
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg., 09 September 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.944980
Background: Primary abdominal “egg-shelled” lesions with positive anti-echinococcus IgG antibody were misdiagnosed as echinococcosis.
Case presentation: Herein we report two cases with primary abdominal “egg-shelled” lesions were misdiagnosed as echinococcosis. Case 1: A 44-year-old woman presented to our department with a history of slight abdominal pain for 4 months. After admission, the laboratory tests indicated a positive anti-echinococcus IgG antibody status. The contrast-enhanced CT scan showed a 12 × 12 cm “me contrast-mass located in the hepatorenal area. The patient had the entire mass and the right adrenal gland resected. This patient recovered smoothly and was discharged uneventfully 20 days after the operation. The pathologic diagnoses was adrenal lymphangioma. Case 2: A 55-year-old woman was admitted with a history of an abdominal mass for over 10 years. After admission, her anti-echinococcus IgG antibody was positive. The contrast-enhanced CT scan revealed a heterogeneous, solid mass measuring 10 × 9 × 8 cm in the right hepatic lobe. A laparoscopic exploration was performed, and the surgery revealed that the mass arose from the retroperitoneal tissue rather than the liver. Finally, the pathologic diagnoses were paraganglioma with necrosis and cystic changes.
Conclusion: Enhanced CT scan and contrast-enhanced ultrasound scans are important for distinguishing echinococcosis disease from the other “egg-shelled” lesions. Surgical resection is the main treatment method for this disease. Minimally invasive surgery is recommended but should be performed by experienced surgical teams. Immunohistochemical examination is important for the pathologic diagnosis.
The “egg-shelled” lesion is a mass with an eggshell-like calcified wall, characterized by homogeneous intracapsular necrotic material, that will not or will only be slightly enhanced on contrast-enhanced computed tomography (CT) scans. Many primary lesions could present as egg-shelled lesions with calcification including retroperitoneal pseudocyst, adrenal tuberculosis, pelvic schwannoma, and acute calcific retropharyngeal tendinitis (1–5). As a heterogeneous primary disease, an abdominal “egg-shelled” mass is easily misdiagnosed as echinococcosis based on the similar imaging findings (6, 7).
Echinococcosis is an aggressive and potentially lethal zoonotic infection that often occurs in the hepatic lobe(s) (8). Based on the different diseases associated with Echinococcus, human echinococcosis can be divided into two types: cystic echinococcosis (CE, caused by Echinococcus granulosus) and alveolar echinococcosis (AE, caused by Echinococcus multilocularis) (9). However, egg-shelled calcifications may be present in the imaging of CE or AE cases and many other diseases. The geographical history and positive anti-echinococcus IgG antibody status also can mislead the diagnosis (10).
Herein, we present two cases that had “egg-shelled” lesions that were misdiagnosed as hepatic echinococcosis. As abdominal “egg-shelled” masses are rare and can be present in different diseases, including echinococcosis, the purpose of this paper is to present our experience with treatment and to reduce the misdiagnosis rate. This work was supported by the Ethics Committee of Sichuan Provincial People's Hospital.
A 44-year-old woman presented to our department with a history of slight abdominal pain for 4 months. She had a long history of living in an area with echinococcosis, and there was no significant familial medical history for this patient. The medical examination found a mass located in the middle right abdominal region with tenderness and unclear boundaries.
After admission, the laboratory tests indicated normal white blood cell (WBC) [4.74 × 109/L, (references 3.50–9.5 × 109/L)] and absolute eosinophil counts [0.45 × 109/L, (references 0.02–0.52 × 109/L)], the serum tumor markers were normal and a positive anti-echinococcus IgG antibody status. The contrast-enhanced CT scan showed a 12 × 12 cm “egg-shelled” mass located in the hepatorenal area (Figure 1A). This mass had a well-distributed calcified wall wrapping around the homogeneous content with point calcifications. The content showed mild enhancements during arterial phases (Figure 1B). The primary diagnosis was abdominal AE disease. This patient underwent laparotomy surgery for a mass resection. During surgery, we found a large calcified mass located near the upper right kidney arising from the right adrenal gland adjacent to the lower right hepatic lobes. The patient had the entire mass and the right adrenal gland resected. This patient recovered smoothly and was discharged uneventfully 20 days after the operation.
