
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Surg., 31 August 2022
Sec. Visceral Surgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.943544
This article is part of the Research TopicBariatric Surgery: Its influence on the development, diagnosis and treatment of tumorsView all 5 articles
 Robert C. Ross1
Robert C. Ross1 Yetunde M. Akinde2
Yetunde M. Akinde2 Philip R. Schauer3
Philip R. Schauer3 Carel W. le Roux4
Carel W. le Roux4 Donal Brennan5
Donal Brennan5 Amelia M. Jernigan6
Amelia M. Jernigan6 Marco Bueter7
Marco Bueter7 Vance L. Albaugh1,3*
Vance L. Albaugh1,3*
The obesity pandemic continues to contribute to a worsening burden of disease worldwide. The link between obesity and diseases such as diabetes, cardiovascular disease, and cancer has been well established, yet most patients living with obesity remain untreated or undertreated. Metabolic and bariatric surgery is the most effective and durable treatment for obesity, is safe, and may have a protective benefit with respect to cancer incidence. In this review, an overview of the link between obesity, metabolic surgery, and cancer is discussed with emphasis on indications for endometrial cancer, the malignancy most strongly associated with obesity. Considerable evidence from retrospective and prospective cohort studies supports a decreased risk of endometrial cancer in patients with obesity who undergo bariatric surgery compared with nonsurgical controls. Survivors of endometrial cancer are at increased risk of poor health outcomes associated with obesity, and women with endometrial cancer are more likely to die of cardiovascular disease and other obesity-related illnesses than of the malignancy itself. Recent advances in anticancer drug therapies have targeted pathways that may also be therapeutically altered with metabolic surgery. Metabolic surgery has significant potential to enter the treatment paradigm for endometrial cancer, and gynecologic oncologist visits present an opportunity to identify patients who may benefit the most.
Obesity, defined by the World Health Organization as excess or abnormal fat that causes a deterioration in health, has surpassed epidemic proportions worldwide and continues to contribute to a worsening burden of diseases like diabetes and cardiovascular disease (1). Aside from its significant financial impact, patients with a BMI >30 kg/m2 have been associated with a decreased quality of life and a 25% reduction of disease-free life years (2). Although the link to cardiovascular mortality is long-established, a BMI >30 kg/m2 has more recently been linked to cancer (3, 4). Despite this evidence of the association of obesity with cardiovascular disease and cancer, most patients living with obesity remain untreated or undertreated (5).
Metabolic and bariatric surgery is by far the most effective and durable treatment for obesity but remains grossly underutilized. Various benefits of bariatric surgery have been demonstrated in numerous clinical studies that have uniformly identified surgical obesity treatment with increased longevity (6), improved quality of life (7), and decreased cardiovascular morbidity and mortality (8–12). In addition to the studies identifying weight loss in general with cancer-specific benefits (13), bariatric surgery may also have a protective benefit with respect to cancer incidence (14). Thus, recent scientific interest has shifted focus to the potential mechanisms underlying these clinical benefits.
In the following, a general overview of the link between obesity and cancer is reviewed, as well as a focus on the potential for cancer protection following bariatric surgery, specifically with respect to endometrial cancer—the leading malignancy most strongly associated with obesity. We then explore the promise and potential for bariatric surgery to fit into the treatment algorithm for endometrial cancer.
