
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
POLICY AND PRACTICE REVIEWS article
Front. Surg., 26 December 2022
Sec. Otorhinolaryngology - Head and Neck Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.944223
 Mai Thy Truong1
Mai Thy Truong1 Yi-Chun Carol Liu2,3
Yi-Chun Carol Liu2,3 Jocelyn Kohn4
Jocelyn Kohn4 Sivakumar Chinnadurai5,6
Sivakumar Chinnadurai5,6 David A. Zopf7
David A. Zopf7 Melissa Tribble8
Melissa Tribble8 Paul B. Tanner9
Paul B. Tanner9 Kathleen Sie10
Kathleen Sie10 Kay W. Chang1*
Kay W. Chang1*
Objectives: To present recommendations for the coordinated evaluation and management of the hearing and reconstructive needs of patients with microtia and aural atresia.
Methods: A national working group of 9 experts on microtia and atresia evaluated a working document on the evaluation and treatment of patients. Treatment options for auricular reconstruction and hearing habilitation were reviewed and integrated into a coordinated care timeline.
Results: Recommendations were created for children with microtia and atresia, including diagnostic considerations, surgical and non-surgical options for hearing management and auricular reconstruction, and the treatment timeline for each option. These recommendations are based on the collective opinion of the group and are intended for otolaryngologists, audiologists, plastic surgeons, anaplastologists, and any provider caring for a patient with microtia and ear canal atresia. Close communication between atresia/hearing reconstruction surgeon and microtia repair surgeon is strongly recommended.
• Standardize the evaluation, including hearing evaluation, for a newborn with microtia and aural atresia
• Review hearing amplification options, including worn devices and surgically implanted devices for patients with microtia and aural atresia
• Review reconstructive options for microtia and the timeline that each option entails
• Review issues concerning aural atresia repair, especially in coordination with microtia repair.
• Create a coordinated timeline for hearing management and microtia reconstruction to reduce the total number of surgeries required and avoid potential deleterious impacts of hearing surgeries on microtia repair.
Pediatric and adult patients with microtia and aural atresia
(a) Otolaryngologists: pediatric otolaryngologists, otologists, facial plastic surgeons
(b) Plastic surgeons
(c) Oral/Maxillofacial surgeon
(d) Audiologists
(e) Anaplastologists
(f) Pediatricians
(g) Any provider caring for patients with microtia, aural atresia, and craniofacial abnormalities
Each of the authors provided expert opinions. The primary authors (MT, KC) wrote a draft of the manuscript with additional input provided by the contributing authors in specific niche interests and practice areas. A final consensus was obtained on the management recommendations reviewed and approved by all contributors.
Microtia is a congenital malformation of the outer ear or pinna whereby the ear is reduced in size, has underdeveloped landmarks, or is misshapen (1). Microtia is often associated with the absence of the external auditory canal (canal atresia or aural atresia) or an extremely narrow ear canal (canal stenosis). Classically, microtia is classified into four grades, ranging from mild malformation to the complete absence of the external ear (Figure 1). The most common presentation is grade 3, consisting of significant hypoplasia of the entire cartilaginous framework with a lobule remnant. Microtia ears can also be described as “lobular-type,” when there is a predominant lobule remnant or “conchal-type,” where a conchal bowl remnant is present. “Conchal-type” microtia is often associated with the presence of a stenotic canal.
Microtia occurs in about 1 in 10,000 births, though rates vary depending on ethnic background (1). Ninety percent of cases are unilateral, with the right side being affected more frequently than the left, and it is more prevalent in males than females. The exact cause of microtia and atresia is unknown, and there may be a variety of different etiologies. There is evidence for both environmental and genetic causes of microtia. This malformation is associated with a syndrome in 35%–55% of patients, many of which have an identified genetic mutation (2). In non-syndromic or sporadic cases, no single genetic mutation has been identified. Maternal exposure to medications during pregnancy, such as retinoids and thalidomide, have been identified (3, 4). Microtia also has an increased incidence in countries at a higher elevation (5). An association between maternal diabetes and microtia has also been suggested (6).
Deficiencies of the ear canal, also called aural atresia, are often present with microtia. Varying degrees of aural atresia can be classified from Type A (Stenosis), in which both the fibrocartilaginous and bony parts of EAC are present but narrow, to Type B (Partial Atresia), in which only some part of the fibrocartilaginous or bony EAC is present with the tympanic membrane being missing or rudimentary, to Type C (Total Atresia), in which both fibrocartilaginous and bony parts of the EAC as well as the tympanic membrane are absent (7). In most cases it is possible to classify the degree of atresia from careful physical exam, however in some unusual cases, the opening to the remnant ear canal may appear only as a tiny pit on the skin, or be hidden by overlying microtic skin remnants. In these cases, a CT scan (Figure 2) can better delineate that a stenotic, epithelium-lined bony ear canal is still present, posing a risk for an eventual canal cholesteatoma.
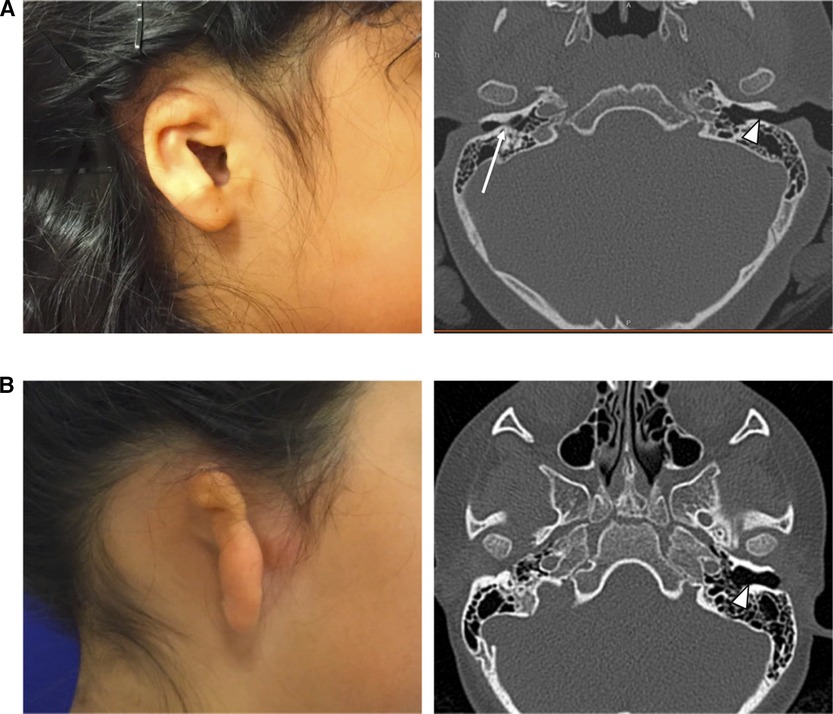
Figure 2. (A) Child with visible canal opening on the right, but severe bony external canal stenosis demonstrated on non-contrast CT scan (long white arrow). Normal canal size is noted on the contralateral side (short white arrow). (B) Child with complete atresia on the right, demonstrated on non-contrast CT scan. Normal canal size is noted on the contralateral side (short white arrow).
History: Clinicians should perform a complete history including ear infections, speech and language milestones, and family history of hearing loss and ear anomalies.
Physical exam: A complete physical exam of the infant should be performed looking for associated syndromic findings (Table 1). It is also essential to monitor the middle ear status in the typically formed ear to ensure optimal hearing is maintained for the better hearing ear.
Hearing evaluation: When infants present with microtia and aural atresia, the involved ear(s) will typically fail the newborn hearing screen (NHS). Regardless of the NHS results, these infants should be referred directly to an audiologist to undergo an outpatient diagnostic Auditory Brainstem Response (ABR) test (8). Newborn hearing screening is mandated and performed universally in the United States, but often by non-medical volunteers. Regardless of the presence or absence of an ear canal, the infant with microtia or with a severe auricular malformation should be referred to a pediatric audiologist (or a general audiologist comfortable doing office sleep ABRs) for diagnostic hearing testing.