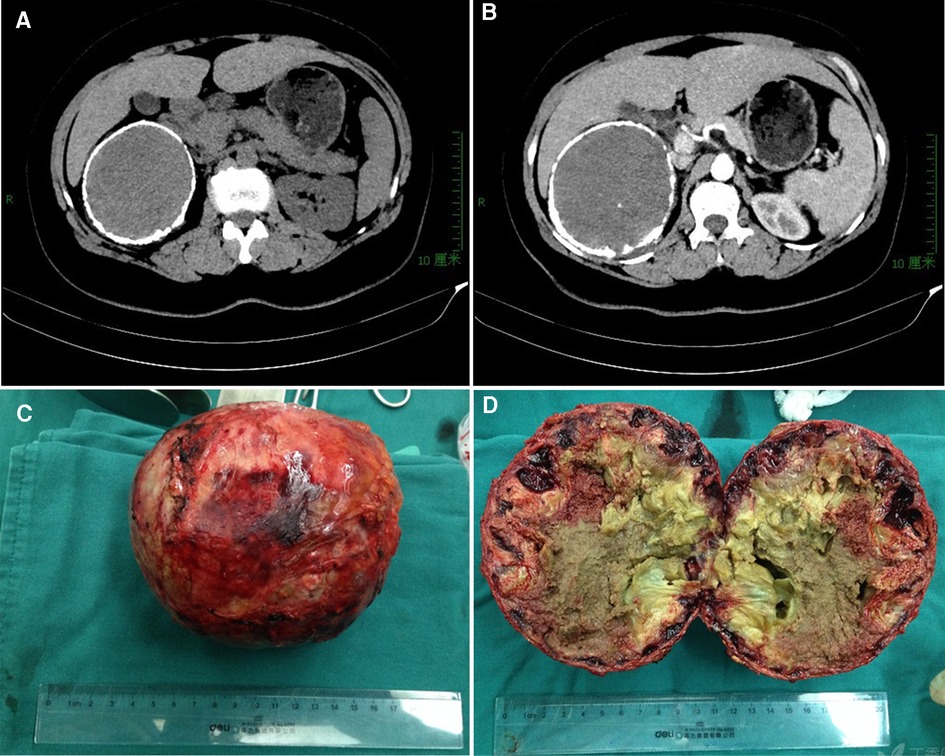
Figure 1. Ct imaging and specimen for case 1. (A) an “egg-shell” mass locating in the right subhepatic area; (B) mild enhanced on contrast-enhanced CT scans for the mass in the artery phase; (C) the resected specimen with a calcific shell; (D) homogeneous necrotic content with point calcifications.
The resected specimen had a calculated shell (Figure 1C), which wrapped around the homogeneous necrotic content with point calcifications (Figure 1D). The pathological examination revealed calcification of the surface, necrosis and sclerotization of the contents. The immunohistochemical examination revealed positive expressions of CD-34, CD-31 and D2–40 but no expression of CK. These findings supported a pathologic diagnosis of adrenal lymphangioma with hemorrhage and hematoma formation. There were no significant findings in the follow-up 6 years after surgery for this patient.
A 55-year-old woman was admitted with a history of an abdominal mass for over 10 years. The patient had unremarkable medical and family histories, and her physical examination was normal. After admission, the laboratory evaluations indicated normal WBC (7.25 × 109/L) and absolute eosinophil counts (0.32 × 109/L), and the other significant index were negative, except her serum anti-echinococcus IgG antibody was positive. The contrast-enhanced CT scan revealed a heterogeneous, solid mass measuring 10 × 9 × 8 cm in the right hepatic lobe (Figure 2A). The mass had a clearly calcified edge wrapping around the homogeneous content with multipoint calcifications and calcified separations (Figure 2B). Furthermore, three-dimensional CT reconstructed imaging revealed that the right hepatic vein and hepatic pedicle were involved from the dorsal to the ventral part but were not invaded into or encapsulated (Figures 2C,D). The primary diagnosis was hepatic CE (CE-5 type).