The link between a BMI >30 kg/m2 and cancer is well established. Globally, an estimated 3.6% of all cancers and 12.8% of obesity-related cancers are associated with increasing BMI (15). In general, the effect of obesity on cancer physiology varies depending on the malignancy. As the second and third leading causes of death in the United States, obesity has been implicated as a causal factor in colorectal cancer (16, 17) as well as pancreatic cancer (18, 19). There are 13 types of cancer in the organs strongly related to obesity, namely, esophageal (esophagus), gastric (stomach), colorectal (colon), liver, gallbladder, pancreatic (pancreas), kidney, prostate, breast, ovary, endometrial (endometrium), cervical (cervix), and the hematopoietic system (4). This obesity-cancer link has been demonstrated and confirmed by numerous reviews and meta-analyses, specifically that for every 5 kg/m2 increase in BMI >30 kg/m2, there is an associated increased risk of developing cancer. This obesity-cancer risk ranges from as low as 9% increased risk for colorectal cancer to as high as a 56% increase of biliary cancers (20). For endometrial cancer, every 10% increase in the waist-to-hip ratio increases endometrial cancer risk by 21% (20). Overall, data continue to mount demonstrating significant proclivity of cancer development in preclinical and clinical obesity studies (21–26).
The overarching question then focuses on “why” obesity is associated with such a proclivity for cancer development. Although there may be unique factors that trigger the development of some malignancies greater than others, general changes associated with obesity and the excess adiposity likely contribute to an increased overall propensity toward cancer growth. Obesity has been described as a chronic inflammatory state, and potentially a state of immunoparalysis (27), which is associated with numerous circulating inflammatory mediators and other growth factors that are elevated relative to a normal weight individual. Insulin, predominately known for its effects on regulating glycemia, is also a strong growth factor proposed to be a driver of carcinogenic pathways. Obesity and insulin resistance are associated with increasing concentrations of circulating insulin that parallel elevated body weight and excess fat mass. This relationship of insulin has been demonstrated in breast cancer studies, as well as other circulating mediators that originate from excess adipose tissue production of other inflammatory mediators (i.e., IL-6, TNF-alpha). Increased obesity and excess adipose tissue provide a feed-forward mechanism that worsens insulin resistance and subsequently further increases insulin secretion from the pancreatic beta cells. This continued worsening of insulin resistance drives excess fatty acid release from adipocytes, as a driver of breast cancer proliferation and migration (28, 29). Increased adipose tissue release of fatty acids is also associated with hepatic insulin resistance and excess hepatic glucose production that can worsen glycemia and drive a further worsening of insulin resistance peripherally (29). Collectively, these inflammatory mediators and the worsening hyperinsulinemia also increase the release of insulin growth factor-1 (IGF-1), a driver of cell growth and proliferation (30), culminating in a milieu of inflammatory and proliferative signals.
Metabolic and bariatric surgery provides the most substantial and durable treatment for obesity (6, 31, 32). Typical patients undergoing metabolic surgery are women (80%) between 35 and 54 years of age (33). In terms of operations (Figure 1), vertical sleeve gastrectomy (VSG) accounts for approximately two-thirds of procedures in the USA. Roux-en-Y gastric bypass (RYGB) accounts for most of the remaining operations, with biliopancreatic diversion with duodenal switch (BPD/DS) making up the remaining primary surgical procedures (34). Bariatric operations successfully treat the disease of obesity through biological mechanisms, many of them remain to be completely elucidated (35–37).
Compared with intensive medical therapy alone, metabolic surgery is associated with more robust weight loss, decreased glycated hemoglobin levels, better glycemic control, decreased requirement for glucose-lowering medications, decreased triglyceride levels, increased HDL cholesterol levels, decreased urinary albumin to creatinine ratio, and an improvement in the quality of life in patients with BMI ≥27 kg/m2 (34, 38). A meta-analysis of 17 studies including 174,772 matched participants showed a 49.2% reduction in the hazard rate of death and an increase of 6.1 years of life expectancy in those who underwent metabolic–bariatric surgery compared with usual care (39).
Aside from this remarkable clinical efficacy, metabolic surgery is safe and has a lower 30-day postoperative mortality rate than laparoscopic appendectomy or cholecystectomy (0.3% vs. 0.5% and 0.7%, respectively) and a complication rate similar to that of laparoscopic hysterectomy (3.4% vs. 3.5%) (40). These safety measures have been documented in large retrospective studies (41, 42) and confirmed by prospective clinical trials (43, 44). Even in patients who might be at increased risk secondary to organ failure (heart, lung, kidney failure), the potential benefit of weight loss surgery as a bridge to transplantation has been shown to be remarkably safe and effective (45–48).
