
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Surg. , 03 October 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.945126
This article is part of the Research Topic Reviews in Surgical Oncology View all 18 articles
Background: RDW might be an easy and cost-effective pre-operative prognostic factor for cancer patients. The aim of the current study was to analyze whether red blood cell distribution width (RDW) was a prognostic factor for colorectal cancer (CRC) patients who underwent radical surgery.
Methods: We conducted the searching strategy in three databases including the PubMed, Embase and Cochrane Library from the inception to May 07, 2022, to find eligible studies. In this meta-analysis, we focused on the prognosis. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for overall survival (OS), disease-free survival (DFS) and cancer-specific survival (CSS).
Results: A total of seven studies involving 7,541 patients were included in this meta-analysis. After pooling up the HRs, red blood cell distribution width-coefficient of variation (RDW-CV) was not an independent prognostic factor of OS (HR = 1.48, I2 = 90%, 95% CI = 0.93 to 2.36, P = 0.10), however, red blood cell distribution width-standard deviation (RDW-SD) was an independent prognostic factor of OS (HR = 1.99, I2 = 0%, 95% CI = 1.59 to 2.49, P < 0.01). As for DFS, we found that RDW-CV (HR = 1.51, I2 = 83%, 95% CI = 0.94 to 2.43, P = 0.09 < 0.10) and RDW-SD (HR = 1.77, I2 = 56%, 95% CI = 0.91 to 3.43, P = 0.09 < 0.10) were both the independent prognostic factors. In terms of CSS, we found that RDW-CV was not an independent prognostic factor (HR = 1.23, I2 = 95%, 95% CI = 0.72 to 2.10, P = 0.46).
Conclusion: RDW-SD was an independent prognostic factor of OS and DFS, and RDW-CV was an independent prognostic factor of DFS.
The incidence of colorectal cancer (CRC) was 38.7 per 100,000 and the mortality rate was 13.9 per 100,000% (1). Among them, CRC was the third most common cancer in males and the second in females (2). The treatments of CRC include surgery, chemotherapy, radiotherapy, surgery, targeted therapy and immunotherapy (3–8). Nowadays, radical surgery is the cornerstone treatment of CRC (9, 10), which not only can treat cancer, but also help in the improvement of some comorbidities (11, 12).
Red blood cell distribution width (RDW) is a hematological parameter which can be divided into two types as follows: RDW standard deviation (RDW-SD) and RDW coefficient of variation (RDW-CV), whose unit was FL and %, respectively (13). RDW can reflect the heterogeneity of red blood cell size (14), and it has been applied to predict anemia, chronic inflammation and cardiovascular disease (15–18). Recent studies reported that RDW could predict the prognosis of patients with esophageal cancer, gastric cancer and liver cancer (19–22).
Some studies reported the relationship between RDW and CRC patients as well, however, whether RDW could affect the prognosis of CRC was controversial (13–26). Furthermore, the prognostic value of RDW-SD and RDW-CV might be inconsistent. Thus, it is necessary to analyze the exact impact of RDW (RDW-SD and RDW-CV) on CRC.
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (27).
Two authors conducted the searching strategy in three databases including the PubMed, Embase and Cochrane Library independently. The searching date was May 07, 2022. As for RDW, the searching strategy included: “red blood cell distribution width” OR “red cell distribution width” OR “RDW”; As for CRC, the searching strategy included: “colorectal cancer” OR “colon cancer” OR “rectal cancer” OR “colorectal neoplasm” OR “colon neoplasm” OR “rectal neoplasm” OR “colorectal tumor” OR “colon tumor” OR “rectal tumor”. The language was limited to English and the searching scope was limited to titles and abstracts.
The inclusion criteria were as follows: 1, CRC patients who underwent primary and radical surgery; 2, Pre-operative RDW (RDW-CV or RDW-SD) was tested; and 3, Overall survival (OS), disease-free survival (DFS) or cancer-specific survival (CSS) was reported. The exclusion criteria were as follows: 1, The type of article was letters, case reports, comments, reviews, or conference; 2, Repeated or overlapped data; and 3, Insufficient data reporting the prognosis including OS, DFS or CSS.
Two authors conducted the study selection independently. Firstly, the titles and abstracts were looked through by authors to find potentially relevant studies; Secondly, the full texts were read and discussed by the two authors based on the inclusion and exclusion criteria. If there was a disagreement, another author was due to make a final judgment.
