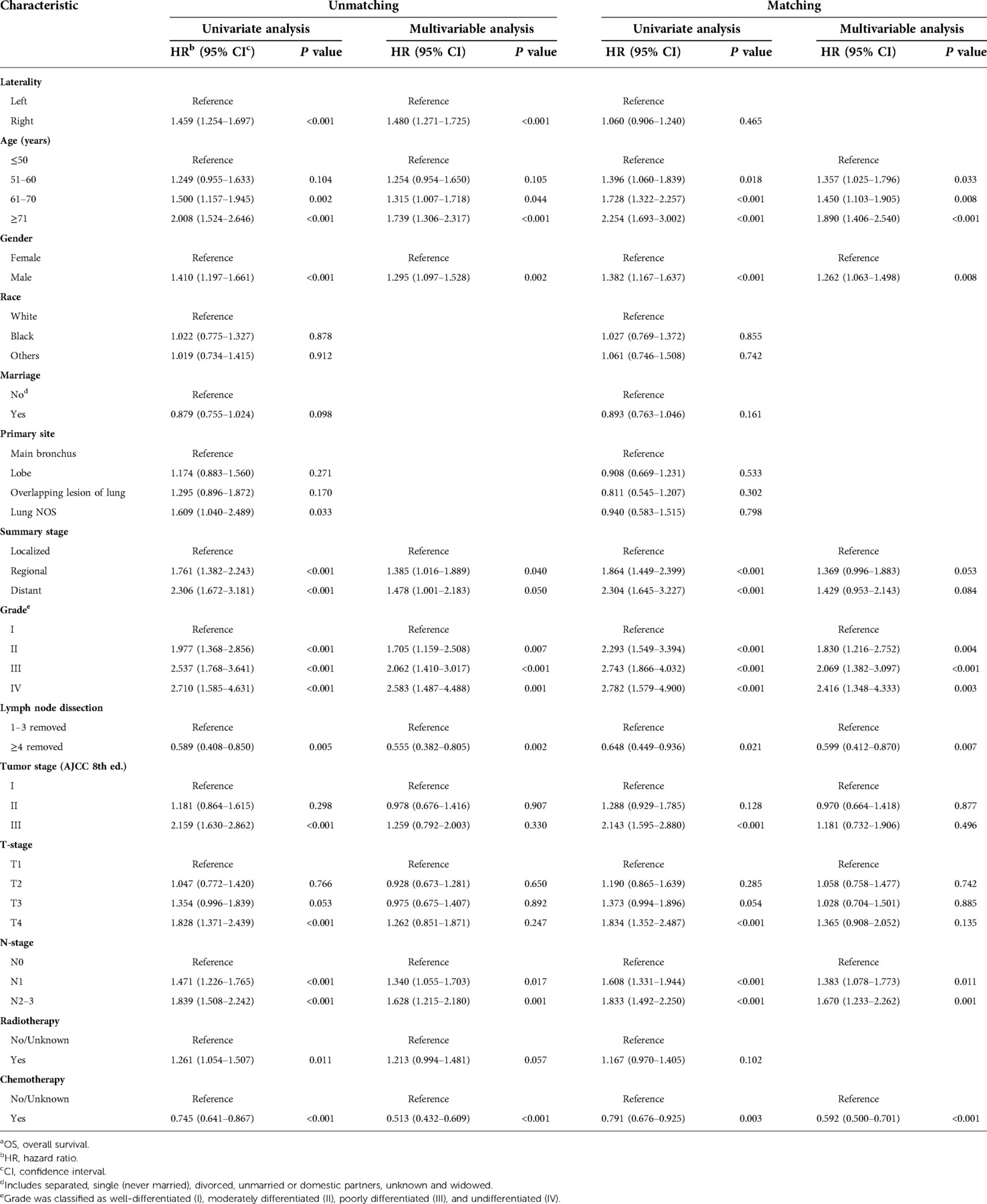
We used univariate analysis to identify possible prognostic factors in PNs for NSCLC and found statistically significant (P < 0.05) correlations between OS and laterality, age, gender, summary stage, grade, tumor stage, T-stage, N-stage, lymph node dissection, radiotherapy, and chemotherapy (Table 2). Chemotherapy was associated with a better prognosis and radiotherapy was associated with a worse prognosis (Figures 3A,C). Race, marriage, and primary site were not significant prognostic factors in our univariate analysis (P > 0.05). Compared with right PNs, the characteristics of those with left PNs were as follows: age ≤50 years (P = 0.004), 61–70 years (P < 0.001); female (P = 0.009), male (P < 0.001); white (P < 0.001); unmarried (P = 0.004), married (P < 0.001); single lobe (P < 0.001) and overlapping lesion (P = 0.003); II (P = 0.002), III (P < 0.001) and IV (P = 0.027); localized (P = 0.018) and regional (P < 0.001); number of regional lymph nodes dissected ≥4 (P < 0.001); tumor stage II (P < 0.001) and III (P = 0.002); T2 (P = 0.005) and T4 (P = 0.001); N0 (P < 0.001) and N1 (P < 0.001); no/unknown radiotherapy (P < 0.001); no/unknown chemotherapy (P < 0.001) and chemotherapy (P = 0.006).