As the link between obesity and cancer continues to be defined, it remains to be conclusively identified how and to what extent metabolic surgery might modify an individual's cancer risk or even response to cancer treatment. Patients with obesity who undergo bariatric surgery have reduced overall cancer risk compared with controls (49–53), which may be more pronounced in women than in men for reasons not fully understood yet (14). Cancer mortality also decreases, suggesting that metabolic surgery not only protects against developing cancer but also improves patient outcomes after cancer development (52). Individuals who have already developed comorbidities associated with increased cancer risk may also benefit from bariatric surgery. Patients with nonalcoholic fatty liver disease (NAFLD) and a BMI ≥40 kg/m2 who undergo bariatric surgery are 16% less likely to develop any cancer and 35% less likely to develop obesity-related cancers than their nonsurgical counterparts (54). In one of the longest studies looking at bariatric surgery and cancer to date with a median follow-up of 21.3 years, Sjoholm et al. reported data from the Swedish Obese Subjects (SOS) study on the effect of bariatric surgery on cancer risk. Incidence of all cancer types was 9.1 per 1,000 person-years in patients who underwent bariatric surgery and 14.1 per 1,000 person-years in the control group (Adjusted HR 0.63, 95% CI 0.43–0.84, P = 0.008) (55). The benefits of metabolic surgery on long-term cancer risk are significant, though they remain to be definitively proven through prospective, randomized clinical trials.
Responses to different types of malignancy following bariatric surgery have been increasingly reported as the overall effect of bariatric surgery on cancer development has been identified (Table 1). Although squamous cell carcinoma of the skin has been suggested to have an inverse relationship with obesity and melanoma has had little evidence of any correlation to obesity (56), reported data from the SOS study showed a decreased incidence of both types of skin cancer in patients with obesity who underwent bariatric surgery when compared with nonsurgical matched controls (57). Obesity increases the risk of developing pancreatic cancer, but conclusive data on bariatric surgery patients and ongoing risk reduction are limited (48, 52, 53, 58). Similarly, the relationship of bariatric surgery and colorectal cancer has had conflicting evidence, with some studies demonstrating decreased risk (53, 59, 60), while others showing no change (52, 61) or even a possible increased risk with some but not all procedures (62, 63). Large, prospective clinical trials are necessary to fully identify these effects and whether there may be procedure-specific differences.
Above all other cancers, endometrial cancer has the strongest association with obesity, and data suggest that significant weight loss may even lead to a regression of premalignant pathology (64–66). A decreased incidence of endometrial, breast, and prostate cancer (hormonally responsive cancers) has been associated with RYGB, gastric banding, and VSG (63). The largest reduction in the risk of hormone-related cancers, however, is seen in gastric bypass, suggesting a possible alternative mechanism or variable effect on an underlying mechanism of risk reduction among different types of bariatric surgery (63). A decreased risk of breast cancer has been independently associated with patients who have undergone bariatric surgery compared with nonsurgical controls (52, 67–69). Bariatric surgery has also been associated with a 53% decreased risk of ovarian cancer in women living with obesity (67).
Considerable evidence from retrospective and prospective cohort studies supports a decreased risk of endometrial cancer in patients with obesity who undergo bariatric surgery compared with nonsurgical matched controls (52, 53, 67, 70–72). A prospective pilot study of the effects of bariatric surgery on the risk of endometrial pathology in women undergoing laparoscopic RYGB identified a 6.8% preoperative prevalence of occult hyperplastic endometrium, considered a precursor lesion for endometrial cancer, upon biopsy. At one-year follow-up, half of the patients with identified endometrial hyperplasia had resolution (66).