The data were extracted by two authors. The extracted article information included the first author, publishing country and publishing year. The extracted patients' data included RDW type, sample size, cut-off value of RDW, OS, DFS and CSS.
As for clinical-pathological characteristics, two authors collected the data independently. The third author was responsible for checking the information to ensure their accuracy and completeness. Only variables which were reported by more than two studies were allowed. The baseline characteristics included age, gender, carcinoembryonic antigen (CEA), tumor location, histological differentiation, Tumor Node Metastasis (TNM) stage, vascular invasion, and adjuvant chemotherapy.
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included studies (28). The score equaled 9 points represented high quality, the score equaled 7 or 8 points represented medium-quality and the score which was less than 7 points represented low quality.
In this meta-analysis, we focused on the prognosis. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for OS, DFS and CSS. The I2 value and the results of the chi-squared test were used to assess the statistical heterogeneity (29, 30). High heterogeneity was considered when I2>50%; in such cases, the random effects model was used, and P < 0.1 was considered statistically significant. The fixed effects model was used when I2≤50%, and P < 0.05 was considered statistically significant. This meta-analysis was performed with RevMan 5.3 (The Cochrane Collaboration, London, United Kingdom).
A total of 76 studies were found in the databases, including 25 studies in the PubMed, 50 studies in the Embase and 1 study in the Cochrane Library. Finally, seven studies (23–26, 31–33) were included for final analysis. The flow chart of the study selection was shown in Figure 1.
Seven studies included 7,541 patients were included in this meta-analysis. The publication year ranged from 2018 to 2022. Two studies were from China, two studies were from Japan, one study was from Italy, one study was from United Kingdom and one study was from Switzerland. The study date was from 2001 to 2020. Three studies reported RDW-SD and five studies reported RDW-CV. The cut-off values and NOS were shown in Table 1.
After pooling up the odds ratio and 95% CI, there were more older patients, higher CEA level, and more TNM stage II in the high RDW group than in the low RDW group. Other characteristics including gender, tumor location, histological differentiation, TNM stage III, vascular invasion, and adjuvant chemotherapy were not significantly different between the two groups (Table 2).
Four studies reported OS of RDW-CV, after pooling up the HRs, RDW-CV was not an independent prognostic factor of OS (HR = 1.48, I2 = 90%, 95% CI = 0.93 to 2.36, P = 0.10) (Figure 2a).
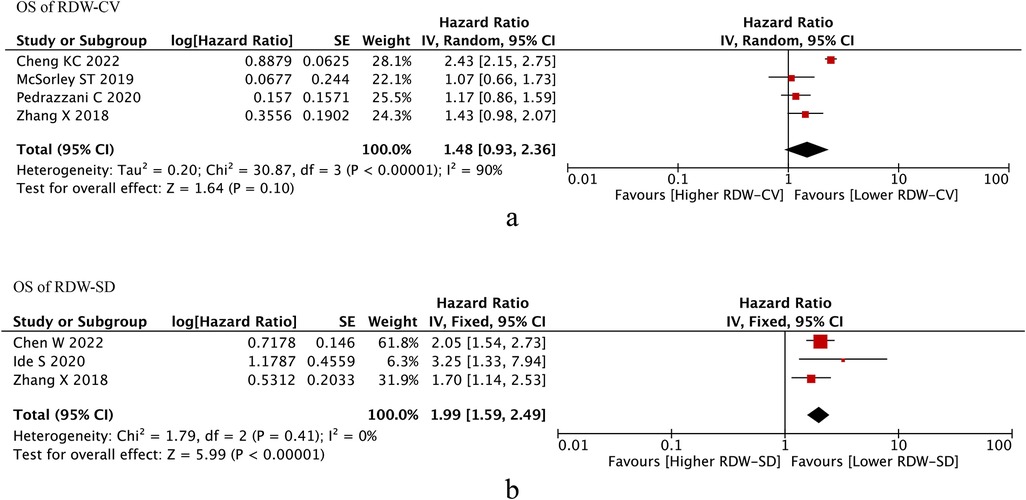
Figure 2. Os of RDW. (A) OS of RDW-CV; (B) OS od RDW-SD. Abbreviations: OS, overall survival; RDW, red blood cell distribution width.
Three studies reported OS of RDW-SD, after pooling up the HRs, RDW-CV was an independent prognostic factor of OS (HR = 1.99, I2 = 0%, 95% CI = 1.59 to 2.49, P < 0.01) (Figure 2b).