A diagnostic ABR should be performed as early as possible. The Early Hearing Detection and Intervention (EDHI) program goals recommend a diagnostic ABR by 3 months of age. For clinics and centers meeting this metric, they should strive for a diagnostic ABR by 2 months of age (8). At a minimum, this diagnostic ABR should include thresholds for broadband stimulus such as a click or CE-Chirp, as well as tone burst thresholds between 500 Hz and 4,000 Hz. Air conduction and bone conduction testing should performed. Specifically, masked bone conduction testing will best isolate the response from the cochlea of the affected ear to ensure the bone conduction response is indeed coming from the test ear. In cases of bilateral atresia, only unmasked bone conduction can be measured due to the inability to appropriately mask the bone conduction response. Otherwise, in cases of unilateral ear canal stenosis or atresia, masked bone conduction testing should be performed for the affected ear. Additionally, tympanometry should be performed (1000 Hz tympanometry to demonstrate the mobility of the eardrum and 226 Hz tympanometry to confirm ear canal volume) and distortion product otoacoustic emissions in the ear with a patent ear canal.
In most infants from birth to up to 6 months of age, this diagnostic ABR can be done while the infant is sleeping without sedation (Figure 3). Beyond six months of age, audiologic assessments should be performed using developmentally appropriate behavioral test methods (visual reinforcement audiometry, conditioned play audiometry, standard audiometry). The behavioral audiologic evaluation is essential to confirm hearing thresholds (by masked bone conduction) for the ear with microtia/atresia as well as confirm and monitor hearing sensitivity for the non-microtic/atretic ear. Children who are challenging to assess behaviorally may require a sedated ABR. Hearing sensitivity in this population should be monitored annually or bi-annually until age 5.
In cases of bilateral microtia and atresia, patients should be referred to audiology as early as possible. Diagnostic audiological assessment should be completed by 2–3 months of age and the infant provided with hearing amplification by 4 months of age and enrolled in early intervention (EI) by 3–6 months of age to optimize speech and language development.
Imaging studies: A CT scan of the temporal bone is not recommended in the newborn period. CT should be reserved for just before hearing reconstructive surgical options are discussed (approximately age five years, depending on the surgical plan) or if there is a concern for cholesteatoma. A high-resolution CT scan of the temporal bone without contrast is recommended before any microtia reconstruction to assess candidacy for atresia repair and to rule out the presence of canal or congenital middle ear cholesteatoma (Figure 4), though it may not be necessary if the family has absolutely no interest in atresia repair. Clinical concern for cholesteatoma, often seen in cases with severe canal stenosis, may warrant diffusion-weighted imaging (DWI) MRI to help confirm the diagnosis. Renal ultrasound in the newborn period can be performed to rule out kidney abnormalities associated with syndromic causes of microtia (9).
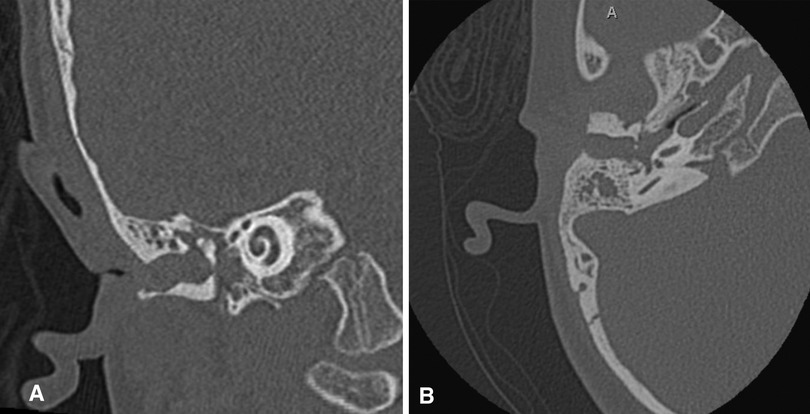
Figure 4. (A) Coronal and (B) axial non-contrast temporal bone CT scan demonstrating severe EAC stenosis with cholesteatoma.
Consultations: Newborns should be referred to audiology and otolaryngology to consult on the child's hearing status. In addition, a genetics referral is recommended if the child appears to be syndromic at birth (Tables 1, 2). These referrals can be made as an outpatient, though many parents are comforted to meet with an otolaryngologist after birth, particularly to address hearing concerns. An adolescent with hemifacial microsomia may require a plastic surgery or oral and maxillofacial surgery (OMFS) referral regarding mandibular hypoplasia and malocclusion issues. This referral may be through a Craniofacial clinic. Parents or patients desiring more information about the actual microtia reconstruction should be referred to an experienced microtia surgeon; this may be a plastic surgeon/craniofacial surgeon, pediatric otolaryngologist, or facial plastic surgeon. Recommend evaluations for speech and language delay and Early Intervention programs that are available from birth to age three years in every state for children with developmental delays and disabilities (10), including isolated hearing loss. After age 3, it is important to pursue an Individualized Education Plan (IEP) or 504 (c) plan through the school system to set the child up for success in the classroom.
Parents of a newborn with microtia may experience intense emotions, especially guilt and frustration. It is important to counsel parents on theoretical causes of microtia, the hearing impact and then guide them on hearing and reconstructive options and timelines. In most cases, the cause of microtia and atresia is not known. Parental self-blame should be mitigated to the extent possible. Advising parents on ways to talk to the affected child, friends, acquaintances, or other family members about the patient's “little ear” is also paramount to prevent feelings of shame. Families should be encouraged to explore support groups or online communities and forums (e.g., www.earcommunity.org) to minimize feelings of isolation and provide positive examples of how children with microtia and atresia can grow to be productive, healthy adults.
Children with microtia/atresia are most commonly found to have conductive hearing loss (CHL) on the side of the microtic ear. The hearing loss is confirmed at the time of the diagnostic audiologic evaluation. Amplification options for children with microtia and atresia can be non-surgical or surgical. Before the age of five years, the non-surgical amplification option is recommended as some surgically implanted device options are approved by the FDA for children of age five years and over.
A variety of worn hearing devices are available to help children with the conductive hearing loss associated with microtia and atresia (Figure 5). Bone conduction sound processors (BCSP) such as Cochlear BAHA® or Ponto can be worn on an accessory known as a softband (11). Another option is the MedEl ADHEAR sound processor, which can be worn on an adhesive gel pad or a designated softband. In children over five years of age, the Cochlear BAHA® SoundArc may be used in conjunction with the Cochlear BAHA® Sound processors and is worn on the posterior aspect of the head as an alternative to the headband.

Figure 5. Non-Surgical Bone Conduction Devices. Bone conduction sound processors. (A) Cochlear BAHA® with softband. (B) Ponto® with softband. (C) Med-El ADHEAR® with adhesive gel pad. (D) Cochlear BAHA SoundArc®.
Clinicians should encourage early use of these worn bone conduction devices for infants with CHL. In bilateral microtia/atresia cases, infants should be fit for a device by an audiologist by 4 months of age (8). In patients with unilateral microtia/atresia, where the non-microtic ear has normal hearing, a device consultation with a pediatric audiologist to discuss bone conduction options and encourage early use to facilitate early adaptation to hearing aid use. Although children under three years old are seldom exposed to challenging hearing environments, it is the experience of most centers that successful use of bone conduction aids in school-aged children correlates with earlier fitting and adaptation. Parents should be made aware that they may not notice a behavioral difference in the early fitting of bone conduction aids, and some may feel that it “doesn't make a difference.” The goal of the early fitting is early adaptation to amplified sound for sensory integration into the child's experience in preparation for school age. While there is an increasing body of literature that indicates that the lack of sound localization in children with unilateral hearing loss is associated with increased difficulties with speech recognition in noise often found in the typical classroom environment (12–17), whether or not children with unilateral hearing loss from aural atresia actually gain benefit from amplification is still an emerging area of research with at least one group finding that bone conduction devices improved both sound localization and speech recognition in children with unilateral aural atresia (18).
Parents should be made aware that there may be challenges with insurance coverage for bone conductive hearing devices in patients with unilateral microtia. They should also be counseled that toddler years can be challenging for successfully using worn devices, and a goal of 2–4 h a day use is encouraged until the child is more tolerant of more extended periods of use. Many programs have the minimal goal of daily use of the hearing device while in school.
Once patients reach five years of age, they are candidates to use BCSP with a surgically placed osseointegrated implant retention system, using either a subcutaneous magnet or a percutaneous abutment (Figure 6). This implant allows the child to use a bone conduction sound processor without a softband or an adhesive gel pad.