Information about bariatric or metabolic surgery and cancer has primarily been confined to retrospective or prospective cohort studies. Although attempts have been made to parse out cancer outcomes after bariatric surgery from randomized controlled trials, the relative rarity of these studies, combined with small sample sizes and short follow-up periods, has limited their usefulness (73). Thus, a better understanding of endometrial and other hormonally sensitive cancers, including breast and prostate, is needed.
More than half of women diagnosed with endometrial cancer in the United States have a BMI >25 kg/m2 (74). It is not surprising, therefore, that endometrial cancer survivors are at risk for experiencing poor health outcomes such as type 2 diabetes mellitus, dyslipidemia, obstructive sleep apnea, and cardiovascular morbidity and mortality (75). In women who fail nonsurgical weight loss approaches, bariatric surgery has been shown to reduce the incidence of obesity-related disease and long-term all-cause mortality (76). Because of the excellent cancer-specific outcomes and preponderance of obesity-related complications, women with endometrial cancer are more likely to die of cardiovascular disease and other obesity-related illnesses than endometrial cancer itself (77). This makes an endometrial cancer diagnosis a critical teachable moment and emphasizes the importance of actively managing the underlying issue of obesity in the endometrial cancer survivorship period.
The endometrial cancer survivorship period begins at the time of diagnosis and includes cancer treatment, management of chronic or intermittent disease, and addressing end-of-life care as needed. During this period, patients with endometrial cancer develop close relationships with their gynecologist oncologists. Therefore, it is an opportune time to address approaches to weight loss as patients typically see their oncologist on average at least every 3–12 months. Endometrial cancer survivors report that it is acceptable for their gynecologic oncologist to discuss weight loss strategies (75). However, there are gaps in gynecologic oncologists’ practice patterns as it relates to weight loss counseling. Only 60% of gynecologic oncologists report feeling adequately prepared to provide weight loss counseling and only 50% actually provide it (78, 79). Furthermore, only 10% of gynecologic oncologists report receiving formal training in obesity management (78, 79). Importantly, women are more likely to accept a bariatric referral when it is offered early in the course of endometrial cancer survivorship (75). Thus, gynecologic oncologist visits offer an opportune juncture for interventions focused on correction of metabolic disease, and bariatric referrals are most likely to be acted upon when offered early in the course of cancer care and treatment.
As noted above, obesity plays a crucial role in endometrial carcinogenesis, and an effective intervention has tremendous potential for improving the overall health of endometrial cancer survivors living with obesity. The standard treatment for most early-stage endometrial cancers is hysterectomy, using minimally invasive approaches when possible. This is followed by adjuvant radiotherapy and/or chemotherapy as needed. Unlike women who have healthy weight, women with obesity experience significant intraoperative challenges such as difficulty with optimal positioning, abdominal insufflation, initial abdominal access, and tolerating steep positioning (80). Thus, women with obesity are less likely to undergo minimally invasive approaches and are at a much higher risk for developing postoperative complications (e.g., wound infection, venous thromboses, and prolonged length of stay (81)). As obesity rates continue to rise and childbearing is increasingly deferred, growing numbers of endometrial cancer may be found in women desiring uterine preservation and progestin based therapies may be appropriate (82). Bariatric surgery is effective in achieving weight loss, restoring the hypothalamic–pituitary axis which is deranged in many of these women, and optimizing pregnancy outcomes (83). With the ability to use conservative measures, like the levonorgestrel-releasing intrauterine device (IUD) to buy time, there is tremendous interest in utilizing bariatric surgery to help women lose weight and reach their goals, whether that be candidacy for a safer minimally invasive hysterectomy or optimizing their chances at fertility and healthy pregnancy outcomes.
There is tremendous enthusiasm for employing bariatric surgery early during cancer care. This has been demonstrated to be feasible as a component of delayed hysterectomy as women either pursue pregnancy or weight loss to optimize themselves for a minimally invasive hysterectomy (27). In one case, an adolescent patient treated with the levonorgestrel-releasing IUD for fertility preservation, bariatric surgery with sleeve gastrectomy was employed and she subsequently reached normal body weight and showed complete response to the IUD (84). Another patient with obesity underwent bariatric surgery to become eligible for hysterectomy after failing hormonal treatment with the levonorgestrel-releasing IUD. She successfully lost ∼18% of her total body weight within 8 weeks of her laparoscopic sleeve gastrectomy and was able to have an uncomplicated hysterectomy and bilateral salpingo-oophorectomy for stage IA grade 1 endometrial cancer (85). In a compelling series by Dyck et al., 6/9 women with endometrial cancer treated with VSG experienced a regression of the tumor, suggesting that there may be a therapeutic benefit to surgical weight loss (27). Concurrent Roux-en-Y with a robotic hysterectomy, bilateral salpingo-oophorectomy, and bilateral sentinel lymph node biopsies in the setting of early-stage endometrial cancer treatment has been reported; the woman experienced a subsequent 30% total weight loss as well as reduction in antihypertensive and antidiabetic medication requirements (86). Coordination of these cases is complex and requires both a multidisciplinary team and a well-counseled and well-informed patient. Criteria for optimal patient selection and long-term outcomes in larger patient cohorts remain to be described.
Tremendous strides have been made in recent years with regard to the treatment of advanced and recurrent endometrial cancers, with new drug approvals and therapeutic approaches in the immunotherapy, targeted therapy, and hormonal therapy spaces. While enthusiasm for bariatric surgery is likely to be limited to women with early-stage disease with expected excellent cancer-specific outcomes, this wealth of new information in the advanced disease space offers the opportunity to refine our approach to early-stage endometrial cancer. Many novel therapeutics work on the same pathways that are altered with obesity and surgical weight loss, which presents the possibility of pivoting off these advances to improve an understanding of how bariatric surgery might benefit women with endometrial cancer.
KEYNOTE 158 showed a robust and durable response to pembrolizumab for advanced mismatch repair deficient or microsatellite instable endometrial cancer, and the FDA has subsequently granted approval for treatment in this setting (87, 88). Pembrolizumab is a humanized monoclonal antibody that binds PD1 on T cells. Ligands on tumor cells interact with PD1 to downregulate the immune response to the tumor, but with immune checkpoint inhibition, this interaction is blocked, resulting in T-cell recognition of the tumor cell as “foreign” and subsequent antitumor immune response (Figure 2) (89). Importantly, of the four molecular types of endometrial cancers (POLE, mismatch repair deficient, p53 abnormal, or no specific molecular profile), most obese women are likely to be no specific molecular profile. These mismatch repair proficient endometrial cancers do not show the same robust response to single-agent immune checkpoint inhibition. However, pembrolizumab, in combination with lenvatinib, a multiple tyrosine kinase inhibitor, has activity and is an approved second-line therapy in this setting (90). Everolimus is an mTOR kinase inhibitor. It is believed that resistance to hormonal therapy for women with endometrial cancer may be secondary to PI3Kinase pathway activation and that mTOR kinase inhibition may help overcome endocrine resistance. In fact, the combination of everolimus and letrozole has yielded promising results in women with advanced endometrial cancer (91). The addition of metformin to everolimus and letrozole has also demonstrated promise in this setting (92). Furthermore, CDK4/6 inhibitors are being avidly explored in combination with aromatase inhibitors in ER+ endometrial cancer with some preliminary success (93).

Figure 2. Pembrolizumab mechanism of action. Pembrolizumab prevents PD-1 binding and blocking T-cell and tumor cell interaction, allowing for immune-mediated tumor cell destruction.
Obesity is also associated with T-cell anergy and a state of “inflammaging”, which is characterized by marked chronic inflammation with an impaired immune response (94–97). Some reports suggest an enhanced immune checkpoint inhibition in patients with cancer and obesity (98), suggesting that this immune dysfunction may be uniquely targetable in this population. Bariatric surgery alters immune cell counts, lipids, and oxidation products, generally shifting from a pro-inflammatory to an anti-inflammatory phenotype (99, 100). In the series by Dyck et al. that demonstrated a 66.6% rate of regression of endometrial cancer after bariatric surgery, the authors also reported that BMI was negatively correlated with CD8 T-cell infiltration of the tumor (27). Furthermore, the addition of lenvatinib seems to enhance the efficacy of immune checkpoint inhibition, especially in the DNA mismatch repair proficient population. Lenvatinib works as a multiple kinase inhibitor acting on VEGFR 1–3 and FDGFR 1–4 among other receptors (Figure 3). One primary effect is the inhibition of angiogenesis, the markers of which are also reduced in bariatric surgery, variable with the amount of weight lost (101). Obesity and chronic states of excess insulin are implicated in carcinogenesis through the PI3Kinase pathway and excess unopposed estrogen (102, 103). Increases in estrogen and insulin seen in women with obesity lead to an activation of downstream PI3Kinase and MAPKinase pathways through a phosphorylation of AKT and ERK leading to increased cell proliferation and inhibited apoptosis (104). Successful weight loss after bariatric surgery is associated with reductions in aromatase conversion of androgens to estrogen as well as alterations in the mTOR and PI3K pathways (74, 105–108) (Figure 4).
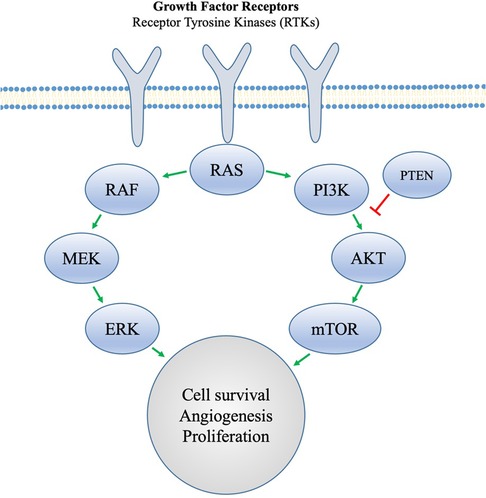
Figure 3. Tyrosine kinase receptors involved in angiogenesis and cell proliferation. Obesity promotes proangiogenic factors that act at the receptor level. Cancer cells that are able to evade hormonal resistance are proposed to do so through an activation of the PI3K pathway, but MTOR inhibition can be used to overcome this.
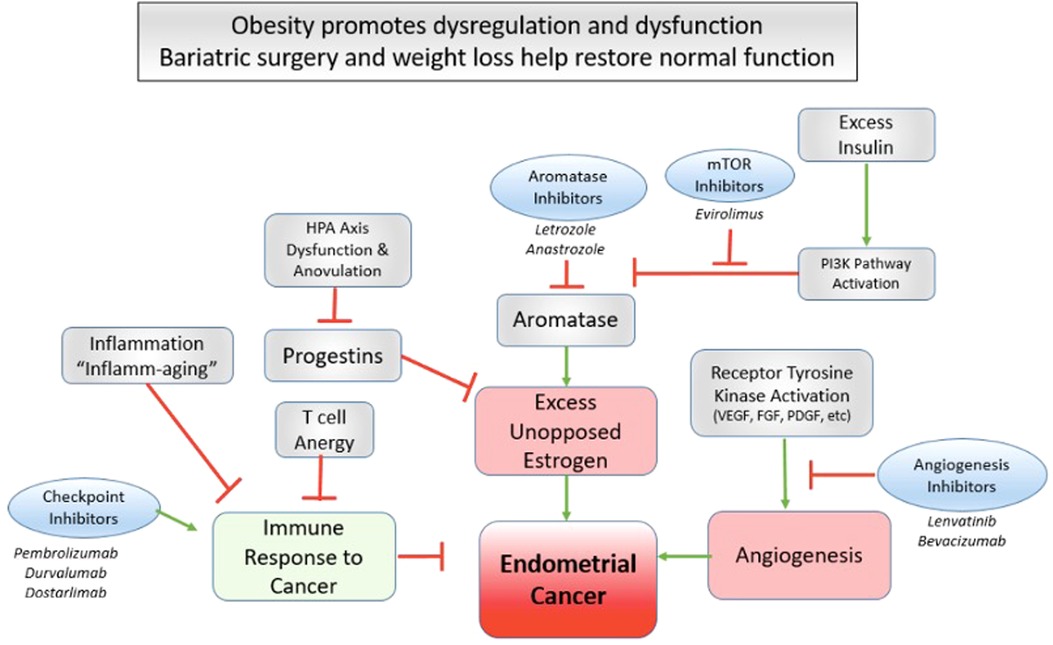
Figure 4. Bariatric surgery as a targeted therapy for endometrial cancer amelioration. Several novel advancements in targeted therapy for advanced endometrial cancer highlight critical pathways that may be in kind targeted effectively by bariatric surgery and successful weight loss.
The link between obesity and endometrial cancer is clear and obesity complicates and confounds our ability to offer the best anticancer therapies to such patients. Most women with endometrial cancer will do well from a cancer standpoint but are at a very high risk of poor outcomes due to their obesity-related complications. Recent advances in anticancer therapies highlight actionable pathways such as T-cell response, anti-estrogen, and mTOR manipulation and angiogenesis; these very pathways may be therapeutically altered with bariatric surgery and weight loss. Bariatric surgery is a safe and effective treatment for obesity. We are therefore challenged to consider and explore how and where bariatric surgery might fit into the cancer treatment paradigm, particularly for early-stage patients. We may be able to harness this powerful intervention to prevent a progression of premalignant lesions to cancer, bridge patients to a better endometrial cancer surgery, effect better reproductive outcomes, prevent secondary malignancies, lead to healthier survivorship, or even produce swifter response or more durable endometrial cancer outcomes. Early metabolic surgery, particularly gastric bypass as it demonstrates the greatest risk reduction in hormonal cancers, may represent a powerful targeted therapy for patients with obesity and endometrial cancer. Well-designed, prospective, and mechanistic studies are needed to clarify the most appropriate candidates and time for bariatric surgery in this population.
PRS, CWL, DB, AMJ, MB, and VLA contributed to the conception and design of the study. RCR and YMA performed the literature review and wrote the first draft of the manuscript. AMJ and VLA wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. (2020) 360:1–8. PMID: 32487284 https://pubmed.ncbi.nlm.nih.gov/32487284/
2. Nyberg ST, Batty GD, Pentti J, Virtanen M, Alfredsson L, Fransson EI, et al. Obesity and loss of disease-free years owing to major non-communicable diseases: a multicohort study. Lancet Public Heal. (2018) 3:e490–7. doi: 10.1016/S2468-2667(18)30139-7
3. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. (2002) 3:565–74. doi: 10.1016/S1470-2045(02)00849-5
4. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
5. Ciciurkaite G, Moloney ME, Brown RL. The incomplete medicalization of obesity: physician office visits, diagnoses, and treatments, 1996-2014. Public Health Rep. (2019) 134:141–9. doi: 10.1177/0033354918813102
6. Carlsson L, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Svensson P-A, Taube M, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med. (2020) 383:1535–43. doi: 10.1056/NEJMoa2002449
7. Aminian A, Kashyap SR, Wolski KE, Brethauer SA, Kirwan JP, Nissen SE, et al. Patient-reported outcomes after metabolic surgery versus medical therapy for diabetes: insights from the STAMPEDE randomized trial. Ann Surg. (2021) 274:524–32. doi: 10.1097/SLA.0000000000005003
8. Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. (2019) 322:1271–12. doi: 10.1001/jama.2019.14231
9. Moussa O, Ardissino M, Heaton T, Tang A, Khan O, Ziprin P, et al. Effect of bariatric surgery on long-term cardiovascular outcomes: a nationwide nested cohort study. Eur Heart J. (2020) 41:2660–7. doi: 10.1093/eurheartj/ehaa069.32188981
10. Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O’Connor PJ, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. (2018) 320:1570–82. doi: 10.1001/jama.2018.14619
11. Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs. usual care obesity management with all-cause mortality. JAMA. (2018) 319:279. doi: 10.1001/jama.2017.20513
12. Eliasson B, Liakopoulos V, Franzén S, Näslund I, Svensson A-M, Ottosson J, et al. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol. (2015) 3:847–54. doi: 10.1016/S2213-8587(15)00334-4
13. Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. (2006) 296:193–201. doi: 10.1001/jama.296.2.193
14. Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surg Endosc. (2013) 27:4449–56. doi: 10.1007/s00464-013-3127-9
15. Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. (2015) 16:36–46. doi: 10.1016/S1470-2045(14)71123-4
16. Bull CJ, Bell JA, Murphy N, Sanderson E, Smith GD, Timpson NJ, et al. Adiposity, metabolites, and colorectal cancer risk: Mendelian randomization study. BMC Med. (2020) 18:396. doi: 10.1186/s12916-020-01855-9
17. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
18. Xu M, Jung X, Hines OJ, Eibl G, Chen Y. Obesity and pancreatic cancer. Pancreas. (2018) 47:158–62. doi: 10.1097/MPA.0000000000000974
19. Zhou B, Wu D, Liu H, Du L, Wang Y, Xu J, et al. Obesity and pancreatic cancer: an update of epidemiological evidence and molecular mechanisms. Pancreatology. (2019) 19:941–50. doi: 10.1016/j.pan.2019.08.008
20. Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. Br Med J. (2017) 356:j477. doi: 10.1136/bmj.j477
21. Choi IY, Choi YJ, Shin DW, Han KD, Jeon KH, Jeong S, et al. Association between obesity and the risk of gastric cancer in premenopausal and postmenopausal women: a nationwide cohort study. J Gastroen Hepatol. (2021) 36:2834–40. doi: 10.1111/jgh.15558
22. Inagaki-Ohara K. Gastric leptin and tumorigenesis: beyond obesity. Int J Mol Sci. (2019) 20:2622. doi: 10.3390/ijms20112622
23. Poorolajal J, Moradi L, Mohammadi Y, Cheraghi Z, Gohari-Ensaf F. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. (2020) 42:e2020004. doi: 10.4178/epih.e2020004
24. Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, et al. Risk factors for renal cell carcinoma in the VITAL study. J Urol. (2013) 190:1657–61. doi: 10.1016/j.juro.2013.04.130
25. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol. (2018) 75:74–84. doi: 10.1016/j.eururo.2018.08.036
26. Gild P, Ehdaie B, Kluth LA. Effect of obesity on bladder cancer and renal cell carcinoma incidence and survival. Curr Opin Urol. (2017) 27:409–14. doi: 10.1097/MOU.0000000000000425
27. Dyck L, Prendeville H, Raverdeau M, Wilk MM, Loftus RM, Douglas A, et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J Exp Med. (2022) 219:e20210042. doi: 10.1084/jem.20210042
28. Kang C, LeRoith D, Gallagher EJ. Diabetes, obesity, and breast cancer. Endocrinology. (2018) 159:3801–12. doi: 10.1210/en.2018-00574
29. Balaban S, Shearer RF, Lee LS, Geldermalsen Mv, Schreuder M, Shtein HC, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. (2017) 5:1. doi: 10.1186/s40170-016-0163-7
30. Argolo DF, Hudis CA, Iyengar NM. The impact of obesity on breast cancer. Curr Oncol Rep. (2018) 20:47. doi: 10.1007/s11912-018-0688-8
31. Ceriani V, Sarro G, Micheletto G, Giovanelli A, Zakaria AS, