
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Surg. , 27 September 2022
Sec. Genitourinary Surgery and Interventions
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.944561
This article is part of the Research Topic Optimizing Outcomes of Surgical Interventions in Urologic Oncology View all 7 articles
We reviewed the evolving strategies, practice patterns, and recent advancements aimed at improving the perioperative and surgical outcomes in patients undergoing robot-assisted radical prostatectomy for the management of localized prostate cancer.
Robot-assisted radical prostatectomy (RARP) using the DaVinci Surgical System (Intuitive Surgical, Sunnyvale, CA) was initially described at the turn of the century (1–3), and popularized by Dr. Mani Menon with the establishment of a robotic program at our home institution (4–6). The success of the robotic platform in the surgical management of prostate cancer enabled the dissemination of this technology within urology and other surgical fields. By 2015, RARP accounted for near 70%–85% of all radical prostatectomies performed in United States (US) (7–10). Since its initial approval by the US Food and Drug Administration (FDA) in 2000 for use in urological conditions, the DaVinci Surgical System has gone through several iterations (Standard, S, Si, Xi, X, and SP). Likewise, the RARP technique has been the subject of ongoing refinements aimed at improving surgical, oncologic, and functional outcomes. In this review, we described recent advancements in surgical technique and peri-operative care in patients undergoing RARP.
Radical prostatectomy will invariably result in erectile dysfunction and urinary incontinence, which can negatively impact the quality of life of affected patients (11). The advent of RARP allowed for an intricate dissection of the prostate due to the improved and magnified vision of the robotic platform paving the way for many refinements in surgical technique. Despite these efforts, post-prostatectomy urinary incontinence has been reported anywhere between 1% and 69% of patients depending the definition and length of follow up (12, 13). Similarly, a report from a high-volume center suggested that post-prostatectomy potency rates have not significantly improved in the past 20 years despite a robotic approach and improvements in post-operative management including penile rehabilitation programs. Erectile function recovery rates were 27% and 34% at 12 and 24 months, respectively, and defined as ≥24 on a scale of 30 on a validated questionnaire, the International Index of Erectile Function 6 (IIEF-6) as reported by patients (14).
Return of urinary continence in the post-operative setting following radical prostatectomy is multifactorial. Structures believed to play a role, such as the endopelvic fascia, neurovascular bundle, puboprostatic ligaments, dorsal vascular complex, are all typically violated to some extent during the conventional RARP or open radical retropubic prostatectomy (ORRP). With this in mind, Bocciardi et al. described the Retzius-Sparing RARP (RS-RARP) in 2010 as a way to minimize iatrogenic urinary incontinence (15). Ensuing prospective studies by two different groups showed improved early urinary continence rates at 1 month as high as 92% (16, 17), which was corroborated by a randomized controlled trial (RCT) completed at our institution where RS-RARP was associated with higher continence rates at 1 month compared to the conventional anterior RARP (83% vs. 67%) (18). Improved early continence rates in RS-RARP have also been reported by other groups (19, 20); however, urinary control appears to be equivalent by 12 months (21, 22). Furthermore, RS-RARP limits iatrogenic damage to the bladder neck, which may have a synergistic effect in the early return of urinary continence as suggested by older studies in patients who underwent ORRP with bladder neck preservation (23–26). Additionally, RS-RASP was noted to have lower post-operative urinary dysfunction bother scores up to 1 month after catheter removal, suggesting an earlier return to baseline status compared to the anterior approach.
Despite its improved continence control, adoption of the RS-RASP approach remains limited due to its technical difficulty and prolonged learning curve. An alternative is to perform Retzius space reconstruction at time of anterior RARP as some have reported improved continence rates (27). However, more studies are indicated to corroborate the efficacy of such technique. More recently, Retzius-Sparing using transvesical, extraperitoneal, and transperitoneal approach via a single-port robotic platform been described (28–30), but no direct comparison to the multi-port RS-RARP currently exists. Furthermore, RS-RARP is associated with questionable oncological control. A high rate of positive surgical margins (PSM) has been consistently reported on RS-RARP series (14%–28%) (16–19, 31, 32). A post hoc analysis of the RCT by Dalela et al. showed that the rates of biochemical recurrence free survival were comparable between RS-RARP and conventional RARP (22). In our practice, we routinely use a GelPOINT (Applied Medical, Rancho Santa Margarita, CA) access port when performing RARP (33). This is particularly useful during the Retzius-Sparing approach (1) as it allows intraoperative extracorporeal bimanual examination of the prostate to guide decision to obtain wider margins, (2) and provides greater intraoperative maneuverability of the camera port aiding the surgeon as the dissection approaches the prostatic apex and/or individuals with a deep pelvis (Figure 1). It should be noted that our institution has moved away from using regional pelvic hypothermia as internal review of our data revealed inconclusive results regarding post-operative functional outcomes. Added costs of the GelPOINT device should be considered ($550 at our institution). Furthermore, the potential need for a specimen retrieval bag remains ($36), which can be overcome via appropriate surgical planning as it relates to the size of midline skin/fascia incision relative to prostate size, and having an experienced bedside assistant that can successfully maneuver the extraction of the specimen.