Figure 3. (Top row) Kaplan–Meier survival curves for chemotherapy in patients with non-small cell lung cancer (NSCLC) after a PN. (A) Before propensity score matching (PSM), HR: 0.745; 95% CI 0.641–0.867; P < 0.001; (B) after PSM, HR: 0.791; 95% CI 0.676–0.925; P = 0.003. (Bottom row) Kaplan–Meier survival curves for radiotherapy in NSCLC patients after PN. (C) Before PSM, HR: 1.261; 95% CI 1.054–1.507; P = 0.011; (D) after PSM, HR: 1.167; 95% CI 0.970–1.405; P = 0.102.
Multivariable analysis performed with the Cox regression model included laterality, age, gender, summary stage, grade, lymph node dissection, tumor stage, T-stage, N-stage, radiotherapy, and chemotherapy. The results showed that laterality, age, gender, grade, lymph node dissection, N-stage, radiotherapy, and chemotherapy were independent predictors of survival time in OS (P < 0.05; Table 2), with radiotherapy appearing as a negative prognostic factor with increased risk of death for OS (HR: 1.261; 95% CI 1.054–1.507; P = 0.011) and chemotherapy appearing as an independent predictor of improved for OS (HR: 0.745; 95% CI 0.641–0.867; P < 0.001).
Propensity score matching survival analysis
All the variables were well balanced between the two groups after 1:1 PSM. The propensity scores before matching were 0.399 ± 0.082 for left PNs and 0.430 ± 0.089 for right PNs (P < 0.001). After matching, the propensity scores were 0.419 ± 0.085 for left PNs and 0.424 ± 0.084 for right PNs (P = 0.288). Finally, a total of 1,100 patients (550 with left PNs and 550 with right PNs) were included in our study. We found there were no significant differences in baseline characteristics between the matched groups (Table 1). The mean follow-up time was 31.78 ± 24.20 months (35.10 ± 25.18 months, left PNs and 28.46 ± 22.71 months, right PNs). Median OS was 35 (95% CI 29.44–40.56) months following a left PN, vs. 32 (95% CI 23.98–40.02) months following a right PN. One-, three-,and five-year OS rates for all patients, left and right PN patients were 72.5%, 47.7%, and 39.5%; 76.2%, 48.2%, and 39.1%; 68.8%, 47.4%, and 40.3%, respectively. Kaplan–Meier survival analysis implied between-group OS was not significantly different after matching (HR: 1.060; 95% CI 0.906–1.240; P = 0.465; Figure 2B).
Subgroup analysis in matched groups
Univariate analysis to identify possible prognostic factors after matching found statistically significant correlations between OS and age, gender, summary stage, grade, lymph node dissection, tumor stage, T-stage, N-stage, and chemotherapy (P < 0.05) (Figure 4). However, the results showed that radiotherapy was not an independent prognostic factor (HR: 1.167; 95% CI 0.970–1.405; P = 0.102; Figure 3D). The subsequent multivariable Cox regression model showed that age ≥51 (P ≤ 0.005), male (P = 0.008), higher tumor grade (P ≤ 0.004), <4 lymph node dissections removed, and higher N-stage (P < 0.05) were significant independent negative prognostic factors. The results also revealed that chemotherapy was an independent predictor of improved OS (HR: 0.791; 95% CI 0.676–0.925; P = 0.003; Figure 3B). The forest plot of individual HRs for OS in patients with left PNs vs. right PNs (Figure 5).
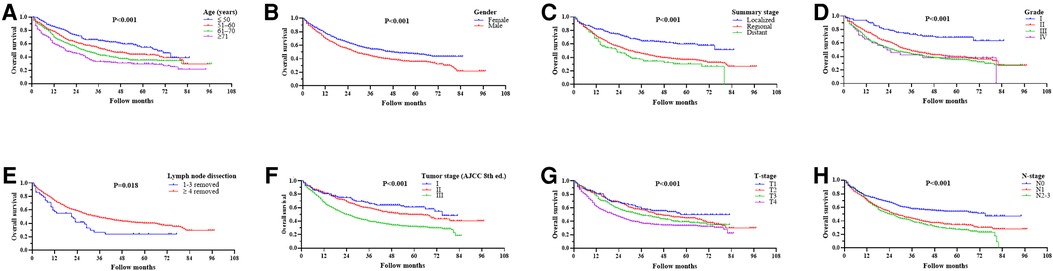
Figure 4. Ording to age (A), gender (B), summary stage (C), grade (D), lymph node dissection (E), tumor stage (F), T-stage (G), and N-stage (H).
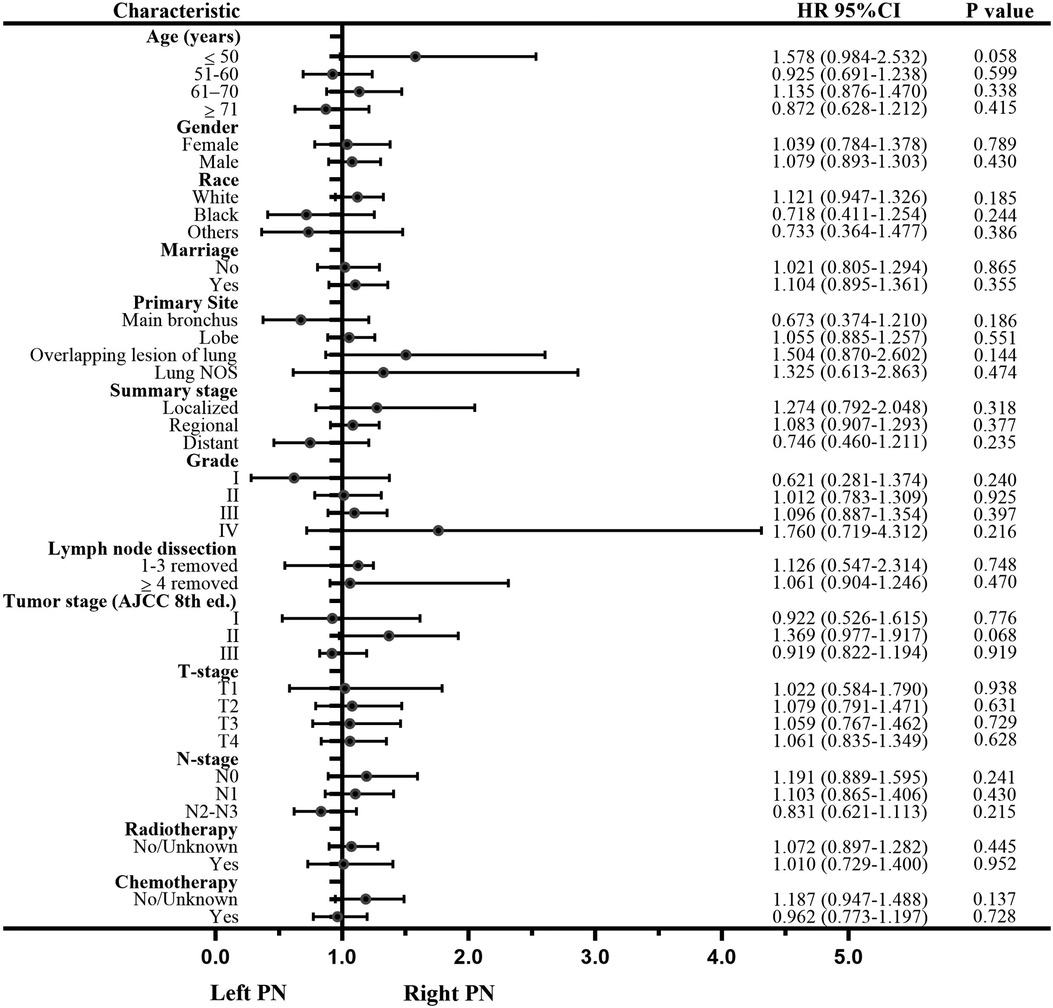
Figure 5. Forest plot of individual hazard ratios for the overall survival in left vs. right pneumonectomies.
Discussion
Anatomic surgical resection is currently the preferred method of treating lung cancer. Central tumors may be amenable to lobectomy or bronchial sleeve lobectomy (12, 13). With the advancement of technology, the wide application of high-resolution spiral computed tomography and the improvement of economic level, more lung cancer patients receive early surgical intervention. Some patients might receive direct radiotherapy and chemotherapy, target therapy or immunotherapy and other medical interventions but no PN treatment. In recent years, the clinical application of pneumonectomy has gradually decreased. But in patients with large tumors, tumor invasion of the left or right main bronchus and tumors crossing lung fissures, anatomic resection cannot be completed and a PN is required to achieve a clinical effect (14, 15). Nonetheless, PNs have relatively high morbidity and mortality (5.0%–10.0%) in the treatment of lung cancer (16). The operation is traumatic and the risk of postoperative complications including cardiac arrhythmias, cardiac failure, pulmonary infection, bronchopleural fistula, and acute respiratory distress syndrome (ARDS) is high (6, 7, 17, 18). Complication rates are generally higher after a right PN and these may affect long-term survival outcomes. However, due to the defects of the SEER database itself, we did not conduct further analysis. Martin et al. (19) had a total mortality rate of 3.8% after a PN (18/470), with an overall incidence of PNs of 38.1% (179/470). Ludwig et al. (20) reported that the 5-year OS rate after a PN was 27%, while Wang et al. (21) reported a post-PN 5-year survival rate of 46.3% in patients with pIII-N2 NSCLC. In this study, 1-, 3-, and 5-year OS rates after left and right PN were 82.0%, 58.4%, and 49.0%; and 67.6%, 46.3%, and 39.3%, respectively. Previously, PSM and OS were worse after right PNs vs. left PNs (HR: 1.459; 95% CI 1.254–1.697; P < 0.001). However, after matching in this study, 1-, 3-, and 5-year OS rates were 76.2%, 48.2%, and 39.1%; 68.8%, 47.4%, and 40.3%, respectively. Between-group OS was not significantly different after matching (HR: 1.060; 95% CI 0.906–1.240; P = 0.465). Yang et al. (22) reported similar findings, but they did not find a significant difference in the 5-year survival rate between left and right PNs before or after matching. We found that laterality did not affect survival. The reasons may be as follows: (1) there is a bias in patient selection, for example, patients with right PN are younger, have better lung function, and have no history of cardiovascular and cerebrovascular diseases; (2) for right PN patients in perioperative management more standardized.
In our study, several factors affecting PNs were analyzed. Generally speaking, the older the age, the greater the perioperative death and the worse the long-term survival prognosis (23). However, Bernet et al. found that age has nothing to do with long-term survival prognosis (24). Therefore, when a PN is required for elderly patients, the choice should be made cautiously in terms of postoperative oncology results and loss of physiological function. In this study, the long-term prognosis of male patients was poor. However, some scholars believe that gender is not a factor affecting prognosis (25).
Usually, the higher the degree of tumor differentiation, the worse the prognosis. Mediastinal lymph node metastasis is an unfavorable factor affecting the prognosis of lung cancer patients (26). We reported that relative N0, N1, and N2–3 had poor prognoses. Therefore, for patients undergoing a PN, strict staging should be performed before surgery, and methods such as PET-CT should be used to assess lymph node metastasis. In addition, the greater the number of lymph nodes dissected, the longer the OS may be; this is representative of the real-world situation (27). For patients with persistent hemoptysis, medical and interventional therapy are ineffective; or when intraoperative lobectomy and sleeve resection fail in patients with preoperative neoadjuvant therapy, PN is also required for lymph node advanced. In some cases, the stage can be lowered with neoadjuvant treatment before surgery (28, 29), but for patients whose tumors cannot be completely removed, PNs should be abandoned. Currently, neoadjuvant therapy is also controversial (30). More clinical trials are needed for further exploration in the future. It is generally believed that OS after a right PN is shorter than for a left PN. This may be related to the development of ARDS and bronchopleural fistulas after right PNs (22). Similar to a previous study, in the field of laterality (31), our study showed that after PSM, there is no difference in OS between the left and right PNs (P = 0.763).
Patients undergoing right PNs lose more lung capacity than those undergoing left PNs because the right lung accounts for 55%–60% of the total lung volume. Therefore, preoperative optimization of cardiopulmonary function before a right PN is particularly important. However, Deslauriers et al. (32) reported that expiratory lung function decreased by approximately 30% following a PN regardless of the operation side, indicating that even though the proportion of lung volume loss is greater after a right PN, long-term postoperative adjustments in pulmonary function may allow patients to adapt and lead near-normal lives. Ilonen et al. (33) also reported that there was no significant difference in pulmonary function after right vs. left PNs. Nonetheless, the relationship between lung function and survival prognosis after a PN remains controversial and there may be a poorer prognosis after a right PN vs. left PN. In this study, we did not compare the difference in lung function in relation to long-term survival after a PN because of the shortcomings of the database itself.
With the continuous advancement of thoracoscopy technology, which is more minimally invasive and leads to a more rapid recovery compared to traditional open surgery, a thoracoscopic lobectomy has better perioperative results and the same survival prognosis, such as fewer postoperative complications, less postoperative pain, aesthetics, and a shorter hospital stay. Whether thoracoscopic PNs will benefit patients remains unclear. Flores et al. (34) and Bendixen et al. (35) showed that there was no statistical difference between the two groups in long-term prognosis. Al Sawalhi et al. (36) reported that compared with open surgery, complications and oncologic outcomes were similar to uniportal video-assisted thoracoscopic surgery (VATS) PN. But shorter length of hospital stays, lower postoperative pain and superior OS with the uniportal VATS PN. Robotic PN has been gradually developed in recent years. The robotic approach offers many technical advantages, such as accurate identification of tissue planes, better optics, natural movement of the operator's hand is used to control the instrument for safe dissection and more ergonomic (37). May be better in long-term overall survival of PN. However, most are case reports (38–40) due to safety, surgical technique, and oncological factors, there are few single-center or multi-center reports of a series of robotic PN. In the future, multi-center randomized controlled trials should be carried out to further prove the advantages and safety of robotic PN.
This study was based on public data from the SEER database, even if we used PSM analysis, there could be bias and errors. Several limitations were found in this study: (1) The study lacked detailed information regarding complications, and adjuvant therapy (chemotherapy, radiotherapy, targeting, immunotherapy, etc.), regardless of whether it was pre- or post-operative. (2) There are data biases because we grouped the no or unknown variables into one group. (3) We used the 8th AJCC staging system, which replaced the 6th and 7th editions, leading to inconsistencies in the data transformation process. (4) The SEER database lacked information on tumor markers, smoking history, imaging, etc. and although our study did not address the impact of these factors on the prognosis in patients with PNs, they may play a significant role.
Conclusion
There was no significant difference in long-term survival between patients with NSCLC undergoing a left vs. right PN. Laterality was not a prognostic factor for survival after a PN. Both neoadjuvant and adjuvant chemotherapy can prolong postoperative survival and either can be recommended if a PN is indicated. Additional long-term survival and outcomes analyses should be conducted on larger numbers of patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LLW drafted the manuscript. The data acquisition was performed by LLW, LHG and GFZ. LLW and ZYW designed the analysis. YR and YYL participated in the conception and design. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Liaoning Province Science and Technology Program Project (2019JH8/10300089) who did not participate in the study design, data collection, analysis, or interpretation and was not involved in writing the manuscript.
Acknowledgments
We acknowledge the SEER*Stat team for providing the patient information required for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Dhanasopon AP, Salazar MC, Hoag JR, Rosen JE, Kim AW, Detterbeck FC, et al. Fate of pneumonectomy patients variably captured by non-small cell lung cancer staging system. Ann Thorac Surg. (2017) 104:1829–36. doi: 10.1016/j.athoracsur.2017.06.073
3. Wang L, Liu D, Lu J, Zhang S, Yang X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer. (2017) 17:75. doi: 10.1186/s12885-017-3069-z
4. Takeda S, Maeda H, Koma M, Matsubara Y, Sawabata N, Inoue M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: Trends over time and 20-year institutional experience. Eur J Cardiothorac Surg. (2006) 29:276–80. doi: 10.1016/j.ejcts.2005.12.017
5. Wu LL, Chen WT, Liu X, Jiang WM, Huang YY, Lin P, et al. A nomogram to predict long-term survival outcomes of patients who undergo pneumonectomy for non-small cell lung cancer with stage I-IIIB. Front Surg. (2021) 8:604880. doi: 10.3389/fsurg.2021.604880
6. Wang C, Wang S, Li Z, He W. A multiple-center nomogram to predict pneumonectomy complication risk for non-small cell lung cancer patients. Ann Surg Oncol. (2022) 29:561–9. doi: 10.1245/s10434-021-10504-1
7. Wang G, Liu L, Zhang J, Li S. The analysis of prognosis factor in patients with non-small cell lung cancer receiving pneumonectomy. J Thorac Dis. (2020) 12:1366–73. doi: 10.21037/jtd.2020.02.33
8. Gu C, Wang R, Pan X, Huang Q, Luo J, Zheng J, et al. Comprehensive study of prognostic risk factors of patients underwent pneumonectomy. J Cancer. (2017) 8:2097–103. doi: 10.7150/jca.19454
9. Jia B, Zheng Q, Li J, Zhao J, Wu M, An T, et al. Evaluation of different treatment strategies between right-sided and left-sided pneumonectomy for stage I–IIIA non-small cell lung cancer patients. J Thorac Dis. (2021) 13:1799–812. doi: 10.21037/jtd-21-264
10. Minervini F, Kocher GJ, Bertoglio P, Kestenholz PB, Gálvez Muñoz C, Patrini D, et al. Pneumonectomy for lung cancer in the elderly: Lessons learned from a multicenter study. J Thorac Dis. (2021) 13:5835–42. doi: 10.21037/jtd-21-869
11. Liang J, Hu Z, Zhan C, Wang Q. Using propensity score matching to balance the baseline characteristics. J Thorac Oncol. (2021) 16:e45–6. doi: 10.1016/j.jtho.2020.11.030
12. Park JS, Yang HC, Kim HK, Kim K, Shim YM, Choi YS, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol. (2010) 5:517–20. doi: 10.1097/JTO.0b013e3181d0a44b
13. Kim YT, Kang CH, Sung SW, Kim JH. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg. (2005) 79:1153–61; discussion 1153–61. doi: 10.1016/j.athoracsur.2004.09.011
14. Wang L, Pei Y, Li S, Zhang S, Yang Y. Left sleeve lobectomy versus left pneumonectomy for the management of patients with non-small cell lung cancer. Thorac Cancer. (2018) 9:348–52. doi: 10.1111/1759-7714.12583
15. Dancewicz M, Kowalewski J, Peplinski J. Factors associated with perioperative complications after pneumonectomy for primary carcinoma of the lung. Interact Cardiovasc Thorac Surg. (2006) 5:97–100. doi: 10.1510/icvts.2005.118125
16. Battoo A, Jahan A, Yang Z, Nwogu CE, Yendamuri SS, Dexter EU, et al. Thoracoscopic pneumonectomy: An 11-year experience. Chest. (2014) 146:1300–9. doi: 10.1378/chest.14-0058
17. Thomas PA, Berbis J, Baste JM, Le Pimpec-Barthes F, Tronc F, Falcoz PE, et al. Pneumonectomy for lung cancer: Contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg. (2015) 149:73–82. doi: 10.1016/j.jtcvs.2014.09.063
18. Steger V, Spengler W, Hetzel J, Veit S, Walker T, Mustafi M, et al. Pneumonectomy: Calculable or non-tolerable risk factor in trimodal therapy for stage III non-small-cell lung cancer? Eur J Cardiothorac Surg. (2012) 41:880–5. doi: 10.1093/ejcts/ezr160
19. Martin J, Ginsberg RJ, Abolhoda A, Bains MS, Downey RJ, Korst RJ, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: The risks of right pneumonectomy. Ann Thorac Surg. (2001) 72:1149–54. doi: 10.1016/S0003-4975(01)02995-2
20. Ludwig C, Stoelben E, Olschewski M, Hasse J. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg. (2005) 79:968–73. doi: 10.1016/j.athoracsur.2004.08.062
21. Wang W, Men Y, Wang J, Zhou Z, Chen D, Xiao Z, et al. Postoperative radiotherapy is effective in improving survival of patients with stage pIII-N2 non-small-cell lung cancer after pneumonectomy. BMC Cancer. (2019) 19:478. doi: 10.1186/s12885-019-5692-3
22. Yang CJ, Shah SA, Lin BK, VanDusen KW, Chan DY, Tan WD, et al. Right-sided versus left-sided pneumonectomy after induction therapy for non-small cell lung cancer. Ann Thorac Surg. (2019) 107:1074–81. doi: 10.1016/j.athoracsur.2018.10.009
23. Bernard A, Deschamps C, Allen MS, Miller DL, Trastek VF, Jenkins GD, et al. Pneumonectomy for malignant disease: Factors affecting early morbidity and mortality. J Thorac Cardiovasc Surg. (2001) 121:1076–82. doi: 10.1067/mtc.2001.114350
24. Bernet F, Brodbeck R, Guenin MO, Schüpfer G, Habicht JM, Stulz PM, et al. Age does not influence early and late tumor-related outcome for bronchogenic carcinoma. Ann Thorac Surg. (2000) 69:913–8. doi: 10.1016/S0003-4975(99)01439-3
25. Martini N, Rusch VW, Bains MS, Kris MG, Downey RJ, Flehinger BJ, et al. Factors influencing ten-year survival in resected stages I to IIIa non-small cell lung cancer. J Thorac Cardiovasc Surg. (1999) 117:32–6; discussion 37–8. doi: 10.1016/S0022-5223(99)70467-8
26. Alexiou C, Beggs D, Rogers ML, Beggs L, Asopa S, Salama FD. Pneumonectomy for non-small cell lung cancer: Predictors of operative mortality and survival. Eur J Cardiothorac Surg. (2001) 20:476–80. doi: 10.1016/S1010-7940(01)00823-5
27. Zhu Z, Mei W, Song Z, Jiao W, Huang Q, Zhu L, et al. A standard for hilar and intrapulmonary lymph node dissection and pathological examination in early non-small cell lung cancer. Transl Lung Cancer Res. (2021) 10:4587–99. doi: 10.21037/tlcr-21-959
28. Brunelli A, Rocco G, Szanto Z, Thomas P, Falcoz PE. Morbidity and mortality of lobectomy or pneumonectomy after neoadjuvant treatment: An analysis from the ESTS database. Eur J Cardiothorac Surg. (2019) 57(4):740–6. doi: 10.1093/ejcts/ezz287
29. Couñago F, Montemuiño S, Martin M, Taboada B, Calvo-Crespo P, Samper-Ots MP, et al. Prognostic factors in neoadjuvant treatment followed by surgery in stage IIIA-N2 non-small cell lung cancer: A multi-institutional study by the oncologic group for the study of lung cancer (spanish radiation oncology society). Clin Transl Oncol. (2019) 21:735–44. doi: 10.1007/s12094-018-1976-3
30. Kim AW, Boffa DJ, Wang Z, Detterbeck FC. An analysis, systematic review, and meta-analysis of the perioperative mortality after neoadjuvant therapy and pneumonectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg. (2012) 143:55–63. doi: 10.1016/j.jtcvs.2011.09.002
31. Jia B, Zheng Q, Qi X, Zhao J, Wu M, An T, et al. Survival comparison of right and left side non-small cell lung cancer in stage I-IIIA patients-A surveillance epidemiology and End results (SEER) analysis. Thorac Cancer. (2019) 10:459–71. doi: 10.1111/1759-7714.12959
32. Deslauriers J, Ugalde P, Miro S, Ferland S, Bergeron S, Lacasse Y, et al. Adjustments in cardiorespiratory function after pneumonectomy: Results of the pneumonectomy project. J Thorac Cardiovasc Surg. (2011) 141:7–15. doi: 10.1016/j.jtcvs.2010.09.010
33. Ilonen IK, Räsänen JV, Sihvo EI, Knuuttila A, Sovijärvi AR, Sintonen H, et al. Pneumonectomy: Post-operative quality of life and lung function. Lung Cancer. (2007) 58:397–402. doi: 10.1016/j.lungcan.2007.07.008
34. Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, Rizk NP, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. (2009) 138:11–8. doi: 10.1016/j.jtcvs.2009.03.030
35. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomized controlled trial. Lancet Oncol. (2016) 17:836–44. doi: 10.1016/S1470-2045(16)00173-X
36. Al Sawalhi S, Gysling S, Cai H, Zhao L, Alhadidi H, Al Rimawi D, et al. Uniportal video-assisted versus open pneumonectomy: A propensity score-matched comparative analysis with short-term outcomes. Gen Thorac Cardiovasc Surg. (2021) 69:1291–302. doi: 10.1007/s11748-021-01626-0
37. Scheinerman JA, Jiang J, Chang SH, Geraci TC, Cerfolio RJ. Extended robotic pulmonary resections. Front Surg. (2021) 8:597416. doi: 10.3389/fsurg.2021.597416
38. Spaggiari L, Galetta D. Pneumonectomy for lung cancer: A further step in minimally invasive surgery. Ann Thorac Surg. (2011) 91:e45–7. doi: 10.1016/j.athoracsur.2010.12.008
39. Rodriguez JR. Total portal robotic pneumonectomy. Gen Thorac Cardiovasc Surg. (2013) 61:538–41. doi: 10.1007/s11748-012-0150-z
Keywords: pneumonectomy, SEER database, non-small cell lung cancer, propensity score matching, forest plot
Citation: Wang L, Ge L, Zhang G, Wang Z, Liu Y and Ren Y (2022) Clinical characteristics and survival outcomes of patients with pneumonectomies: A population-based study. Front. Surg. 9:948026. doi: 10.3389/fsurg.2022.948026
Received: 19 May 2022; Accepted: 27 July 2022;
Published: 9 August 2022.
Edited by:
Alessandro Brunelli, Leeds Teaching Hospitals NHS Trust, United KingdomReviewed by:
Sibu Saha, University of Kentucky, Lexington, United StatesAndrea De Vico, Azienda Usl Teramo, Italy
Mehmet Ali Bedirhan, Yedikule Teaching Hospital, Turkey
Jon Andri Lutz, Fribourg Cantonal Hospital, Switzerland
© 2022 Wang, Ge, Zhang, Wang, Liu and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Ren c3l4a3h3a0AxNjMuY29t
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Linlin Wang
Linlin Wang Lihui Ge2
Lihui Ge2 Ziyi Wang
Ziyi Wang Yi Ren
Yi Ren