Then, we conducted meta-analysis of RDW (RDW-CV/RDW-SD) on DFS. We found that RDW-CV (HR = 1.51, I2 = 83%, 95% CI = 0.94 to 2.43, P = 0.09 < 0.10) and RDW-SD (HR = 1.77, I2 = 56%, 95% CI = 0.91 to 3.43, P = 0.09 < 0.10) were both independent prognostic factors of DFS (Figures 3A,B).

Figure 3. DFS of RDW. (A) DFS of RDW-CV; (B) DFS od RDW-SD. Abbreviations: DFS, disease-free survival; RDW, red blood cell distribution width.
Four studies reported RDW-CV on the prognostic roles on CSS, and we found that RDW-CV was not an independent prognostic factor (HR = 1.23, I2 = 95%, 95% CI = 0.72 to 2.10, P = 0.46) (Figure 4). However, no information was found about RDW-SD on the prognostic roles on CSS.
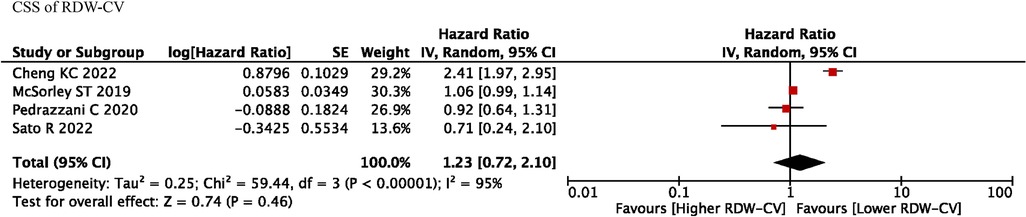
Figure 4. CSS of RDW-CV. Abbreviations: CSS, cancer-specific survival; RDW, red blood cell distribution width.
Repeated meta-analysis was performed by excluding one study at a time, and the exclusion of any one study did not significantly alter the results.
A total of seven studies involving 7541 patients were included in this meta-analysis. After pooling up the HRs, RDW-CV was not an independent prognostic factor of OS, however, RDW-SD was an independent prognostic factor of OS. As for DFS, we found that RDW-CV and RDW-SD were both independent prognostic factors. In terms of CSS, we found that RDW-CV was not an independent prognostic factor. As for clinical characteristics, the high RDW group had more older patients, higher CEA level, and more TNM stage II than the low RDW group.
RDW can reflect the heterogeneity of red blood cell size (14), and the primary role of RDW is to diagnose anemia (13). The increase of RDW could accompanied by other cancer prognostic risk factors including age, later TNM stage and higher tumor markers level (34, 35). Furthermore, RDW is also associated with various diseases such as heart disease, lung disease, and even trauma (14, 36). In addition, RDW is also considered as an indicator for some inflammatory diseases including pancreatitis and hepatitis (35, 36). However, the mechanism has not been clearly demonstrated.
Previous studies had reported the relationship between RDW and the prognosis of CRC (23–26, 31–33). Zhang X et al. (23) reported that elevated RDW could be an independent factor for non-metastatic rectal cancer; Cheng KC et al. (37) analyzed 5,315 CRC patients and did propensity score matching analysis, they found that RDW was a predictor of OS, DFS and CSS. However, Pedrazzani C et al. (25) reported that RDW did not seem to influence OS or CSS, independently. Moreover, McSorley ST et al. (26) reported the same results that RDW was not a predictor of prognosis. Therefore, it is necessary to analyze the exact impact of RDW on CRC (38).
There were many factors which could affect the prognosis of CRC, including tumor stage, tumor size, age, body mass index (BMI), type 2 diabetes mellitus and so on (39–44). Prognostic indicators related to blood examination included lymphocyte count ratio (NLR), platelet count and lymphocyte count ratio (PLR), etc (31, 45, 46). The main reason that NLR and PLR could affect the prognosis was that they were important markers of systemic inflammation (23,24). Furthermore, PLR and NLR levels increased the body's inflammatory response, promoted tissue infiltration and angiogenesis (47). Similarly, in our meta-analysis, RDW could also affect the prognosis of CRC, the mechanism might be that RDW was another important marker of systemic inflammation as well.