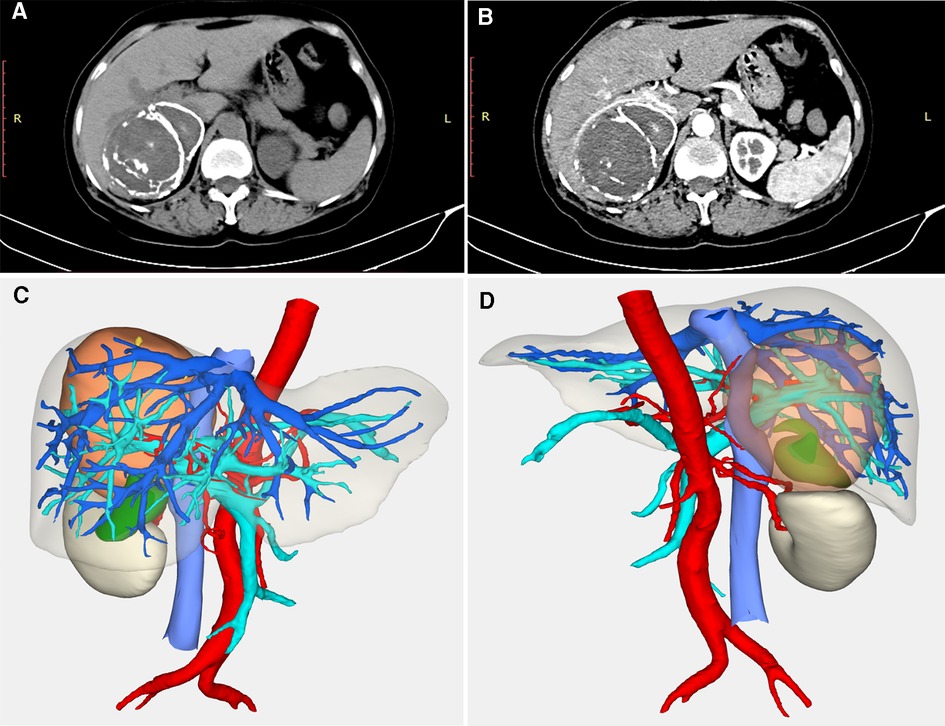
Figure 2. Imaging examination for case 2. (A) an “egg-shell” mass locating in the right subhepatic area; (B) the mass had a calcified edge wrapping around the homogeneous content with multipoint calcifications and calcified separations in the artery phase; (C) three-dimensional CT reconstructed imaging revealed that the right hepatic vein and hepatic pedicle were compressed from the dorsal to the ventral part (anterior); (D) three-dimensional CT reconstructed imaging (posterior).
After an appropriate preoperative preparation, a laparoscopic exploration was performed, and the surgery revealed that the mass arose from the retroperitoneal tissue rather than the liver. There was an obvious calcification of this mass which was pressing against the right kidney and lower right hepatic lobes (Figure 3A), the edge of which was clear (Figure 3B). A laparoscopic retroperitoneal mass resection was successful, and the patient recovered smoothly and was discharged uneventfully 7 days after the operation.
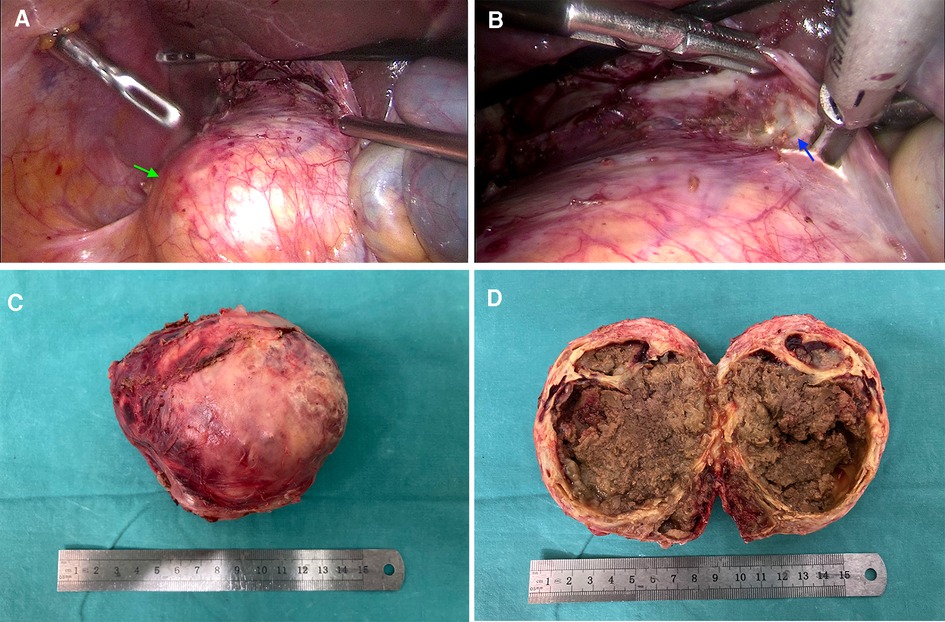
Figure 3. Laparoscopic exploration and specimen for case 2. (A) an “egg-shell” mass (green arrow) arose from the retroperitoneal tissue; (B) there is a clear edge between the mass and the hepatic lobes (blue arrow); (C) the resected specimen with a calcific shell; (D) homogeneous necrotic content with point calcifications and calcified separations.