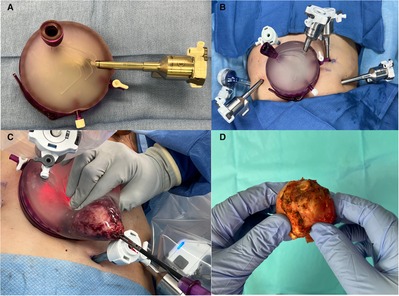
Figure 1. (A) GelPOINT access port for use in robot-assisted radical prostatectomy. For orientation purposes the insufflation port point to the left side of the patient, and the latch should point towards the feet when open. Robotic (camera port) and assistant trocars are pre-placed. (B) GelPOINT access port is placed via an infraumbilical 4 cm transverse incision. An additional five port incisions are made including a 12 and 5 mm assistant ports, both on the right side of the patient. For better triangulation during Retzius-Sparing robot-assisted radical prostatectomy, our preference is to medialize the most lateral left port site, and move the left paramedian port site 1–2 cm in the cephalad direction. (C) Specimen retrieval. A laparoscopic grasper is used to obtain a firm grip of the specimen through the urethra as it enters the apical prostate. The camera robotic arm is moved to an extracorporeal position within the GelPOINT and then lateralized to provide an unobstructed path for specimen retrieval through the assistant port. Note that the GelPOINT assistant port is usually extended sharply at time of placement in order to accommodate the retrieval of larger prostates. (D) Extracorporeal bimanual examination of prostate during robot-assisted radical prostatectomy.
Post-prostatectomy erectile dysfunction arises from the iatrogenic denervation of eretogenic nerves around the prostate including the neurovascular bundle (NVB) (34, 35). Initially pioneered by Dr. Patrick Walsh in the early 1980s (36), performing a nerve-sparing approach (NSA) became the standard of care in patients undergoing radical prostatectomy for localized prostate cancer if technically and oncologically feasible. Current perspectives on nerve-sparing are abound and generally fall into three categories: extent of dissection, minimizing iatrogenic nerve injuries, and adjuncts to guide dissection (37). Standard nerve-sparing aims at preserving each NVB. Patients with favorable oncological characteristics and mild to no erectile dysfunction may be candidates for an augmented nerve-sparing technique known as the “Veil of Aphrodite”, which is associated with improved potency rates following RARP (38–40). This technique preserves accessory nerves within the lateral prostatic fascia by developing an intra-fascial plane between the lateral pelvic fascia and the prostatic capsule. The dissection can be done in a retrograde fashion starting at the antero-apical aspect of the prostate and work towards the posterolateral base of the prostate, or vice versa (antegrade). The retrograde approach is associated with higher potency rates (41); while the antegrade approach may expediate the removal of prostate (40). A “Super Veil” technique has also been described, and involves sparing of the puboprostatic ligaments, which creates an avascular “hood” that preserves additional accessory nerves (42). Moreover, iatrogenic injury to erectogenic, or caversonal, nerves can arise from different sources. Excessive tissue traction may lead to ischemic injury (43). Additionally, judicious use of surgical clips and electrocautery should be advocated when dissecting close to the NVB and obtaining control of the prostatic pedicle as excessive usage could nullify the benefits of a nerve-sparing dissection. Furthermore, caversonal nerves are unmyelinated and particularly prone to thermal injury (37). A study from 2008 reported a 5-fold improvement in early return of sexual function following RARP with athermal nerve-sparing dissection (44). To this effect, a recent single-surgeon series reported on the use of clips vs. bipolar energy for control of the prostatic pedicle during RARP and found no difference in post-operative complications, and short-term functional and oncological outcomes between the two approaches (45). More recently, visual adjuncts have been developed that can potentially allow for a more intricate dissection of the prostate (37). The use of indocyanine green (ICG) may help in the surgical preservation of pertinent blood vessels as it can help identify the prostatic artery and other accessory arterial branches that may play role in maintaining erectogenic function. On the other hand, multi-parametric magnetic resonance imaging (MRI) of the prostate improves patient selection as it helps guide the extent, or lack thereof, of nerve-sparing techniques in up to 47% of patients undergoing RARP (46). Similarly, augmented reality (AR) is a novel technology that has shown promise in patients undergoing RARP (47). Its role is analogous to that of a prostate MRI fusion biopsy where a three-dimensional rendering of the prostate is obtained via MRI and fused with the live feed on the robotic console, which allows the surgeon to adjust its dissection plane in real-time.
Despite improved surgical techniques, concerns for urinary incontinence and erectile dysfunction persist both in patients and provider, prompting many to pursue alternative management strategies such as active surveillance and focal therapy that are associated with a more favorable side-effect profile. Recently, Dr. Mani Menon developed the Precision Prostatectomy (MPP), a new organ-preserving surgical approach for the management of low to intermediate-risk prostate cancer (48, 49). The MPP involves subtotal resection of the prostate with a radical excision on the side of the index lesion while a thin 5–10 mm rim of tissue, including the prostatic capsule and seminal vesicle, is deliberately preserved on the contralateral side with less cancer burden. This procedure is intended for patients with favorable risk prostate cancer who are pre-operatively potent, and are willing to follow an active surveillance protocol post-operatively. Early results are promising with 85% of all-comers and 90% of the pre-operatively potent men being potent at 12 months, with rates of residual cancer and need for secondary procedures appear to be equivalent or superior to those of who undergo high-intensity focused ultrasound (HIFU) (48). The precision prostatectomy offers a favorable cancer control compared to less invasive focal therapy techniques, and minimizes the risk of overtreatment associated with whole gland treatments such as radiation or radical prostatectomy.
Post-operative bladder drainage in patients who undergo RARP can be achieved via urethral or suprapubic catheter (SPC). Our institutional SPC technique was initially described in 2009 and associated with less patient discomfort (50). Overall, there was a 4.4% rate of complications attributable to SPC with most taking place in the immediate post-operative period and managed conservatively or with the conversion to urethral catheter. In rare occasions, patient required need for prolonged catheterization (<0.6%) or had formation of bladder neck contracture (<0.3%) (51, 52). These findings have been corroborated by other high-volume centers and a recent meta-analysis (53–55). To this date, SPC remains a safe and viable option for interested patients. Currently, we use a 14F Ultrathane® with Mac-Loc (Cook Medical, Bloomington, IN) SPC that is typically removed at seven days without the need of a urethral catheter at any point in the post-operative setting.
Pain management in the post-operative setting is of the utmost importance. We employed a multimodal approach that minimizes opioid analgesics. At the conclusion of each case, and when not clinically contraindicated, patients receive incisional local anesthetic installation, insertion of a belladonna and opium suppository, and administration of intravenous (IV) ketorolac. Post-operatively, patients remain on staggered scheduled ketorolac and a combination of acetaminophen and methocarbamol. On discharge, patients receive a pro re nata 10-day supply of acetaminophen, ibuprofen, and methocarbamol. Our approach is similar to that of recently published studies reporting on the excellent analgesic effect of opioid-sparing protocols following RARP with the exception that we do not use pre-operative rehabilitation pathways (56, 57). The caveat is that post-surgical pain-control in patients after a radical prostatectomy tends to be favorable regardless of approach. A prospective study from 2005 in patients undergoing RARP vs. open-radical prostatectomy found that pain scores on POD 1 were no different between the two groups (58).
There has been a shift towards same-day discharge (SDD) for patients undergoing RARP. First reported in 2007, a select group of 11 patients had favorable outcomes following extraperitoneal RARP and able to be discharged home the same day (59). Since then, multiple groups have corroborated the safety and feasibility of SDD-RARP; however, study sample sizes were generally small until recently (60–62). Large single and multi-centered studies in patient undergoing RARP have reported favorable results following SDD (63, 64). The commonality amongst these studies is the importance of established protocols that promote the multidisciplinary collaboration between the surgeon, anesthesia team, and nursing staff to ensure a safe discharge to home. Predictors of successful SDD include being first or second case of the day (61, 63, 64), which allows for an extended period of monitoring prior to discharge later in the day. Recently, single (SP) and multiport (MP) robotic approaches have been described in patients undergoing SDD RARP with rate of SDD higher in those undergoing SP procedures (65). Furthermore, it appears that minimizing iatrogenic peritoneal irritation either from an underlying pneumoperitoneum, transperitoneal incisions, and mobilization of bladder or peritoneal contents, maximizes chances of successful SDD following RARP. This can be achieved by employing ultra-low pneumoperitoneum of 6 mm Hg as commonly done by Abaza et al. using either SP and MP robotic platforms (63, 65, 66), or by employing alternative methods and performing RARP via extraperitoneal (88% SDD) (67) or transvesical (65% SDD) (29) approaches via the SP robotic platform. On the contrary, there is disagreement if a pelvic lymph node dissection impairs chances of SDD but it appears to be less of an issue as experience with SDD grows (63, 64, 68). At our institution, SDD-RASP is routinely performed on well-motivated patients using the SP or MP robotic platform with extraperitoneal or transperitoneal approaches available depending on surgeon's preference and patient factors.
The DaVinci SP Surgical System (Intuitive Surgical, Sunnyvale, CA) is the latest and most advanced iteration of the robotic surgical platform commonly used to perform urological procedures (SP Robot). Single-port prostatectomy was initially described in 2008 in patients with large-volume benign prostatic hyperplasia (BPH) who underwent laparoscopic transvesical enucleation of the prostate, which was followed by a small series reporting on the feasibility of single-port laparoscopic radical prostatectomy by the same group (69, 70). In the ensuing decade, multiple groups described the use of premarketing versions of the SP Robot (SP999 and SP1098) in the successful completion of RARP and other urological procedures in both clinical and pre-clinical settings (71, 72). This should not be confused with the DaVinci Single Site (Intuitive Surgical, Sunnyvale, CA) technology that uses a single incision, a GelPOINT or its equivalent, and utilizes curved instruments that are compatible with DaVinci MP robotic platforms. Single-site approaches for RARP and other urological procedures have been described; however, its relevance remains uncertain but may be an option where the SP robotic platform is not available (73–76).
Usage of the SP Robot involves an “adjusting” curve as most surgeons transitioning to the SP platform likely have significant experience performing MP-RARP. The Endowrist® technology in the SP platform maintains 7 degrees of freedom albeit using a different mechanism, which can dramatically change the dynamics of operating in confined spaces. Furthermore, the reduced footprint of the SP limits the ability of a bedside assistant to aid the surgeon with retraction and suction throughout the procedure. Multiple approaches have been developed to circumvent around these limitations. The remotely operated suction irrigation (ROSI) system is surgeon-controlled and minimizes reliance on a bedside assistant for suctioning in patients undergoing SP or MP surgery including RARP (77). The use of a non-invasive magnetic retraction device (Levita™ Magentic Surgical System, San Mateo, CA) has been described in RARP using both SP and reduced-port approaches (78, 79). Furthermore, not all SP-RARP are truly single-port as in some instances an additional port may be placed at the surgeon's discretion, an approach commonly known as the SP + 1. Both pure SP (28, 65, 80) and SP + 1 (67, 79, 81–83) approaches have been reported by prominent groups. In our experience, the SP + 1 was commonly used early in our experience with the SP robotic platform, but now it is reserved for complex cases where dynamic bedside assistance may be warranted.
Following FDA approval of the SP Robot in 2018 for use in urological procedures, a multitude of groups across the globe have reported their initial experience performing RARP with the SP robot (65, 67, 79, 83, 84). Not surprisingly, various approaches and techniques have emerged. The conventional transperitoneal RARP is the most commonly done approach using the SP platform and it usually involves the use of an additional port incision for bedside assistance (67, 79, 81–83, 85–87), albeit the largest series of SP-RARP outcomes was performed using a pure single-port transperitoneal approach (63). Second most common SP-RARP modality is via an extraperitoneal approach (87), which was described using pure SP that allows for SDD (28), SP + 1 with a drain that is removed on POD 1 (88), and single-site using a MP robotic platform (74, 76). The extraperitoneal approach may be indicated if planning for SDD as it minimizes peritoneal irritation, or for men with hostile abdomen and/or comorbidities at odds with maintaining a prolonged pneumoperitoneum (28, 88). Transperitoneal Retzius-sparing SP-RARP has been reported with varying degrees of success, which was defined as avoiding conversion to an anterior approach (80, 81). Other lesser known SP-RARP approaches include the transperineal and transvesical approaches, as previously described by Dr. Jihad Kaouk et al. A transperineal SP-RARP was described in 26 patients with relative contraindications for a retropubic approach and noted to be technically challenging; however, it was associated with higher rates of positive surgical margins (23% vs. 65%) and comparable functional and oncological outcomes to those of the conventional MP-RARP at 12 months (89). A purely transvesical SP-RARP with limited pelvic lymph node dissection was initially described as an option for patients with low risk of lymph node metastases and a “Frozen Pelvis” that limits access whether it be transperitoneal or extraperitoneal, and where the intravesical lumen provides a big-enough space for maneuvering needed to perform a SP-RARP (29). Advantages include the Retzius-sparing nature of this approach and associated improved urinary control along with avoidance of peritoneal contents and a theoretical lower risk of complications that make this approach and attractive option in all patients regardless of history of prior abdominal surgery (29). To date, the use of SP-RARP offers similar outcomes compared to MP-RARP in terms of urinary control and erectile function; longer follow-up is needed to determine if they are oncologically equivalent (87).
Robot-assisted radical prostatectomy is a safe procedure that can be performed in a myriad of ways using the SP or MP robotic platforms. Specific techniques and approaches will vary depending on the surgeons preference, patient expectations, clinical factors, and tumor characteristics. Nonetheless, the goal should be to obtain oncological control, maintain sexual potency, and minimize urinary incontinence.
IP-Z — manuscript. FA — review, revision. CR — review, revision. WJ — manuscript, review, revision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Abbou CC, Hoznek A, Salomon L, Lobontiu A, Saint F, Cicco A, et al. [Remote laparoscopic radical prostatectomy carried out with a robot. Report of a case]. Prog Urol. (2000) 10(4):520–3. Prostatectomie radicale laparoscopique realisee a distance par robot. A propos d'un cas.11064890
2. Rassweiler J, Frede T, Seemann O, Stock C, Sentker L. Telesurgical laparoscopic radical prostatectomy. Initial experience. Eur Urol. (2001) 40(1):75–83. doi: 10.1159/000049752
3. Pasticier G, Rietbergen JB, Guillonneau B, Fromont G, Menon M, Vallancien G. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol. (2001) 40(1):70–4. doi: 10.1159/000049751
4. Menon M, Shrivastava A, Tewari A, Sarle R, Hemal A, Peabody JO, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. (2002) 168(3):945–9. doi: 10.1097/01.ju.0000023660.10494.7d
5. Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. (2002) 60(5):864–8. doi: 10.1016/s0090-4295(02)01881-2
6. Tewari A, Peabody J, Sarle R, Balakrishnan G, Hemal A, Shrivastava A, et al. Technique of da Vinci robot-assisted anatomic radical prostatectomy. Urology. (2002) 60(4):569–72. doi: 10.1016/s0090-4295(02)01852-6
7. Lowrance WT, Eastham JA, Savage C, Maschino AC, Laudone VP, Dechet CB, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. (2012) 187(6):2087–92. doi: 10.1016/j.juro.2012.01.061
8. Mazzone E, Mistretta FA, Knipper S, Tian Z, Larcher A, Widmer H, et al. Contemporary national assessment of robot-assisted surgery rates and total hospital charges for major surgical uro-oncological procedures in the United States. J Endourol. (2019) 33(6):438–47. doi: 10.1089/end.2018.0840
9. Leow JJ, Chang SL, Meyer CP, Wang Y, Hanske J, Sammon JD, et al. Robot-assisted versus open radical prostatectomy: a contemporary analysis of an all-payer discharge database. Eur Urol. (2016) 70(5):837–45. doi: 10.1016/j.eururo.2016.01.044
10. Cole AP, Leow JJ, Chang SL, Chung BI, Meyer CP, Kibel AS, et al. Surgeon and hospital level variation in the costs of robot-assisted radical prostatectomy. J Urol. (2016) 196(4):1090–5. doi: 10.1016/j.juro.2016.04.087
11. Tyson MD 2nd, Koyama T, Lee D, Hoffman KE, Resnick MJ, Wu XC, et al. Effect of prostate cancer severity on functional outcomes after localized treatment: comparative effectiveness analysis of surgery and radiation study results. Eur Urol. (2018) 74(1):26–33. doi: 10.1016/j.eururo.2018.02.012
12. Radadia KD, Farber NJ, Shinder B, Polotti CF, Milas LJ, Tunuguntla H. Management of postradical prostatectomy urinary incontinence: a review. Urology. (2018) 113:13–9. doi: 10.1016/j.urology.2017.09.025
13. Averbeck MA, Woodhouse C, Comiter C, Bruschini H, Hanus T, Herschorn S, et al. Surgical treatment of post-prostatectomy stress urinary incontinence in adult men: report from the 6th international consultation on incontinence. Neurourol Urodyn. (2019) 38(1):398–406. doi: 10.1002/nau.23845
14. Capogrosso P, Vertosick EA, Benfante NE, Eastham JA, Scardino PJ, Vickers AJ, et al. Are we improving erectile function recovery after radical prostatectomy? Analysis of patients treated over the last decade. Eur Urol. (2019) 75(2):221–8. doi: 10.1016/j.eururo.2018.08.039
15. Galfano A, Ascione A, Grimaldi S, Petralia G, Strada E, Bocciardi AM. A new anatomic approach for robot-assisted laparoscopic prostatectomy: a feasibility study for completely intrafascial surgery. Eur Urol. (2010) 58(3):457–61. doi: 10.1016/j.eururo.2010.06.008
16. Galfano A, Di Trapani D, Sozzi F, Strada E, Petralia G, Bramerio M, et al. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of the first 200 patients with >/= 1 year of follow-up. Eur Urol. (2013) 64(6):974–80. doi: 10.1016/j.eururo.2013.06.046
17. Lim SK, Kim KH, Shin TY, Han WK, Chung BH, Hong SJ, et al. Retzius-sparing robot-assisted laparoscopic radical prostatectomy: combining the best of retropubic and perineal approaches. BJU Int. (2014) 114(2):236–44. doi: 10.1111/bju.12705
18. Dalela D, Jeong W, Prasad MA, Sood A, Abdollah F, Diaz M, et al. A pragmatic randomized controlled trial examining the impact of the retzius-sparing approach on early urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. (2017) 72(5):677–85. doi: 10.1016/j.eururo.2017.04.029
19. Asimakopoulos AD, Topazio L, De Angelis M, Agro EF, Pastore AL, Fuschi A, et al. Retzius-sparing versus standard robot-assisted radical prostatectomy: a prospective randomized comparison on immediate continence rates. Surg Endosc. (2019) 33(7):2187–96. doi: 10.1007/s00464-018-6499-z
20. Qiu X, Li Y, Chen M, Xu L, Guo S, Marra G, et al. Retzius-sparing robot-assisted radical prostatectomy improves early recovery of urinary continence: a randomized, controlled, single-blind trial with a 1-year follow-up. BJU Int. (2020) 126(5):633–40. doi: 10.1111/bju.15195
21. Rosenberg JE, Jung JH, Edgerton Z, Lee H, Lee S, Bakker CJ, et al. Retzius-sparing versus standard robot-assisted laparoscopic prostatectomy for the treatment of clinically localized prostate cancer. BJU Int. (2021) 128(1):12–20. doi: 10.1111/bju.15385
22. Menon M, Dalela D, Jamil M, Diaz M, Tallman C, Abdollah F, et al. Functional recovery, oncologic outcomes and postoperative complications after robot-assisted radical prostatectomy: an evidence-based analysis comparing the retzius sparing and standard approaches. J Urol. (2018) 199(5):1210–7. doi: 10.1016/j.juro.2017.11.115
23. Klein EA. Early continence after radical prostatectomy. J Urol. (1992) 148(1):92–5. doi: 10.1016/s0022-5347(17)36519-9
24. Lowe BA. Comparison of bladder neck preservation to bladder neck resection in maintaining postrostatectomy urinary continence. Urology. (1996) 48(6):889–93. doi: 10.1016/s0090-4295(96)00324-x
25. Deliveliotis C, Protogerou V, Alargof E, Varkarakis J. Radical prostatectomy: bladder neck preservation and puboprostatic ligament sparing–effects on continence and positive margins. Urology. (2002) 60(5):855–8. doi: 10.1016/s0090-4295(02)01956-8
26. Selli C, De Antoni P, Moro U, Macchiarella A, Giannarini G, Crisci A. Role of bladder neck preservation in urinary continence following radical retropubic prostatectomy. Scand J Urol Nephrol. (2004) 38(1):32–7. doi: 10.1080/00365590310017280
27. Abu-Ghanem Y, Dotan Z, Ramon J, Zilberman DE. Retzius space reconstruction following transperitoneal laparoscopic robot-assisted radical prostatectomy: does it have any added value? J Robot Surg. (2018) 12(3):475–9. doi: 10.1007/s11701-017-0768-z
28. Wilson CA, Aminsharifi A, Sawczyn G, Garisto JD, Yau R, Eltemamy M, et al. Outpatient extraperitoneal single-port robotic radical prostatectomy. Urology. (2020) 144:142–6. doi: 10.1016/j.urology.2020.06.029
29. Kaouk J, Beksac AT, Abou Zeinab M, Duncan A, Schwen ZR, Eltemamy M. Single port transvesical robotic radical prostatectomy: initial clinical experience and description of technique. Urology. (2021) 155:130–7. doi: 10.1016/j.urology.2021.05.022
30. Bassett JC, Salibian S, Crivellaro S. Single-port retzius-sparing robot-assisted radical prostatectomy: feasibility and early outcomes. J Endourol. (2022) 36(5):620–5. doi: 10.1089/end.2021.0542
31. Sayyid RK, Simpson WG, Lu C, Terris MK, Klaassen Z, Madi R. Retzius-sparing robotic-assisted laparoscopic radical prostatectomy: a safe surgical technique with superior continence outcomes. J Endourol. (2017) 31(12):1244–50. doi: 10.1089/end.2017.0490
32. Checcucci E, Veccia A, Fiori C, Amparore D, Manfredi M, Di Dio M, et al. Retzius-sparing robot-assisted radical prostatectomy vs the standard approach: a systematic review and analysis of comparative outcomes. BJU Int. (2020) 125(1):8–16. doi: 10.1111/bju.14887
33. Jeong W, Sood A, Ghani KR, Pucheril D, Sammon JD, Gupta NS, et al. Bimanual examination of the retrieved specimen and regional hypothermia during robot-assisted radical prostatectomy: a novel technique for reducing positive surgical margin and achieving pelvic cooling. BJU Int. (2014) 114(6):955–7. doi: 10.1111/bju.12573
34. Kiyoshima K, Yokomizo A, Yoshida T, Tomita K, Yonemasu H, Nakamura M, et al. Anatomical features of periprostatic tissue and its surroundings: a histological analysis of 79 radical retropubic prostatectomy specimens. Jpn J Clin Oncol. (2004) 34(8):463–8. doi: 10.1093/jjco/hyh078
35. Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. (2004) 94(7):1071–6. doi: 10.1111/j.1464-410X.2004.05106.x
36. Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. (1983) 4(5):473–85. doi: 10.1002/pros.2990040506
37. Kumar A, Patel VR, Panaiyadiyan S, Seetharam Bhat KR, Moschovas MC, Nayak B. Nerve-sparing robot-assisted radical prostatectomy: current perspectives. Asian J Urol. (2021) 8(1):2–13. doi: 10.1016/j.ajur.2020.05.012
38. Kaul S, Bhandari A, Hemal A, Savera A, Shrivastava A, Menon M. Robotic radical prostatectomy with preservation of the prostatic fascia: a feasibility study. Urology. (2005) 66(6):1261–5. doi: 10.1016/j.urology.2005.06.107
39. Menon M, Kaul S, Bhandari A, Shrivastava A, Tewari A, Hemal A. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. (2005) 174(6):2291–6; discussion 2296. doi: 10.1097/01.ju.0000181825.54480.eb
40. Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti institute prostatectomy: contemporary technique and analysis of results. Eur Urol. (2007) 51(3):648–57; discussion 657–8. doi: 10.1016/j.eururo.2006.10.055
41. Ko YH, Coelho RF, Sivaraman A, Schatloff O, Chauhan S, Abdul-Muhsin HM, et al. Retrograde versus antegrade nerve sparing during robot-assisted radical prostatectomy: which is better for achieving early functional recovery? Eur Urol. (2013) 63(1):169–77. doi: 10.1016/j.eururo.2012.09.051
42. Asimakopoulos AD, Annino F, D’Orazio A, Pereira CF, Mugnier C, Hoepffner JL, et al. Complete periprostatic anatomy preservation during robot-assisted laparoscopic radical prostatectomy (RALP): the new pubovesical complex-sparing technique. Eur Urol. (2010) 58(3):407–17. doi: 10.1016/j.eururo.2010.04.032
43. Tewari A, Srivastava A, Sooriakumaran P, Grover S, Dorsey P, Leung R. Technique of traction-free nerve-sparing robotic prostatectomy: delicate tissue handling by real-time penile oxygen monitoring. Int J Impot Res. (2012) 24(1):11–9. doi: 10.1038/ijir.2011.40
44. Ahlering TE, Rodriguez E, Skarecky DW. Overcoming obstacles: nerve-sparing issues in radical prostatectomy. J Endourol. (2008) 22(4):745–50. doi: 10.1089/end.2007.9834
45. Basourakos SP, Lewicki PJ, Ramaswamy A, Cheng E, Dudley V, Yu M, et al. Clipless robotic-assisted radical prostatectomy and impact on outcomes. Eur Urol Focus. (2021) S2405-4569(21)00175-9. doi: 10.1016/j.euf.2021.06.010
46. Schiavina R, Bianchi L, Borghesi M, Dababneh H, Chessa F, Pultrone CV, et al. MRI displays the prostatic cancer anatomy and improves the bundles management before robot-assisted radical prostatectomy. J Endourol. (2018) 32(4):315–21. doi: 10.1089/end.2017.0701
47. Schiavina R, Bianchi L, Lodi S, Cercenelli L, Chessa F, Bortolani B, et al. Real-time augmented reality three-dimensional guided robotic radical prostatectomy: preliminary experience and evaluation of the impact on surgical planning. Eur Urol Focus. (2021) 7(6):1260–7. doi: 10.1016/j.euf.2020.08.004
48. Sood A, Jeong W, Palma-Zamora I, Abdollah F, Butaney M, Corsi N, et al. Description of surgical technique and oncologic and functional outcomes of the precision prostatectomy procedure (IDEAL stage 1-2b study). Eur Urol. (2022) 81(4):396–406. doi: 10.1016/j.eururo.2021.10.017
49. Sood A, Jeong W, Taneja K, Abdollah F, Palma-Zamora I, Arora S, et al. The precision prostatectomy: an IDEAL stage 0, 1 and 2a study. BMJ Surg Interv Health Technol. (2019) 1(1):e000002. doi: 10.1136/bmjsit-2019-000002
50. Krane LS, Bhandari M, Peabody JO, Menon M. Impact of percutaneous suprapubic tube drainage on patient discomfort after radical prostatectomy. Eur Urol. (2009) 56(2):325–30. doi: 10.1016/j.eururo.2009.04.018
51. Sammon JD, Trinh QD, Sukumar S, Diaz M, Simone A, Kaul S, et al. Long-term follow-up of patients undergoing percutaneous suprapubic tube drainage after robot-assisted radical prostatectomy (RARP). BJU Int. (2012) 110(4):580–5. doi: 10.1111/j.1464-410X.2011.10786.x
52. Ghani KR, Trinh QD, Sammon JD, Jeong W, Simone A, Dabaja A, et al. Percutaneous suprapubic tube bladder drainage after robot-assisted radical prostatectomy: a step-by-step guide. BJU Int. (2013) 112(5):703–5. doi: 10.1111/bju.12071
53. Morgan MS, Ozayar A, Friedlander JI, Shakir N, Antonelli JA, Bedir S, et al. An assessment of patient comfort and morbidity after robot-assisted radical prostatectomy with suprapubic tube versus urethral catheter drainage. J Endourol. (2016) 30(3):300–5. doi: 10.1089/end.2015.0206
54. Martinschek A, Pfalzgraf D, Rafail B, Ritter M, Heinrich E, Trojan L. Transurethral versus suprapubic catheter at robot-assisted radical prostatectomy: a prospective randomized trial with 1-year follow-up. World J Urol. (2016) 34(3):407–11. doi: 10.1007/s00345-015-1678-1
55. Li Z, Li K, Wu W, Wang Q, Ma X, Lin C, et al. The comparison of transurethral versus suprapubic catheter after robot-assisted radical prostatectomy: a systematic review and meta-analysis. Transl Androl Urol. (2019) 8(5):476–88. doi: 10.21037/tau.2019.08.25
56. Ashrafi AN, Yip W, Graham JN, Yu V, Titus M, Widjaja W, et al. Implementation of a multimodal opioid-sparing enhanced recovery pathway for robotic-assisted radical prostatectomy. J Robot Surg. (2021) 16(3):715–21. doi: 10.1007/s11701-021-01268-7
57. Horodyski L, Ball B, Emile C, Rhodes A, Miao F, Reis IM, et al. Safe transition to opioid-free pathway after robotic-assisted laparoscopic prostatectomy. J Robot Surg. (2022) 16(2):307–14. doi: 10.1007/s11701-021-01237-0
58. Webster TM, Herrell SD, Chang SS, Cookson MS, Baumgartner RG, Anderson LW, et al. Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: a prospective assessment of postoperative pain. J Urol. (2005) 174(3):912–4; discussion 914. doi: 10.1097/01.ju.0000169455.25510.ff
59. Martin AD, Nunez RN, Andrews JR, Martin GL, Andrews PE, Castle EP. Outpatient prostatectomy: too much too soon or just what the patient ordered. Urology. (2010) 75(2):421–4. doi: 10.1016/j.urology.2009.08.085
60. Wolboldt M, Saltzman B, Tenbrink P, Shahrour K, Jain S. Same-day discharge for patients undergoing robot-assisted laparoscopic radical prostatectomy is safe and feasible: results of a pilot study. J Endourol. (2016) 30(12):1296–300. doi: 10.1089/end.2016.0552
61. Berger AK, Chopra S, Desai MM, Aron M, Gill IS. Outpatient robotic radical prostatectomy: matched-pair comparison with inpatient surgery. J Endourol. (2016) 30(Suppl 1):S52–6. doi: 10.1089/end.2016.0135
62. Ploussard G, Almeras C, Beauval JB, Gautier JR, Loison G, Salin A, et al. Same-day discharge surgery for robot-assisted radical prostatectomy in the era of ERAS and prehabilitation pathways: a contemporary, comparative, feasibility study. World J Urol. (2020) 40(6):1359–65. doi: 10.1007/s00345-020-03119-w
63. Abaza R, Martinez O, Ferroni MC, Bsatee A, Gerhard RS. Same day discharge after robotic radical prostatectomy. J Urol. (2019) 202(5):959–63. doi: 10.1097/JU.0000000000000353
64. Ploussard G, Dumonceau O, Thomas L, Benamran D, Parra J, Vaessen C, et al. Multi-institutional assessment of routine same day discharge surgery for robot-assisted radical prostatectomy. J Urol. (2020) 204(5):956–61. doi: 10.1097/JU.0000000000001129
65. Abaza R, Murphy C, Bsatee A, Brown DH Jr, Martinez O. Single-port robotic surgery allows same-day discharge in majority of cases. Urology. (2021) 148:159–65. doi: 10.1016/j.urology.2020.08.092
66. Ferroni MC, Abaza R. Feasibility of robot-assisted prostatectomy performed at ultra-low pneumoperitoneum pressure of 6 mmHg and comparison of clinical outcomes vs standard pressure of 15 mmHg. BJU Int. (2019) 124(2):308–13. doi: 10.1111/bju.14682
67. Dobbs RW, Halgrimson WR, Madueke I, Vigneswaran HT, Wilson JO, Crivellaro S. Single-port robot-assisted laparoscopic radical prostatectomy: initial experience and technique with the da Vinci((R)) SP platform. BJU Int. (2019) 124(6):1022–7. doi: 10.1111/bju.14864
68. Banapour P, Elliott P, Jabaji R, Parekh A, Pathak A, Merchant M, et al. Safety and feasibility of outpatient robot-assisted radical prostatectomy. J Robot Surg. (2019) 13(2):261–5. doi: 10.1007/s11701-018-0848-8
69. Desai MM, Aron M, Canes D, Fareed K, Carmona O, Haber GP, et al. Single-port transvesical simple prostatectomy: initial clinical report. Urology. (2008) 72(5):960–5. doi: 10.1016/j.urology.2008.06.007
70. Kaouk JH, Goel RK, Haber GP, Crouzet S, Desai MM, Gill IS. Single-port laparoscopic radical prostatectomy. Urology. (2008) 72(6):1190–3. doi: 10.1016/j.urology.2008.06.010
71. Bertolo R, Garisto J, Gettman M, Kaouk J. Novel system for robotic single-port surgery: feasibility and state of the art in urology. Eur Urol Focus. (2018) 4(5):669–73. doi: 10.1016/j.euf.2018.06.004
72. Kaouk JH, Haber GP, Autorino R, Crouzet S, Ouzzane A, Flamand V, et al. A novel robotic system for single-port urologic surgery: first clinical investigation. Eur Urol. (2014) 66(6):1033–43. doi: 10.1016/j.eururo.2014.06.039
73. Gaboardi F, Pini G, Suardi N, Montorsi F, Passaretti G, Smelzo S. Robotic laparoendoscopic single-site radical prostatectomy (R-LESS-RP) with daVinci Single-Site(R) platform. Concept and evolution of the technique following an IDEAL phase 1. J Robot Surg. (2019) 13(2):215–26. doi: 10.1007/s11701-018-0839-9
74. Chang Y, Lu X, Zhu Q, Xu C, Sun Y, Ren S. Single-port transperitoneal robotic-assisted laparoscopic radical prostatectomy (spRALP): initial experience. Asian J Urol. (2019) 6(3):294–7. doi: 10.1016/j.ajur.2018.08.002
75. Lee HH, Na JC, Yoon YE, Rha KH, Han WK. Robot-assisted laparoendoscopic single-site upper urinary tract surgery with da Vinci Xi surgical system: initial experience. Investig Clin Urol. (2020) 61(3):323–9. doi: 10.4111/icu.2020.61.3.323
76. Li CC, Chien TM, Lee MR, Lee HY, Ke HL, Wen SC, et al. Extraperitoneal robotic laparo-endoscopic single-site plus1-port radical prostatectomy using the da Vinci single-site platform. J Clin Med. (2021) 10(8):1563. doi: 10.3390/jcm10081563
77. Martinez O, Murphy C, Bsatee A, Brown DH Jr, Abaza R. Impact of surgeon-controlled suction during robotic prostatectomy to reduce dependence on bedside assistance. J Endourol. (2021) 35(8):1163–7. doi: 10.1089/end.2020.1059
78. Steinberg RL, Johnson BA, Cadeddu JA. Magnetic-assisted robotic surgery: initial case series of reduced-port robotic prostatectomy. J Robot Surg. (2019) 13(4):599–603. doi: 10.1007/s11701-018-0889-z
79. Steinberg RL, Johnson BA, Meskawi M, Gettman MT, Cadeddu JA. Magnet-assisted robotic prostatectomy using the da Vinci SP robot: an initial case series. J Endourol. (2019) 33(10):829–34. doi: 10.1089/end.2019.0263
80. Alip S, Koukourikis P, Han WK, Rha KH, Na JC. Comparing revo-i and da Vinci in retzius-sparing robot-assisted radical prostatectomy: a preliminary propensity score analysis of outcomes. J Endourol. (2022) 36(1):104–10. doi: 10.1089/end.2021.0421
81. Agarwal DK, Sharma V, Toussi A, Viers BR, Tollefson MK, Gettman MT, et al. Initial experience with da Vinci single-port robot-assisted radical prostatectomies. Eur Urol. (2020) 77(3):373–9. doi: 10.1016/j.eururo.2019.04.001
82. Moschovas M C, Bhat S, Onol F, Rogers T, Patel V. Early outcomes of single-port robot-assisted radical prostatectomy: lessons learned from the learning-curve experience. BJU Int. (2021) 127(1):114–21. doi: 10.1111/bju.15158
83. Ng CF, Teoh JY, Chiu PK, Yee CH, Chan CK, Hou SS, et al. Robot-assisted single-port radical prostatectomy: a phase 1 clinical study. Int J Urol. (2019) 26(9):878–83. doi: 10.1111/iju.14044
84. Kaouk J, Bertolo R, Eltemamy M, Garisto J. Single-port robot-assisted radical prostatectomy: first clinical experience using the SP surgical system. Urology. (2019) 124:309. doi: 10.1016/j.urology.2018.10.025
85. Huang MM, Patel HD, Wainger JJ, Su ZT, Becker REN, Han M, et al. Comparison of perioperative and pathologic outcomes between single-port and standard robot-assisted radical prostatectomy: an analysis of a high-volume center and the pooled world experience. Urology. (2021) 147:223–9. doi: 10.1016/j.urology.2020.08.046
86. Saidian A, Fang AM, Hakim O, Magi-Galluzzi C, Nix JW, Rais-Bahrami S. Perioperative outcomes of single vs multi-port robotic assisted radical prostatectomy: a single institutional experience. J Urol. (2020) 204(3):490–5. doi: 10.1097/JU.0000000000000811
87. Hinojosa-Gonzalez DE, Roblesgil-Medrano A, Torres-Martinez M, Alanis-Garza C, Estrada-Mendizabal RJ, Gonzalez-Bonilla EA, et al. Single-port versus multiport robotic-assisted radical prostatectomy: a systematic review and meta-analysis on the da Vinci SP platform. Prostate. (2022) 82(4):405–14. doi: 10.1002/pros.24296
88. Khalil MI, Joseph JV. Extraperitoneal single-port robot-assisted radical prostatectomy. J Endourol. (2021) 35(S2):S100–5. doi: 10.1089/end.2021.0440
Keywords: Retzius-sparing, precision prostatectomy, single port surgery, robot-assisted radical prostatectomy (RARP), functional outcome, perioperative care
Citation: Palma-Zamora I, Abdollah F, Rogers C and Jeong W (2022) Robot-assisted radical prostatectomy: Advancements in surgical technique and perioperative care. Front. Surg. 9:944561. doi: 10.3389/fsurg.2022.944561
Received: 15 May 2022; Accepted: 1 September 2022;
Published: 27 September 2022.
Edited by:
Richard Naspro, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
José Ignacio Nolazco, Harvard Medical School, United States© 2022 Palma-Zamora, Abdollah, Rogers and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wooju Jeong d2plb25nMUBoZmhzLm9yZw==
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.