Besides the systemic inflammation mechanism, RDW was thought to reflect oxidative stress, malnutrition, dyslipidemia, hypertension, erythrocyte fragmentation and erythropoietin alterations (48). Furthermore, RDW correlated with plasma markers of inflammation, such as high-sensitivity C-reactive protein (hs-CRP) values and erythrocyte sedimentation rate (ESR) (49). RDW was shown to reflect increased levels of circulating cytokines, including interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (50). Thus, these findings suggested that increased RDW might reflect inflammatory responses, malnutrition status and elevated oxidative stress, leading to the hypothesis that RDW was associated with poorer prognosis.
To our knowledge, previous studies had controversy about the effect of RDW on the prognosis of CRC, and this is the first study pooling up all the data to identify the accurate prognostic roles of RDW on CRC patients. Some limitations existed in this study. First, we included seven studies whose sample size was relatively small; Second, the cut-off of RDW-CV and RDW-SD was inconstant, which might cause inaccuracy; Third, small number of studies reporting OS, DFS and CSS, therefore, heterogeneity occurred, random-effects test was adopted.
In conclusion, RDW-SD was an independent prognostic factor of OS and DFS, and RDW-CV was an independent prognostic factor of DFS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Data extraction, Z-LW, D-CX and XZ; quality assessments, XZ; data analysis, XZ and Z-LW; writing-origin draft, Z-LW and D-CX; writing-review and editing, XZ, Z-LW and D-CX. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. (2020) 70(3):145–64. doi: 10.3322/caac.21601
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. (2019) 14(2):89–103. doi: 10.5114/pg.2018.81072
3. Peng D, Cheng YX, Cheng Y. Improved overall survival of colorectal cancer under multidisciplinary team: a meta-analysis. Biomed Res Int. (2021) 2021:5541613. doi: 10.1155/2021/5541613
4. Cheng YX, Tao W, Zhang H, Peng D, Wei ZQ. Does liver cirrhosis affect the surgical outcome of primary colorectal cancer surgery? A meta-analysis. World J Surg Oncol. (2021) 19(1):167. doi: 10.1186/s12957-021-02267-6
5. Ba L, Wang Q, Wang H, Zhu L, Zhang T, Ren J, et al. Survival analysis and prognostic factors of palliative radiotherapy in patients with metastatic colorectal cancer: a propensity score analysis. J Gastrointest Oncol. (2021) 12(5):2211–22. doi: 10.21037/jgo-21-540
6. Lin Y, Kong DX, Zhang YN. Does the microbiota composition influence the efficacy of colorectal cancer immunotherapy? Front Oncol. (2022) 12:852194. doi: 10.3389/fonc.2022.852194
7. Janani B, Vijayakumar M, Priya K, Kim JH, Prabakaran DS, Shahid M, et al. EGFR-Based Targeted therapy for colorectal cancer-promises and challenges. Vaccines (Basel). (2022) 10(4):499. doi: 10.3390/vaccines10040499
8. Liu XY, Yuan C, Kang B, Cheng YX, Tao W, Zhang B, et al. Predictors associated with planned and unplanned admission to intensive care units after colorectal cancer surgery: a retrospective study. Support Care Cancer. (2022) 30(6):5099–105. doi: 10.1007/s00520-022-06939-1
9. Ji X, Zhao Y, Zhu X, Shen Z, Li A, Chen C, et al. Outcomes of stereotactic body radiotherapy for metastatic colorectal cancer with oligometastases, oligoprogression, or local control of dominant tumors. Front Oncol. (2021) 10:595781. doi: 10.3389/fonc.2020.595781
10. Tharin Z, Blanc J, Alaoui IC, Bertaut A, Ghiringhelli F. Influence of first line chemotherapy strategy depending on primary tumor location in metastatic colorectal cancer. J Gastrointest Oncol. (2021) 12(4):1509–17. doi: 10.21037/jgo-20-593
11. Liu XY, Kang B, Cheng YX, Yuan C, Tao W, Zhang B, et al. Higher body mass index was associated with better prognosis in diabetic patients with stage II colorectal cancer. BMC Cancer. (2022) 22(1):596. doi: 10.1186/s12885-022-09691-1
12. Cheng YX, Tao W, Liu XY, Yuan C, Zhang B, Wei ZQ, et al. Hypertension remission after colorectal cancer surgery: a single-center retrospective study. Nutr Cancer. (2022) 74(8):2789–95. doi: 10.1080/01635581.2021.2025256
13. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52(2):86–105. doi: 10.3109/10408363.2014.992064
14. Miyamoto K, Inai K, Takeuchi D, Shinohara T, Nakanishi T. Relationships among red cell distribution width, anemia, and interleukin-6 in adult congenital heart disease. Circ J. (2015) 79(5):1100–6. doi: 10.1253/circj.CJ-14-1296
15. Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. (2017) 2017:7089493. doi: 10.1155/2017/7089493
16. Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. (2009) 169(5):515–23. doi: 10.1001/archinternmed.2009.11
17. Lee WS, Kim TY. Relation between red blood cell distribution width and inflammatory biomarkers in rheumatoid arthritis. Arch Pathol Lab Med. (2010) 134(4):505–6. doi: 10.5858/134.4.505.c
18. Song CS, Park DI, Yoon MY, Seok HS, Park JH, Kim HJ, et al. Association between red cell distribution width and disease activity in patients with inflammatory bowel disease. Dig Dis Sci. (2012) 57(4):1033–8. doi: 10.1007/s10620-011-1978-2
19. Chen GP, Huang Y, Yang X, Feng JF. A nomogram to predict prognostic value of red cell distribution width in patients with esophageal cancer. Mediators Inflamm. (2015) 2015:854670. doi: 10.1155/2015/854670
20. Saito H, Shimizu S, Shishido Y, Miyatani K, Matsunaga T, Fujiwara Y. Prognostic significance of the combination of preoperative red cell distribution width and platelet distribution width in patients with gastric cancer. BMC Cancer. (2021) 21(1):1317. doi: 10.1186/s12885-021-09043-5
21. Fu L, Li Q, Fan Q. Combination of preoperative red cell distribution width and neutrophil to lymphocyte ratio as a prognostic marker for gastric cancer patients. J Gastrointest Oncol. (2021) 12(3):1049–57. doi: 10.21037/jgo-21-271
22. Jing JS, Fu XL, Zhao W, Kong LB. Red cell distribution width as a prognostic factor in patients with hepatocellular carcinoma. Clin Lab. (2020) 66(7). doi: 10.7754/Clin.Lab.2019.191027
23. Zhang X, Wu Q, Hu T, Gu C, Bi L, Wang Z. Elevated red blood cell distribution width contributes to poor prognosis in patients undergoing resection for nonmetastatic rectal cancer. Med (Baltimore). (2018) 97(3):e9641. doi: 10.1097/MD.0000000000009641
24. Ide S, Toiyama Y, Okugawa Y, Omura Y, Kitajima T, Fujikawa H, et al. Clinical significance of an increased red blood cell distribution width in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Surg Today. (2020) 50(6):551–9. doi: 10.1007/s00595-019-01933-3
25. Pedrazzani C, Tripepi M, Turri G, Fernandes E, Scotton G, Conci S, et al. Prognostic value of red cell distribution width (RDW) in colorectal cancer. Results from a single-center cohort on 591 patients. Sci Rep. (2020) 10(1):1072. doi: 10.1038/s41598-020-57721-4
26. McSorley ST, Tham A, Steele CW, Dolan RD, Roxburgh CS, Horgan PG, et al. Quantitative data on red cell measures of iron status and their relation to the magnitude of the systemic inflammatory response and survival in patients with colorectal cancer. Eur J Surg Oncol. (2019) 45(7):1205–11. doi: 10.1016/j.ejso.2019.02.027
27. Moher D, Liberati A, Tetzlaff J. Altman DG; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
28. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–e5. doi: 10.1007/s10654-010-9491-z
29. Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. (2008) 14(5):951–7. doi: 10.1111/j.1365-2753.2008.00986.x
30. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
31. Chen W, Xin S, Xu B. Value research of NLR, PLR, and RDW in prognostic assessment of patients with colorectal cancer. J Healthc Eng. (2022) 2022:7971415. doi: 10.1155/2022/7971415
32. Sato R, Oikawa M, Kakita T, Okada T, Abe T, Yazawa T, et al. Prognostic significance of the mean corpuscular volume (MCV) and red cell distribution width (RDW) in obstructive colorectal cancer patients with a stent inserted as a bridge to curative surgery. Surg Today. (2022). doi: 10.1007/s00595-022-02504-9
33. Cheng KC, Lin YM, Liu CC, Wu KL, Lee KC. High red cell distribution width is associated with worse prognosis in early colorectal cancer after curative resection: a propensity-matched analysis. Cancers (Basel). (2022) 14(4):945. doi: 10.3390/cancers14040945
34. Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. (2010) 65(3):258–65. doi: 10.1093/gerona/glp163
35. Patel R, English L, Liu WK, Tree AC, Ayres B, Watkin N, et al. Red cell differential width (RDW) as a predictor of survival outcomes with palliative and adjuvant chemotherapy for metastatic penile cancer. Int Urol Nephrol. (2020) 52(12):2301–6. doi: 10.1007/s11255-020-02565-0
36. Liu Q, Dang AM, Chen BW, Lv NQ, Wang X, Zheng DY. The association of red blood cell distribution width with anemia and inflammation in patients with takayasu arteritis. Clin Chim Acta. (2015) 438:205–9. doi: 10.1016/j.cca.2014.08.025
37. Fu Y, Mao Y, Chen S, Yang A, Zhang Q. A novel inflammation- and nutrition-based prognostic system for patients with laryngeal squamous cell carcinoma: combination of red blood cell distribution width and body mass Index (COR-BMI). PLoS One. (2016) 11(9):e0163282. doi: 10.1371/journal.pone.0163282
38. Li Z, Hong N, Robertson M, Wang C, Jiang G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep. (2017) 7:43001. doi: 10.1038/srep43001
39. Cheng Y, Cheng YX, Liu XY, Kang B, Tao W, Peng D. The effect of type 2 diabetes Mellitus on the short-term outcomes and prognosis of stage I-III colorectal cancer: a propensity score matching analysis. Cancer Manag Res. (2022) 14:205–14. doi: 10.2147/CMAR.S347242
40. Dell'Aquila E, Rossini D, Galletti A, Stellato M, Boccaccino A, Conca V, et al. Prognostic and predictive role of body mass Index (BMI) in metastatic colorectal cancer (mCRC): a pooled analisys of tribe and tribe-2 studies by GONO. Clin Colorectal Cancer. (2022) 21(3):220–28. doi: 10.1016/j.clcc.2022.02.003
41. Lv MY, Wang W, Zhong ME, Cai D, Fan D, Li CH, et al. DNA Repair-Related gene signature in predicting prognosis of colorectal cancer patients. Front Genet. (2022) 13:872238. doi: 10.3389/fgene.2022.872238
42. Alese OB, Zhou W, Jiang R, Zakka K, Huang Z, Okoli C, et al. Predictive and prognostic effects of primary tumor size on colorectal cancer survival. Front Oncol. (2021) 11:728076. doi: 10.3389/fonc.2021.728076
43. Cheng YX, Liu XY, Kang B, Tao W, Wei ZQ, Peng D. Comparison of surgical and oncologic outcomes in very elderly patients (≥ 80 years old) and elderly (65–79 years old) colorectal cancer patients: a propensity score matching. BMC Gastroenterol. (2022) 22(1):205. doi: 10.1186/s12876-022-02277-y
44. Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and northern America. Cancer Lett. (2021) 522:255–68. doi: 10.1016/j.canlet.2021.09.034
45. Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: a systematic review and meta-analysis. Cancer Med. (2021) 10(17):5983–97. doi: 10.1002/cam4.4143
46. Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. (2014) 31(12):305. doi: 10.1007/s12032-014-0305-0
47. Varkaris A, Katsiampoura A, Davis JS, Shah N, Lam M, Frias RL, et al. Circulating inflammation signature predicts overall survival and relapse-free survival in metastatic colorectal cancer. Br J Cancer. (2019) 120(3):340–5. doi: 10.1038/s41416-018-0360-y
48. Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. (2011) 39(8):1913–21. doi: 10.1097/CCM.0b013e31821b85c6
49. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. (2009) 133(4):628–32. doi: 10.5858/133.4.628
50. Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. (2009) 158(4):659–66. doi: 10.1016/j.ahj.2009.07.024
Keywords: red blood cell distribution width, colorectal cancer, meta-analysis, surgery, survival
Citation: Wen Z-L, Zhou X and Xiao D-C (2022) Is red blood cell distribution width a prognostic factor for colorectal cancer? A meta-analysis. Front. Surg. 9:945126. doi: 10.3389/fsurg.2022.945126
Received: 16 May 2022; Accepted: 16 September 2022;
Published: 3 October 2022.
Edited by:
Veronica De Simone, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Vincenzo Lizzi, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, Italy© 2022 Wen, Zhou and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Da-Chun Xiao ZGFjaHVueGlhbzAwMTFAMTYzLmNvbQ==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.