It was found that the resected mass had a calcified shell (Figure 3C), which wrapped around the bleeding necrotic content with point calcifications and calcified separations (Figure 3D). During a microscopic exam, we found fibroplasia and residual cell components that expressed neuroendocrine markers. The immunohistochemical examination revealed positive expressions of syn, HMB45, CD56, CD68 (weak) and CK (weak) but no expression of SALL4. The percent for Ki-67 was 2%. These findings supported a pathologic diagnosis of paraganglioma with necrosis and cystic changes.
Diseases with “egg-shelled” calcified lesions on imaging are heterogeneously. To date, some primary occurred lesions with typically “eggshell-like” calcification had been reported (1–5, 11). Some infectious disease, including AE and CE disease, also presented “egg-shelled” calcification for some special cases. Based on our experience, hepatic AE disease may present with large “egg-shelled” masses because of the homogeneous intracapsular liquefied necrotic material based on some published research (12) and even our experience (Figures 4A,B). At the same time, it's well known that calcified CE lesions (type-5) present as an “eggshell-like” mass (13). Also, some CE cases with “egg-shelled” wall were found during our surgical exploration (Figures 4C,D). if the patient has been living for a significant time in an area with echinococcosis and/or is positive for anti-echinococcus IgG antibodies, there may be confusion regarding the diagnosis (10).
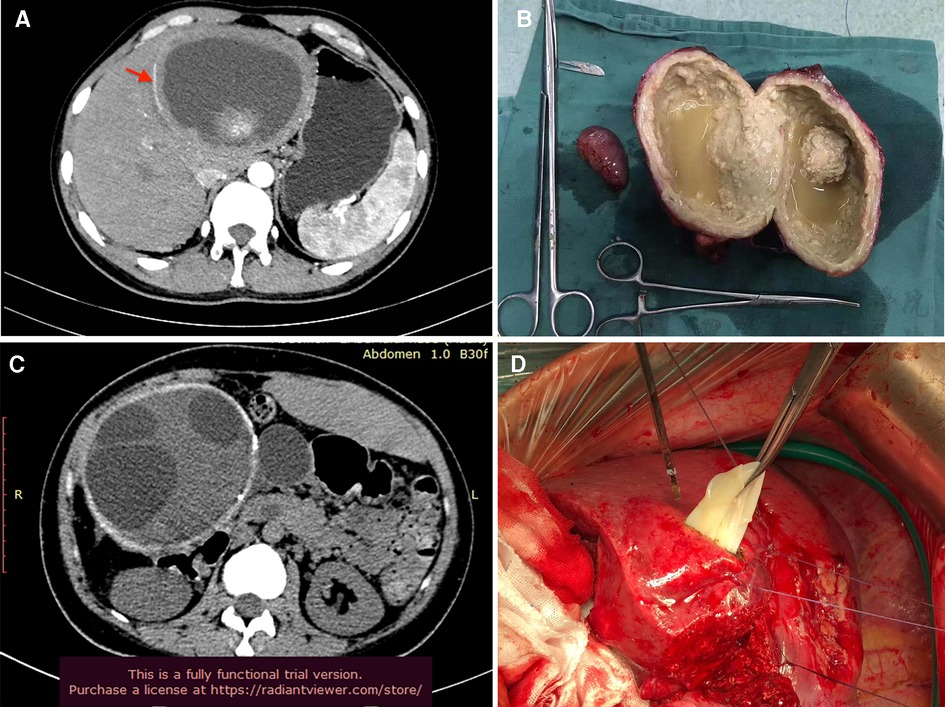
Figure 4. Typical AE and CE with eggshell-like calcification. (A) AE lesion with “egg-shelled” calcification (red arrow); (B) the resected AE lesion; (C) CE lesion with “egg-shelled” calcification and multi-cysts; (D) the cysts of CE during surgery.
There are some common characteristics of the “eggshell like” mass. First, most of the “egg-shelled” lesions are benign rather than malignant. For instance, Imisairi et al reported an adrenal tuberculosis case with eggshell-like calcifications (4). Hepatic CE and AE diseases are conventionally deemed benign despite whose infiltrative growth features. Second, most cases present as asymptomatic or have vague blunt abdominal pain such as these 2 cases. It is assumed that the time for the formation of the calcified shell is very long, and most of the egg-shelled lesion cases conventionally have a long-term history. Third, the relationship between most “egg-shelled” lesions and the adjacent organs is with compressing or by adhering rather than by infiltrating. Therefore, a radical resection is possible for most cases without the need to open the calcified cyst.
Accurate preoperative diagnoses in “egg-shelled” lesions are difficult. This challenge is caused by the similar imaging characteristics, including the presence of a calcified shell and a low-density inner cyst necrotic material. The main reason for the misdiagnoses of the 2 cases presented here is the similar CT findings as in hepatic echinococcosis. Additionally, the positive anti-echinococcus IgG antibody statuses and the geographical histories of the patients misdirected the diagnoses. The positive anti-echinococcus IgG antibody statuses are caused by the antigenic stimulus by the echinococcus when the patients lived in the disease area, rather than by actual hepatic echinococcosis disease (10).
It is important to distinguish echinococcosis lesions from other “egg-shelled” lesions because the overflow of the inner cyst liquid may cause an extensive abdominal hydatid metastasis in CE. Additionally, the overflow of necrotic material in AE lesions could induce an extended abdominal infection. Enhanced CT scans are the most important imaging examinations for distinguishing these tumors. For CE with a calcified shell, many subcysts are often present on imaging. However, the subcysts should not be assumed to be multi-internal septations, such as cystadenomas. On the other hand, AE lesions often have a thick calcified shell, unsmooth inner walls and a homogeneous cyst with necrotic inner material. In addition to CT scans, contrast-enhanced ultrasound scans (CEUS) are also important for distinguishing echinococcosis disease from others. In advanced AE, the cystic components are comprised of metacestodal vesicles and have liquefactive necrosis; the solid components have coagulation necrosis and calcifications with no or little microcirculation. After the injection of a contrast agent, the CEUS shows a nonenhancement, similar to the black hole effect (14). At the same time, AE or CE lesions often occur in the liver rather than in other organs or tissues.
Radical surgical resection is needed for both echinococcosis and other “egg-shelled” lesions. Laparoscopic surgery is a safe technique for radical resection and has been utilized for years. However, if the adjacent tissue (such as the right adrenal gland for the case 1) is easily to be touched during laparoscopic surgery causing serious consequences, laparoscopic mass resection should be recommended. At the same time, the opening of “egg-shelled” cysts for pressure reduction is not recommended by previously reported cases. For some CE cases presented as Figure 4C,D, some activated hydatid eggs may cause abdominal metastasis. For case 2, we successfully performed a laparoscopic surgical resection without opening the cyst. This minimally invasive surgery depends on the experience of the surgical team for a positive outcome in cases suspected of echinococcosis to avoid interoperative hydatid metastasis or abdominal infections. The pathologic diagnosis depends upon the immunohistochemical examination, which determines the future treatment.
In conclusion, “egg-shelled” lesions are rare diseases, and most of them are benign diseases mimicking hepatic echinococcosis. Accurately differentiating nonparasitic lesions from echinococcosis disease is important for surgical planning. Surgical resection is the main method for the treatment of “egg-shelled” lesions. Minimally invasive surgery is recommended and should be performed by experienced surgical teams. Immunohistochemical examination is important for the pathologic diagnosis.
The original contributions presented in the study are included in the article/Supplementary files, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Sichuan Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
YZ designed the study; GZ and YL collected the patient’s clinical data; CY and YZ analyzed the data; YL wrote the paper. All authors contributed to the article and approved the submitted version.
This work was funded by the project of key laboratory of hydatid prevention and control research from Chinese National Health Commission (2020WZK2010).
The authors thank AJE for editing grammar, spelling, and other common errors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Li Z, Li H, Chen H, Gan W. An egg shell-like retroperitoneal pseudocyst. Urology. (2011) 78:561–2. doi: 10.1016/j.urology.2010.12.003
2. Palanivelu C, Rangarajan M, Senthilkumar R, Madhankumar MV, Annapoorni S. Laparoscopic excision of an infected “egg-shelled” retroperitoneal pseudocyst. J Gastrointestin Liver Dis. (2008) 17:465–8. https://www.jgld.ro/jgld/index.php/jgld/article/view/2008.4.1719104712
3. Nakashima T, Tsurumaru D, Nishimuta Y, Miyasaka M, Nishie A, Honda H. A case of pelvic schwannoma presenting prominent eggshell-like calcification. Case Rep Radiol. (2013) 2013:825078. doi: 10.1155/2013/825078
4. Imisairi AH, Hisham AN. Adrenal tuberculosis: the atypical presentations of eggshell-like calcifications. ANZ J Surg. (2009) 79(6):488–9. doi: 10.1111/j.1445-2197.2009.04953.x
5. Yamamoto N, Watari T, Kawasaki K, Matsui Y, Ozaki T. Acute calcific retropharyngeal tendinitis with eggshell-like calcification: case report and literature review on time-course changes in imaging findings. Cureus. (2020) 12(4):e7611. doi: 10.7759/cureus.7611
6. Liu Y, Wang X, Wu J. Continuous long-term albendazole therapy in intraabdominal cystic echinococcosis. Chinese Med J Peking. (2000) 113:827–32.
7. Hosch W, Stojkovic M, Janisch T, Kauffmann GW, Junghanss T. The role of calcification for staging cystic echinococcosis (CE). Eur Radiol. (2007) 17:2538–45. doi: 10.1007/s00330-007-0638-6
8. McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet (London England). (2003) 362:1295–304. doi: 10.1016/S0140-6736(03)14573-4
9. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. (2004) 17:107–35. doi: 10.1128/CMR.17.1.107-135.2004
10. Yang C, Yang H, Deng S, Zhang Y. Hepatobiliary and pancreatic: hepatic cystadenoma mimics hepatic cystic echinococcsis. J Gastroen Hepatol. (2020) 35:1098. doi: 10.1111/jgh.15044
11. Kojima Y, Nakamura M, Koide T, Sonoda T, Haruna Y, Mishima M, et al. [Uncommon calcification of a pheochromocytoma: a case report]. Hinyokika Kiyo. (1989) 35(9):1555–9. hdl.handle.net/2433/1166722683660
12. Takenaka Y, Kogiso T, Ogasawara Y, Koroku M, Yatsuji S, Taniai M, et al. Long-term follow-up of a patient with portal hypertension and hepatic failure due to hepatic hydatid disease. Intern Med. (2021) 60(12):1855–61. doi: 10.2169/internalmedicine.6397-20
13. WHO Informal Working Group. International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. (2003) 85(2):253–61. doi: 10.1016/s0001-706x(02)00223-1
Keywords: “egg-shelled” lesions, hepatic echinococcosis, diagnosis, treatment, surgery
Citation: Liao Y, Zhou G, Yang C and Zhang Y (2022) Uncommon abdominal “egg-shelled” lesions mimic hepatic echinococcosis: Two cases report. Front. Surg. 9:944980. doi: 10.3389/fsurg.2022.944980
Received: 16 May 2022; Accepted: 24 August 2022;
Published: 9 September 2022.
Edited by:
Alexander H. Petter-Puchner, Institute for Experimental and Clinical Traumatology (LBG), AustriaReviewed by:
Wentao Wang, Sichuan University, China© 2022 Liao, Zhou, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhang Njg1MzI4MTVAcXEuY29t Chong Yang eWFuZ2Nob25nQHVlc3RjLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Abbreviations CT, computed tomography; CE, cystic echinococcosis; AE, alveolar echinococcosis; CK, cytokeratin; CEUS, contrast-enhanced ultrasound scans.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.