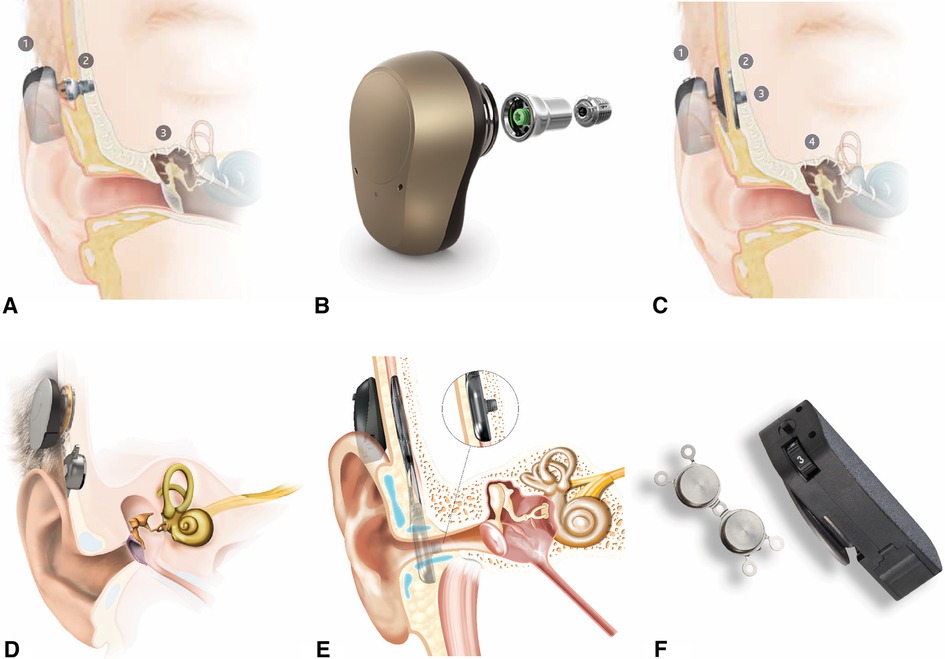
Figure 6. Surgical bone conduction devices. (A) Cochlear BAHA® with osseointegrated percutaneous abutment. (B) Ponto® with abutment and screw for osseointegration. (C) Cochlear BAHA ATTRACT® with subcutaneous magnet. (D) Med-El BoneBridge® with subcutaneous magnet. (E) Cochlear OSIA® with subcutaneous magnet. (F) Medtronic magnetic implant.
The abutment type (BAHA® Connect, Ponto) and the magnetic systems (BAHA® Attract, Medtronic Magnetic Implant) have a similar osseointegrated implant in the skull, but each allows the sound processor to connect to the implant differently. The abutments penetrate the skin, while the magnetic systems do not.
Overall, transcutaneous magnetic systems allow for eliminating an abutment that penetrates the skin. However, the hearing gain is less with a magnet. Different magnet strengths are available to accommodate for varying scalp thickness, though the device may fall off with weaker connections. Occasionally, the scalp needs to be surgically thinned to accommodate the magnetic connection.
In contrast, abutments allow direct bone conduction without any loss of vibration across the skin. Abutments also offer a more secure attachment of the processor to the implant, which can be beneficial for an active child on the playground. However, the skin-abutment interface is susceptible to local skin reactions such as recurrent infections and skin overgrowth. Rates of complications of abutments requiring revision surgery are as high as 44.4% percent, with implant loss ranging up to 25% in pediatric patients (19,20).
Active implants with direct bone conduction without skin penetration (Osia®, BoneBridgeTM) are currently FDA approved for patients 12 years of age and older. At the time of this writing, successful implantation in younger patients has been reported both in and outside of the United States (21,22). These provide optimal hearing amplification without penetrating the skin. These devices also rely on transcutaneous magnetic systems to connect an external microphone. However, the bone conducting transducer is the component implanted under the skin. This allows for separation of the microphone and the active component of the hearing device, eliminating the commonly encountered issue of feedback without the loss of amplification across the scalp skin or issues with skin complications.
Close consultation with the audiology team and the family is essential to determine a patient's candidacy for a surgically implanted hearing device. A child with a history of poor compliance with a softband-retained hearing device, for instance, may not be a good candidate for an implanted device, particularly if the patient does not feel the benefit from the device, does not like the sound, or does not want to wear any device that might attract attention. A child who is a good candidate for ear canal reconstruction may not want to proceed with an implanted device.
The surgeon placing an osseointegrated implant for hearing management should collaborate with the reconstructive surgeon so that the implant surgery does not compromise the fascial flaps or skin that may be critical for microtia repair. It may be preferable to place the implant after microtia reconstruction, though coordinated approaches allow for combined ear reconstructive and hearing augmentation procedures.
If placement is done before microtia reconstruction, it should be placed in the posterior temporal region. It is critical to preserve the vascular integrity of the superficial temporoparietal fascia during implant placement. Doppler of the superficial temporal artery at the time of surgery is recommended for safe placement of the implant posterior to the vessel's course (Figure 7). If a patient and family desiring microtia repair have not yet consulted with a microtia surgeon or have not yet decided on the timing and type of approach desired, deferring surgical implant of a hearing device may be prudent so that all surgeries can be coordinated.
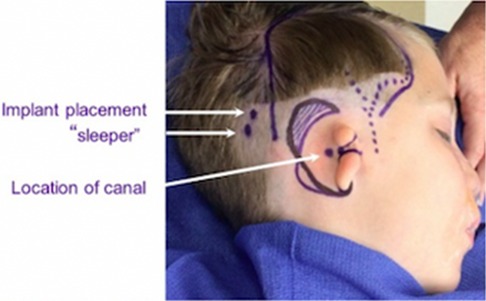
Figure 7. Surgical markings demonstrating course of the superficial temporal artery (dotted line), bone conducting hearing aid primary and “sleeper” implant sites (white arrows).
If a non-surgical, adhesive retained prosthetic ear is desired, some osseointegrated hearing implants can be done at any time after five years of age. If a surgically retained prosthetic (i.e., Vistafix®) is planned, these surgeries can be done concurrently.
If a family is proceeding with alloplastic ear reconstruction, the osseointegrated hearing implant can be placed simultaneously with the alloplastic reconstructive surgery or three months after the ear has healed. For autologous cartilage repair, the implant can be placed concurrently during the stage of surgery in which the newly constructed ear is elevated, which allows access to the posterior auricular region through the same incisions. Alternatively, the osseointegrated hearing implant can be safely placed three months after the final stage of autologous cartilage repair.
Passive bone conduction devices worn on a softband (BAHA®, Ponto) can offer a similar pure-tone average as those worn with an implanted magnet (BAHA Attract®). However, implanted active bone conduction devices (Osia®, BoneBridgeTM) provide better high-frequency gain (23–25). With improved high-frequency fidelity, patients can have improved speech discrimination scores with the active bone conduction implants compared to passive bone conduction devices.
Overall, hearing outcomes of bone conduction devices are reliable, come with less surgical risk, and are typically better than outcomes of ear canal reconstruction (26). However, they rely on a patient's compliance for use and have psychosocial impacts of wearing a hearing device.
Aural atresia repair requires creating an external auditory canal (EAC), a tympanic membrane, lining the neo-tympanum and EAC with squamous epithelium, and liberation or reconstruction of the ossicles of the middle ear. Although parents frequently hope that hearing can “be restored” with this surgery, it is more realistic to prepare for hearing improvement without complete closure of the air-bone gap over the long term (>5 years). Immediate hearing results can often be excellent but can degrade over time (26,27). It is reasonable for experienced surgeons to obtain improved air conduction thresholds with atresia repair for suitable candidates and create the anatomy that would allow the patient to accommodate a traditional behind-the-ear hearing aid.
Candidacy for aural atresia repair is based upon the ear-specific hearing assessment and CT findings. The most common scale used to describe the CT findings is the Jahrsdoerfer scale, a 10-point scale based upon the presence of 9 structures (28,29). Other anatomic factors that may be considered in determining a patient's candidacy for atresia repair include the position of the tegmen and other structures such as venous structures of the temporal bone and the middle ear volume (30–32).
It is absolutely critical that surgeons considering atresia repair closely communicate with the surgeon who plans to manage the microtia reconstruction for a coordinated surgical timeline that optimizes outcomes while minimizing complications.
Ear Prosthetic: atresia reconstruction surgery can be done at any time using an ear prosthetic, whether used with adhesive or surgically retained.
Alloplastic Porous Polyethylene (PPE) reconstruction (e.g., Medpor®, SuPor®): atresia reconstruction surgery is ideally done before or at the time of microtia reconstruction (concurrent surgery). Canal surgery after alloplastic porous polyethylene implant risks damage to the flap vascularity, exposure, and extrusion of the implant with or without infection, which is poorly tolerated by alloplastic implants (7, 33).
Autologous costal cartilage reconstruction: atresia reconstruction surgery is ideally done after microtia reconstruction with cartilage to maintain the viability of the overlying local skin and subcutaneous tissue, which covers the cartilage construct in a tissue envelope (7, 33). However, in cases of canal cholesteatoma, this may not be possible, requiring surgical management of the cholesteatoma and atresia repair prior to auricular reconstruction. In these instances, fibrosis and vascular compromise across old incisions poses an increased risk to skin viability with the subcutaneous pocket of the microtia repair. There can be more difficulty creating a pliable skin pocket around the reconstructed canal due to scarring and limitations to the skin pocket near the newly created tragus.
Careful patient selection, surgeon-specific training and skills, and meticulous technique at every operative step are required for successful ear canal reconstruction surgery (33, 34). Aural atresia can be graded by severity, ranging from a Type A stenosis to Type C total bony atresia (Table 3) (7). The stenoses and partial atresias are at much higher risk for cholesteatoma due to the presence of skin inside the remnant canal (33). However, these allow for surgical approaches that do not require circumferential skin grafting of the reconstructed canal and thus may heal more reliably with better hearing outcomes (35–37).
Careful analysis of the CT scan is critical for determining candidacy for successful ear canal reconstruction. Ears scoring six or less on the Jahrsdoerfer grading scale had only a 45% chance of achieving a postoperative speech reception threshold of 30 decibels (dB) or lower, while ears scoring seven or higher had an 89% chance (28,29). Absence of an oval window uniformly predicts poor hearing results (38). Mesotympanic volume measurement greater than 42 mm3 and middle ear volume measurement greater than 305 mm3 have been demonstrated to predict much better hearing results (30,31). Other anatomic factors that could make ear canal reconstruction impossible or more difficult include a low-lying tegmen, a large malleus-incus complex positioned directly lateral to the stapes rather than the usual anterolateral position, facial nerve obstructing the oval window, and facial nerve turning anterolaterally and obstructing the lateral surgical approach to the attic (39). Poorer hearing outcomes due to need for prosthesis placement has been found with the “boomerang” malleus-incus complex (40).
Average hearing gain from atresiaplasty is about 24 dB in appropriately selected patients, compared to 38 dB gain from osseointegrated implant-retained devices (26). Atresiaplasty achieves less than 30 dB pure tone average (PTA) or air-bone gap (ABG) in about 70% of patients if the hearing is assessed less than six months after surgery. However, this deteriorates to about 50% of patients assessed greater than 12 months after surgery (26,27). Patients with canal stenosis receiving surgery obtain hearing results approximately 10 dB better than patients with total bony atresia (35). Ultimately, these patients with canal stenosis typically require surgery to prevent canal cholesteatoma. Atresiaplasty can be done to create a patent canal which would allow the patient to accommodate an ear level hearing aid.
There is concern for recurrent canal stenosis that occurs in up to one third of patients postoperatively (33). When the ear canal is lined with a skin graft, cerumen and debris typically do not migrate laterally, and therefore the patient requires ongoing and lifelong otologic care for aural hygiene. Parents need to be educated on the required maintenance and care of the reconstructed ear canal. Tolerance of ear cleaning under a microscope in the clinic requires a certain level of maturity in the patient and should be a factor when considering aural atresia repair. Swimming may lead to issues of recurrent ear drainage (7%–24%) after canal reconstruction for atresia (41).
Ear canal reconstruction may be necessary rather than optional when there is canal stenosis and concern for cholesteatoma. The presence of canal cholesteatoma may necessitate atresia repair prior to the normal sequence for optimal microtia reconstruction.
Ultimately, an experienced atresia surgeon must candidly discuss the predicted hearing gain expected from surgery compared to the available implantable bone conduction hearing devices, as well as the option of fitting an ear-level air-conduction hearing aid in the reconstructed ear canal as a good means to further improve hearing.
In discussing the spectrum of reconstructive options, observation should be presented with associated benefits and drawbacks. A select group of patients are satisfied and confident with the microtic ear, elect to wear their hair long and cover the ear, and/or prefer not to undertake the potential surgical and anesthetic risks.
A realistic silicone ear prosthesis can be made for individuals of any age (Figure 8). Parents desiring an ear prosthesis generally seek treatment between four and five years, or school-aged. The appropriate time to consider initiation of prosthetic use is the developmental age at which a child is able to care for and comply with wearing the prosthetic. Depending on the location of microtia ear, an ear prosthesis may be made to cover the ear and provide the desired symmetry with the contralateral ear using Computer-Aided Design and 3D printing. The anaplastologist factors in future tissue growth for the adhesive-held prosthetic to ensure maintaining a proper and secure fit. The anaplastologist then meticulously paints the prosthesis to match the skin (42). This expertise and skill is crucial for a satisfied patient. The prosthetic ear can also be made to cover an ear reconstruction that resulted in a poor cosmetic outcome. No surgery is required for an adhesive-held prosthesis, preserving the choice for more invasive interventions when the patient is mature enough to participate in the decision-making process.
Surgically placed osseointegrated implants can allow for a more secure attachment of the prosthetic by using a bar/clip, snap, or magnetic attachment, and can be implanted after the age of 5 years. The anaplastologist must be involved in the surgical planning as the location of the implant is paramount to a successful outcome of the prosthetic (43). Some patients may have chronic inflammation surrounding the percutaneous abutments, similar to those with osseointegrated percutaneous abutments for bone conduction sound processors. Placing the implants and/or excising the external ear will have serious ramifications, including precluding future repair, on microtia reconstruction. For this reason, it is imperative that the patient be mature enough to participate in the decision-making process.
A successful prosthesis should fit well and be secure enough to use the entire day without worry (44). A prosthesis can be pierced with an earring and enables the use of corrective glasses, oxygen tubes, hearing aids, and face masks. A well-made prosthesis should last two to three years and be remade with a new fitting to account for soft tissue changes. There may be some discoloration over time and degradation of the thin translucent edges of the prosthesis.
Other considerations include the potential need to remove the prosthesis during contact sports and swimming. Care of the prosthetic ear includes removal at night before sleep and daily cleaning.
Parents should be counseled that auricular prostheses and their replacement after normal wear and tear are generally covered by most insurances, though coverage varies. Ear prostheses provide the appearance of a normal outer ear with the psychological benefits of a symmetrical and realistic ear without the risk of surgery. The thought of a prosthesis inadvertently falling off may impact a child's self-image; this can be overcome with counseling and comparisons of the prosthetic ear to other commonly worn medical devices such as glasses and hearing aids.
Alloplastic reconstruction, such as Medpor® and SuPor®, utilizes a high-density porous polyethylene implant covered with a well-vascularized fascial flap, typically the temporoparietal fascia flap (TPFF), which is then covered in remnant temporoparietal skin and skin graft (Figure 9). Alloplastic reconstruction is typically done as a single-stage surgery. Alloplastic reconstruction can be done as early as three years of age (45). Proponents of early reconstruction emphasize the avoidance of psychosocial bullying when the microtic ear is reconstructed prior to integration into school. Critics of early reconstruction emphasize the importance of the child's informed assent in a significant, life-altering, elective procedure. Quality of life improvements have been reported following alloplastic reconstruction as well (46).
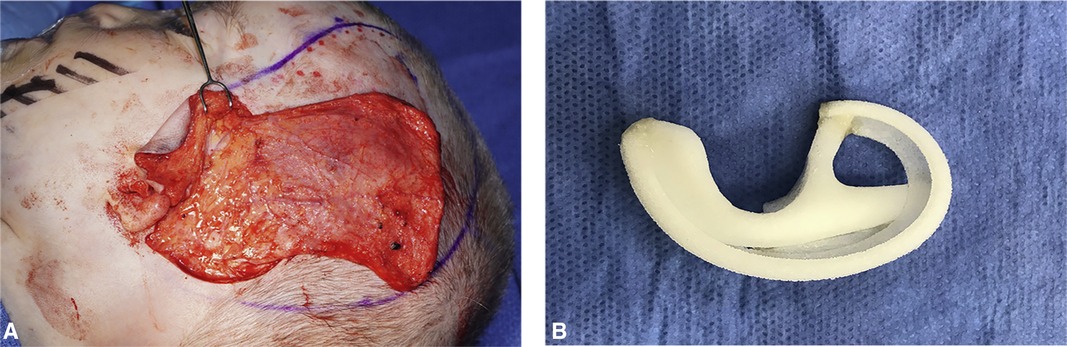
Figure 9. (A) Elevated temporoparietal fascia flap (TPFF). (B) Porous polyethylene auricular framework.
The most compelling advantages of alloplastic reconstruction are that the reconstruction is done in one stage, and the age at the time of surgery is not dependent on the child's growth and size, as is with cartilage reconstruction (see below).
Disadvantages of alloplastic reconstruction include the overall risk of extrusion, fracture, and infection. Some feel this is amplified because the alloplastic reconstructed ear is insensate. Without sensation, the risk of injury, infection, and extrusion may be elevated and is lifelong. If extrusion does happen, the TPFF, often used for salvage in autogenous cartilage reconstruction, has already been exhausted in the initial repair. However, other fascial options such as the occipital artery flap may be utilized in salvage surgery. When the reconstructed ear's vascularity depends on a single vessel in a pedicled fascial flap, pressure may impact the viability. From the patient perspective, comfort with sleeping can be an issue that some children manage by sleeping with a special pillow around the ear to relieve the pressure.
An alloplastic ear reconstruction performed at a younger age will be larger than the opposite ear to account for future ear growth; patients and family must be advised of and agreeable to this initial size discrepancy for younger children. The first Medpor ear was done in 1991. Beyond that time span, we do not have more longitudinal outcomes or long-term data of this reconstruction technique (45).
Cases in which an alloplastic reconstruction may be most advantageous would be in instances of bilateral microtia, reducing the total number of surgeries and issues of symmetry, and for children who are extremely small for age (such as certain syndromic children), which otherwise would require the patient to wait for their teen years to have enough cartilage stock for adequate reconstruction. Also, for children with low hair lines, a technique that relies on coverage with TPFF is advantageous unless laser hair removal is pursued. Auricular reconstruction in older adults may be done with alloplastic reconstruction to eliminate the risk of having calcified cartilage stock which is difficult to carve.
Use of a patient's own, or “autologous,” rib for auricular reconstruction is regarded by many as the gold standard for surgical microtia reconstruction (Figure 10). Decades of follow-up have shown that a properly executed surgery can provide patients with a stable, life-long reconstruction. This surgery is completed in 2–4 stages, depending on the surgeon's technique. Typically, this is started when the patient is at least six years of age.
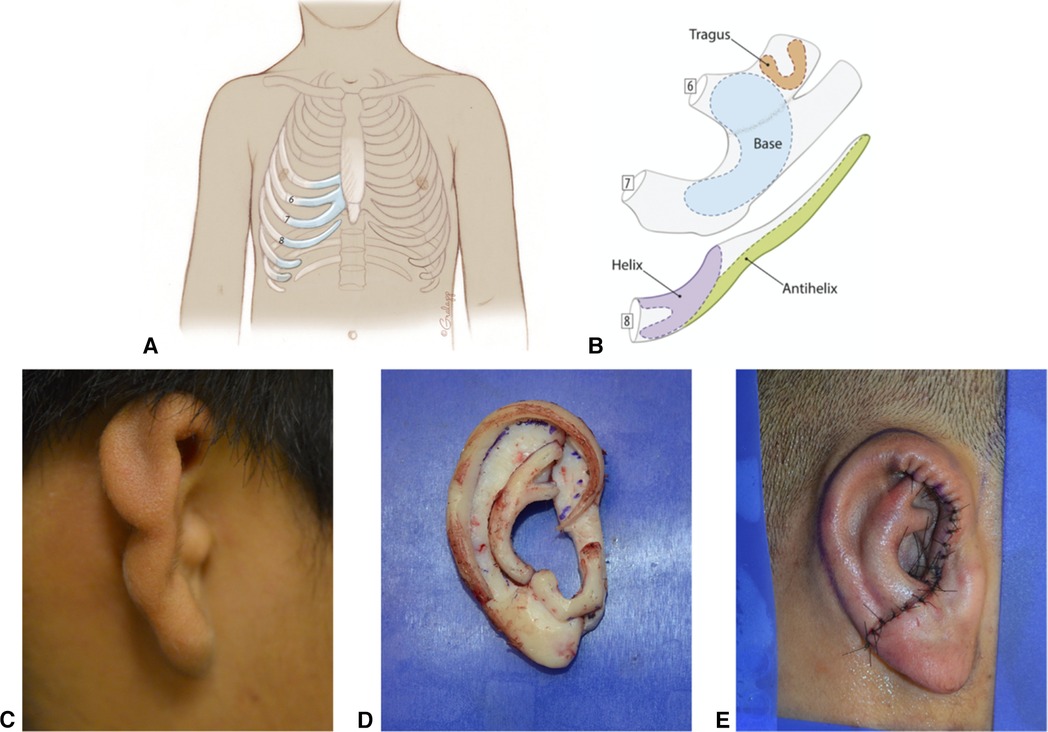
Figure 10. Autologous Cartilage Reconstruction. (A) Typical areas of costochondral cartilage harvest. (B) Example of auricular subunits created from costochondral cartilage. (C) Pre surgical picture of microtic ear. (D) Assembled costochondral auricular framework. (E) Immediate postoperative appearance after Stage 1 costochondral cartilage reconstruction.
Tanzer introduced the surgical techniques, which Brent subsequently refined to achieve acceptable autologous ear reconstruction results (47). Variations on Brent's 4-stage technique remain the most utilized methods in the United States. With the Brent method, a smaller quantity of cartilage than other techniques allows for reconstruction beginning around six years of age. Nagata and Firmin described a 2-stage autologous approach that imparts greater detail but necessitates larger cartilage volumes. Reconstruction using Nagata and Firmin techniques is typically offered at later ages when the child had a chest circumference of at least 60 cm, the standard indicator of sufficient rib stock (48–52).
An advantage of cartilage reconstruction is the extensive knowledge base that has been amassed due to the broad use of this approach worldwide. The reconstructed ear has sensation and vascularity compared to an alloplastic ear. Cartilage reconstruction allows for the creation of an individualized ear, which is carved by the surgeon, to create symmetry with the opposite ear. This is ideal for various forms of microtia, particularly grades of microtia where part of the remnant ear can be saved, and part of the defect is reconstructed, such as in grade 2 microtia. Cartilage reconstruction allows the surgeon to reconstruct only the missing subunits of the ear, preserving part of the microtia ear as desired, which may not be possible with alloplastic techniques.
Some argue that an older child of age 6–10 years can be more involved in the decision-making process, more invested in the surgery, and a more active participant in the care of the ear. In addition, the older child tends to have the opposite ear very close to adult size, allowing for a true size match of the reconstructed ear rather than an approximation based on projected growth. Others argue that later reconstruction in school-aged children can lead to poor self-image and self-concept, though the age that this occurs is controversial and likely different for every child (53).
One disadvantage of cartilage techniques is the requirement for 2–4 stages, separated in time by 3–4 months, depending on the surgeon's technique. The earliest timing of surgery depends on the patient's chest size to allow for enough cartilage rib stock to create the ear, therefore necessitating a longer waiting period before surgery. There is also donor site morbidity at the time of surgery and a scar along the chest, though most patients do not report long-term adverse side effects for donor site issues. Patients do not have chest wall deformities or limitations in future activities or sports after rib cartilage harvest. Long term complications reported include framework resorption and wire extrusion, though overall, comparisons show less long-term complications using autologous cartilage compared to porous polyethylene (54). Pneumothorax is an uncommon short-term complication of rib harvest, and typically easily treated and resolved without lengthening hospital stay or causing any long term consequences.
Several factors should be considered in the timing of cartilage-based reconstruction. The patient's age, size, and maturity are all critical factors.
Age guidelines are general, as many factors such as genetics, diet, and medical comorbidities are strong determinants of a child's size. A chest circumference of 60 cm, measured at the xiphoid level has classically been the minimum indicator of the adequacy of rib stock. Classically, CT scans of the chest were performed to evaluate the costal cartilage, allowing for a view of the synchondrosis and evidence of calcification. However, with increasing awareness of radiation exposure in children with computerized tomography, this has been less practiced. More recently, ultrasound of the cartilage has been reported to accurately assess rib dimensions without the need for ionizing radiation exposure (55). Multidisciplinary evaluation and careful conversation with the child and family also help determine psychological and anatomic readiness.
Timing is critical when considering aural atresia repair in relation to auricular reconstruction. The desire of many families for aural atresia repair at a young age while deferring auricular reconstruction until a later time must be resisted if one wants to pursue autologous cartilage reconstruction. The local tissue changes resulting from atresia repair or a poorly placed osseointegrated implant can complicate or preclude successful cartilage reconstruction (56).
Any technique, alloplastic or cartilage reconstruction, requires a high level of surgical expertise with an experienced surgeon to achieve reliable outcomes. Surgeons who perform microtia reconstruction should regularly perform the surgery and have advanced training.
There are three primary techniques reviewed here, which are commonly used in variations customized by the reconstructive surgeon (Table 4). Historically, Tanzer described a six stage technique which serves as a foundation of the Brent technique (57).
This is the first modern microtia technique to gain wide popularity as adapted from Tanzer. It is traditionally accomplished in 4 stages (58).
• 1st stage: Costal cartilages 6–9 are harvested and carved. The cartilage framework is then placed deep to the retro and peri-auricular skin of the remnant.
• 2nd stage: The lobule of the microtia remnant is transposed to the framework.
• 3rd stage: The ear is elevated with the use of a skin graft placed in the posterior aspect of the ear and the advancement of the retroauricular skin.
• 4th stage: A tragus is created from contralateral conchal cartilage, and the conchal bowl of the construct is deepened.
More recent variations on the Brent technique have reduced the number of stages to 3 by combining the tragus formation with the first stage, creating a tragus with the framework, and often combining the final stage with the placement of an osseointegrated hearing implant (59).
Satoro Nagata revolutionized auricular reconstruction with the description of a 2 staged technique, where reconstruction of the tragus and transposing of the lobule are both done in the first stage (48–50). Françoise Firmin went on to describe what many consider a modified-Nagata technique, characterized by projection pieces of the framework and a formal classification of skin approaches which she calls Type 1, 2, 3a and 3b. This technique provides a standardized approach to auricular reconstruction which takes into account the microtia remnant and missing contours to be reproduced (51,52). These techniques use autologous costal cartilage to reconstruct the ear in 2 stages (48–51, 60–61).
• 1st stage: Costal cartilage 6–9 are harvested and carved to create a framework that includes all the structures of the ear that need to be reconstructed, including the tragus. The cartilage framework is then placed deep to the retro and peri-auricular skin of the remnant with concurrent lobule transposition. Different grades of microtia require variations in skin approaches.
• 2nd stage: Elevation of the ear is performed by incising the skin around the framework. Additional cartilage is placed posteriorly to project the ear. This is either obtained through a second harvest or is derived from excess cartilage banked in the soft tissues of the chest at the time of the first operation. In the Nagata technique, the cartilage used for elevation in the postauricular sulcus is covered with vascularized TPFF and a skin graft.
Firmin describes four techniques to elevate the ear and recreate the retroauricular sulcus, which she categorizes as Type A-D elevation. Type A is the standard Nagata technique utilizing the TPFF fascia to provide vascularity to the elevation cartilage. Type B is the Brent technique, where no cartilage is used, the posterior ear is elevated and covered with a skin graft, and the retroauricular skin is advanced. Type C describes the use of random, posteriorly based fascia from the mastoid region, turned over to provide vascularity to the elevation cartilage, which is then covered by a skin graft. Type D is known as the tunnel technique, where tunnels are created within the enveloping soft tissue beneath the framework, where cartilage blocks are placed to prop the ear forward. As these are tunneled, they do not require coverage with a facial flap. The retroauricular sulcus is then covered with a skin graft. The elevation technique chosen depends on patient factors such as the degree of projection of the other ear, the hairline, and the amount of cartilage available for the second stage.
Ruhong Zhang et al. described the quality of retroauricular fascia for use in staged auricular reconstruction, finding that more superiorly based fascia has better vascularity, elasticity, and lymphatics (62). This led to his description of a posterior-superior fascial flap that is rotated down to cover the elevation cartilage (63).
The advantage of the 2 stage techniques, though they require more expansive cartilage frameworks, is a reconstructed ear with more detail and depth. However, these techniques are more demanding on the skin and may result in increased risk of wound complications such as small areas of dehiscence and problems with skin viability postoperatively. These can typically be managed with local wound care or closures. The advantage of the Brent technique is that each stage allows the skin to heal in between, thus reducing the risk of wound complications.
Figure 11 describes the recommended timing of key events for the hearing management and reconstruction of microtia and aural atresia (Figure 11).
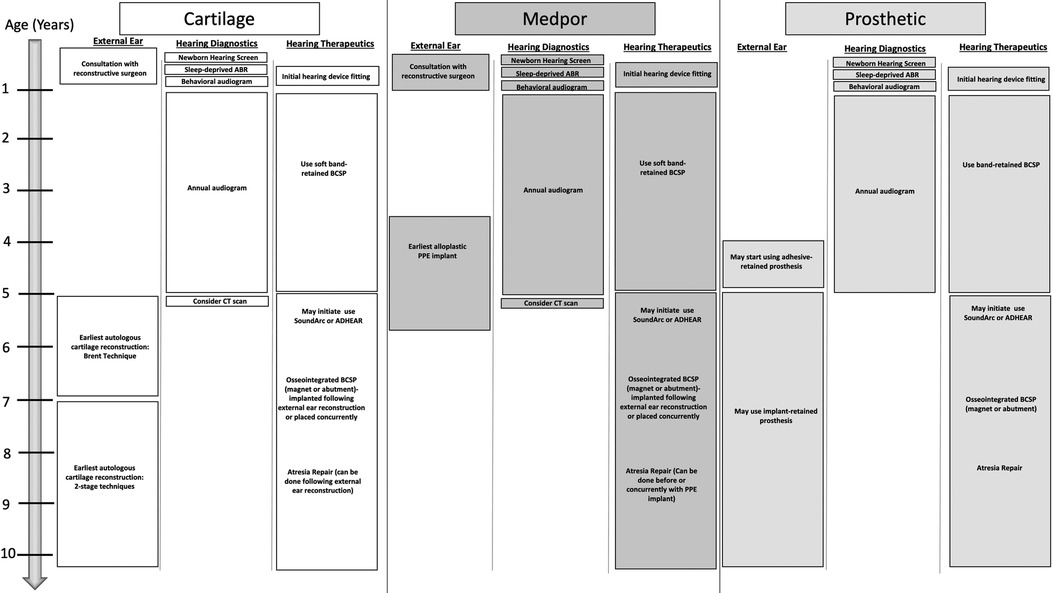
Figure 11. Recommended timing of key events for the hearing management and reconstruction of microtia and aural atresia.
Age: Intervention recommended for the hearing needs of the child
• Newborn: Hearing screening
• 1–3 months: Sleep deprived ABR
• 6–9 months: Behavioral audiogram
• 3–6 months: Consultation on hearing devices (bilateral cases should be fit with softband device by 4–6 months of age)
• 12months-5 years: audiological testing and surveillance every 6–12 months
• 5 years: Consider CT scan for hearing implants and canal reconstruction candidacy. Consider waiting for surgery to implant bone conduction device until after or in coordination with concurrent microtia reconstruction. Consider CochlearTM BAHA® SoundArc or Adhesive gel modes of Bone conduction sound processor use.
Age: Intervention recommended for microtia reconstruction
• Newborn: Consultation with reconstructive surgeons for parental counseling regarding reconstructive options for the future
• 3–5 years: Earliest age for consideration for alloplastic porous polyethylene reconstruction. Any ear canal surgery should be done before or concurrently with alloplastic reconstruction
• 5 years: Earliest age for an osseointegrated anchor for prosthetic use. Prosthetics may be worn with adhesive prior to age 5.
• 5–9 years: Earliest age for autologous cartilage reconstruction. Any ear canal surgery is ideally done after microtia reconstruction.
Caregivers of children born with microtia and atresia face numerous options for their hearing and reconstructive needs, the recommendations for which are often provided by separate and non-communicating disciplines. Plans for hearing augmentative devices, hearing reconstructive surgeries, and auricular reconstruction should be provided in a cohesive manner, with close communication between the atresia repair/hearing rehabilitation surgeon and the microtia reconstruction surgeon. This guideline aims to integrate the hearing and reconstructive recommendations with a timeline that allows for the understanding of all options available, realizing that every patient is unique and requires an individualized plan.
MT T is first author and KW C is the senior and corresponding author. All of the other authors contributed to at least one sub-section of the manuscript and also helped review/edit the others. All authors contributed to the article and approved the submitted version.
Recommendations are based on the collective opinion of members of this group. Any person seeking to consult or apply this report to patient care is expected to use medical judgment in the context of the individual patient and institution. Finally, not all treatment options discussed here may be available or covered by insurance, depending on the region, practice setting, state laws, or patient's insurance company.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer [BK] declared a past collaboration with the author [KC] to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CT, computer tomography; MRI, magnetic resonance imaging; Hz, hertz; 3D, 3 dimensional; ABR, auditory brainstem response; CHL, conductive hearing loss; BCSP, bone conduction sound processor; FDA, food and drug administration, EAC, external auditory canal; TPFF, temporoparietal fascia flap; EI, early intervention.
1. Hartzell LD, Chinnadurai S. Microtia and related facial anomalies. Clin Perinatol. (2018) 45(4):679–97. doi: 10.1016/j.clp.2018.07.007
2. Canfield MA, Langlois PH, Nguyen LM, Scheuerle AE. Epidemiologic features and clinical subgroups of anotia/microtia in Texas. Birth Defects Res A Clin Mol Teratol. (2009) 85(11):905–13. doi: 10.1002/bdra.20626
3. Stern RS, Rosa F, Baum C. Isotretinoin and pregnancy. J Am Acad Dermatol. (1984) 10(5, Part 1):851–4. doi: 10.1016/S0190-9622(84)80142-5
4. Fraser AG, Watson ACH. The surgical treatment of microtia: a long-term review of 17 patients. Br J Plast Surg. (1982) 35(2):185–94. doi: 10.1016/0007-1226(82)90162-X
5. Luquetti DV, Heike CL, Hing AV, Cunningham ML, Cox TC. Microtia: epidemiology and genetics. Am J Med Genet A. (2012) 158A(1):124–39. doi: 10.1002/ajmg.a.34352
6. Van Bennekom CM, Mitchell AA, Moore CA, Werler MM. National birth defects prevention study. Vasoactive exposures during pregnancy and risk of microtia. Birth Defects Res A Clin Mol Teratol. (2013) 97(1):53–9. doi: 10.1002/bdra.23101
7. Zhang TY, Bulstrode N, Chang KW, Cho YS, Frenzel H, Jiang D, et al. International consensus recommendations on microtia, aural atresia and functional ear reconstruction. J Int Adv Otol. (2019) 15(2):204–8. doi: 10.5152/iao.2019.7383
8. Joint committee on infant hearing. Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv. (2019) 4(2):1–44. doi: 10.15142/fptk-b748
9. Koenig JL, Amoils M, Grade MM, Chang KW, Truong MT. Renal ultrasound abnormalities in children with syndromic and non-syndromic microtia. Int J Pediatr Otorhinolaryngol. (2018) 113:173–6. doi: 10.1016/j.ijporl.2018.07.026
10. CDC. Hearing Loss Treatment and Intervention Services | CDC. Centers for Disease Control and Prevention. Published March 21, 2019. Accessed May 13, 2021. https://www.cdc.gov/ncbddd/hearingloss/treatment.html
11. Hol MKS, Cremers CWRJ, Coppens-Schellekens W, Snik AFM. The BAHA softband. A new treatment for young children with bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol. (2005) 69(7):973–80. doi: 10.1016/j.ijporl.2005.02.010
12. Vyskocil E, Liepins R, Kaider A, Blineder M, Hamzavi S. Sound localization in patients with congenital unilateral conductive hearing loss with a transcutaneous bone conduction implant. Otol Neurotol. (2017) 38(3):318–24. doi: 10.1097/MAO.0000000000001328
13. Wazen JJ, Spitzer J, Ghossaini SN, Kacker A, Zschommler A. Results of the bone-anchored hearing aid in unilateral hearing loss. Laryngoscope. (2001) 111(6):955–8. doi: 10.1097/00005537-200106000-00005
14. Kesser BW, Krook K, Gray LC. Impact of unilateral conductive hearing loss due to aural atresia on academic performance in children. Laryngoscope. (2013) 123(9):2270–5. doi: 10.1002/lary.24055
15. Jensen DR, Grames LM, Lieu JEC. Effects of aural atresia on speech development and learning: retrospective analysis from a multidisciplinary craniofacial clinic. JAMA Otolaryngol - Head Neck Surg. (2013) 139(8):797–802. doi: 10.1001/jamaoto.2013.3859
16. Priwin C, Jönsson R, Hultcrantz M, Granström G. BAHA In children and adolescents with unilateral or bilateral conductive hearing loss: a study of outcome. Int J Pediatr Otorhinolaryngol. (2007) 71(1):135–45. doi: 10.1016/j.ijporl.2006.09.014
17. Saliba I, Woods O, Caron C. BAHA Results in children at one year follow-up: a prospective longitudinal study. Int J Pediatr Otorhinolaryngol. (2010) 74(9):1058–62. doi: 10.1016/j.ijporl.2010.06.004
18. Liu Y, Zhao C, Yang L, Chen P, Yang J, Wang D, et al. Characteristics of sound localization in children with unilateral microtia and atresia and predictors of localization improvement when using a bone conduction device. Front Neurosci. (2022) 16:973735. doi: 10.3389/fnins.2022.973735
19. Yin G, Zeng X, Li P. Skin reactions caused by bone-anchored hearing aid (BAHA) implantation. J Otol. (2015) 10(4):159–62. doi: 10.1016/j.joto.2016.01.001
20. Kiringoda R, Lustig LR. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol. (2013) 34(5):790–4. doi: 10.1097/MAO.0b013e318291c651
21. Šikolová S, Urík M, Hošnová D, Kruntorád V, Bartoš M, Motyka O, et al. Two bonebridge bone conduction hearing implant generations: audiological benefit and quality of hearing in children. Eur Arch Otorhinolaryngol. Published online September (2021) 8:3387–98. doi: 10.1007/s00405-021-07068-x
22. You P, Choi A, Drob J, Hunsaker SM, Liu YCC, Silva R. Early outcomes of a new active transcutaneous bone conduction implant in pediatric patients. Otol Neurotol. (2022) 43(2):212–8. doi: 10.1097/MAO.0000000000003426
23. Goldstein MR, Bourn S, Jacob A. Early osia® 2 bone conduction hearing implant experience: nationwide controlled-market release data and single-center outcomes. Am J Otolaryngol. (2021) 42(1):102818. doi: 10.1016/j.amjoto.2020.102818
24. Zernotti ME, Di Gregorio MF, Galeazzi P, Tabernero P. Comparative outcomes of active and passive hearing devices by transcutaneous bone conduction. Acta Otolaryngol. (2016) 136(6):556–8. doi: 10.3109/00016489.2016.1143119
25. Florentine MM, Virbalas J, Chan DK. Early surgical and audiologic outcomes of active, transcutaneous, osseointegrated bone-conduction hearing device (osia 2® system) placement. Int J Pediatr Otorhinolaryngol. (2022) 156:111114. doi: 10.1016/j.ijporl.2022.111114
26. Nadaraja GS, Gurgel RK, Kim J, Chang KW. Hearing outcomes of atresia surgery versus osseointegrated bone conduction device in patients with congenital aural atresia: a systematic review. Otol Neurotol. (2013) 34(8):1394–9. doi: 10.1097/MAO.0b013e3182a36065
27. Imbery TE, Gray L, Champaloux E, Kesser BW. Long-term audiometric outcomes after atresiaplasty for congenital aural atresia. Otol Neurotol. (2020) 41(3):371–8. doi: 10.1097/MAO.0000000000002523
28. Jahrsdoerfer RA, Yeakley JW, Aguilar EA, Cole RR, Gray LC. Grading system for the selection of patients with congenital aural atresia. Am J Otol. (1992) 13(1):6–12. doi: 10.1016/S0196-0709(79)80003-4
29. Shonka DC, Livingston WJ, Kesser BW. The jahrsdoerfer grading scale in surgery to repair congenital aural atresia. Arch Otolaryngol Head Neck Surg. (2008) 134(8):873–7. doi: 10.1001/archotol.134.8.873
30. Oliver ER, Lambert PR, Rumboldt Z, Lee FS, Agarwal A. Middle ear dimensions in congenital aural atresia and hearing outcomes after atresiaplasty. Otol Neurotol. (2010) 31(6):946–53. doi: 10.1097/mao.0b013e3181e8f997
31. Kabadi SJ, Ruhl DS, Mukherjee S, Kesser BW. Semiautomated middle ear volume measurement as a predictor of postsurgical outcomes for congenital aural atresia. AJNR Am J Neuroradiol. (2018) 39(2):355–61. doi: 10.3174/ajnr.A5475
32. Imbery TE, Maldonado M, Mukherjee S, Kesser BW. Relationship between middle ear volume and long-term audiological outcomes in congenital aural atresia repair. Otol Neurotol. (2019) 40(6):782–8. doi: 10.1097/MAO.0000000000002233
33. Kesser BW, Chang KW. Chapter 197: evaluation and management of congenital aural atresia. In: Cummings otolaryngology head and neck surgery. 7th ed. Philadephia, PA: Elsevier; (2020):2935–49.
34. Chang KW, Truong MT. Repair of congenital aural atresia. In: Jackler RK, editor. Ear surgery illustrated. Chapters 3.4. New York, NY: Thieme Medical Publishers; (2019):110–8.
35. Li C, Zhang T, Fu Y, Qing F, Chi F. Congenital aural atresia and stenosis: surgery strategies and long-term results. Int J Audiol. (2014) 53(7):476–81. doi: 10.3109/14992027.2014.890295
36. Chen Y, Zhang T. Modified meatoplasty for external auditory canal stenosis with endoaural-conchal incision. Otol Neurotol. (2015) 36(1):1–3. doi: 10.1097/MAO.0000000000000667
37. Casazza GC, Jonas RH, Kesser BW. Congenital aural stenosis with cholesteatoma. Otol Neurotol. (2022) 43(3):320–7. doi: 10.1097/MAO.0000000000003440
38. de Alarcon A, Jahrsdoerfer RA, Kesser BW. Congenital absence of the oval window: diagnosis, surgery, and audiometric outcomes. Otol Neurotol. (2008) 29(1):23–8. doi: 10.1097/mao.0b013e31815dbb28
39. Dedhia K, Yellon RF, Branstetter BF, Egloff AM. Anatomic variants on computed tomography in congenital aural atresia. Otolaryngol–Head Neck Surg. (2012) 147(2):323–8. doi: 10.1177/0194599812442866
40. Mukherjee S, Kesser BW, Raghavan P. The “boomerang” malleus-incus complex in congenital aural atresia. AJNR Am J Neuroradiol. (2014) 35(11):2181–5. doi: 10.3174/ajnr.A4022
41. Li CL, Dai PD, Yang L, Zhang TY. A meta-analysis of the long-term hearing outcomes and complications associated with atresiaplasty. Int J Pediatr Otorhinolaryngol. (2015) 79(6):793–7. doi: 10.1016/j.ijporl.2015.03.032
42. Tanner P, Leachman S, Boucher K, Ozçelik TB. Depigmented skin and phantom color measurements for realistic prostheses. Skin Res Technol. (2014) 20(1):37–42. doi: 10.1111/srt.12080
43. Ozçelik TB, Tanner PB. A surgical guide for craniofacial implant placement for an implant-retained auricular prosthesis. J Prosthet Dent. (2010) 103(4):253–5. doi: 10.1016/S0022-3913(10)60040-6
44. Tanner PB, Mobley SR. External auricular and facial prosthetics: a collaborative effort of the reconstructive surgeon and anaplastologist. Facial Plast Surg Clin N Am. (2006) 14(2):137–45. vi-vii. doi: 10.1016/j.fsc.2006.01.003
45. Stephan S, Reinisch J. Auricular reconstruction using porous polyethylene implant technique. Facial Plast Surg Clin N Am. (2018) 26(1):69–85. doi: 10.1016/j.fsc.2017.09.009
46. Braun T, Gratza S, Becker S, Schwentner I, Stelter K, Patscheider M, et al. Auricular reconstruction with porous polyethylene frameworks: outcome and patient benefit in 65 children and adults. Plast Reconstr Surg. (2010) 126(4):1201–12. doi: 10.1097/PRS.0b013e3181ebe391
47. Brent B. Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases. Plast Reconstr Surg. (1992) 90(3):355–74.; discussion 375–376. doi: 10.1097/00006534-199209000-00001
48. Nagata S. Modification of the stages in total reconstruction of the auricle: part I. Grafting the three-dimensional costal cartilage framework for lobule-type microtia. Plast Reconstr Surg. (1994) 93(2):221–30.; discussion 267–268. doi: 10.1097/00006534-199402000-00001
49. Nagata S. Modification of the stages in total reconstruction of the auricle: part II. Grafting the three-dimensional costal cartilage framework for concha-type microtia. Plast Reconstr Surg. (1994) 93(2):231–42.; discussion 267–268. doi: 10.1097/00006534-199402000-00002
50. Nagata S. Modification of the stages in total reconstruction of the auricle: part III. Grafting the three-dimensional costal cartilage framework for small concha-type microtia. Plast Reconstr Surg. (1994) 93(2):243–53.; discussion 267-268. doi: 10.1097/00006534-199402000-00003
51. Firmin F, Marchac A. A novel algorithm for autologous ear reconstruction. Semin Plast Surg. (2011) 25(4):257–64. doi: 10.1055/s-0031-1288917
52. Firmin F. Ear reconstruction in cases of typical microtia. Personal experience based on 352 microtic ear corrections. Scand J Plast Reconstr Surg Hand Surg. (1998) 32(1):35–47. doi: 10.1080/02844319850158930
53. Li D, Chin W, Wu J, Zhang Q, Xu F, Xu Z, et al. Psychosocial outcomes among microtia patients of different ages and genders before ear reconstruction. Aesthetic Plast Surg. (2010) 34(5):570–6. doi: 10.1007/s00266-010-9502-1
54. Ronde EM, Esposito M, Lin Y, van Etten-Jamaludin FS, Bulstrode NW, Breugem CC. Long-term complications of microtia reconstruction: a systematic review. J Plast Reconstr Aesthet Surg. (2021) 74(12):3235–50. doi: 10.1016/j.bjps.2021.08.001
55. Miller JE, Farlow JL, Knecht EM, Sanchez R, Kim JC, Zopf DA. Ultrasonographic assessment of costochondral cartilage for microtia reconstruction. Laryngoscope. (2019) 129(5):1078–80. doi: 10.1002/lary.27390
56. Steffen A, Wollenberg B, König IR, Frenzel H. A prospective evaluation of psychosocial outcomes following ear reconstruction with rib cartilage in microtia. J Plast Reconstr Aesthetic Surg JPRAS. (2010) 63(9):1466–73. doi: 10.1016/j.bjps.2009.09.005
57. Tanzer, Radford C. Total reconstruction of the external ear. Plast Reconstr Surg Transplant Bull. (1959) 23(1):1–15. doi: 10.1097/00006534-195901000-00001
58. Brent B. Microtia repair with rib cartilage grafts: a review of personal experience with 1000 cases. Clin Plast Surg. (2002) 29(2):257–71. vii. doi: 10.1016/s0094-1298(01)00013-x
59. Bonilla A. Pediatric microtia reconstruction with autologous rib: personal experience and technique with 1000 pediatric patients with microtia. Facial Plast Surg Clin N Am. (2018) 26(1):57–68. doi: 10.1016/j.fsc.2017.09.008
60. Firmin F, Düsseldorp J, Marchac A. Auricular reconstruction. New York, NY: Thieme Medical Publishers; 2017.
61. Truong MT, Chang KW. Microtia repair. In: Ear surgery illustrated. Chapter 3.3. New York, NY: Thieme Medical Publishers; 2019:93–109.
62. Li Y, Cui C, Zhang R, Zhang Q, Xu Z, Xu F, et al. Anatomical and histological evaluation of the retroauricular fascia flap for staged auricular reconstruction. Aesthetic Plast Surg. (2018) 42(3):625–32. doi: 10.1007/s00266-018-1098-x
Keywords: microtia, aural atresia, aural atresia surgery, microtia surgery, auricular reconstruction, ear canal stenosis, bone conduction implant (BCI), ear canal reconstruction
Citation: Truong MT, Liu YC, Kohn J, Chinnadurai S, Zopf DA, Tribble M, Tanner PB, Sie K and Chang KW (2022) Integrated microtia and aural atresia management. Front. Surg. 9:944223. doi: 10.3389/fsurg.2022.944223
Received: 14 May 2022; Accepted: 16 November 2022;
Published: 26 December 2022.
Edited by:
Sharon L. Cushing, University of Toronto, CanadaReviewed by:
Dylan Chan, University of California, United States© 2022 Truong, Liu, Kohn, Chinnadurai, Zopf, Tribble, Tanner, Sie and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kay W. Chang a2F5Y2hhbmdAc3RhbmZvcmQuZWR1
Specialty Section: This article was submitted to Otorhinolaryngology - Head and Neck Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.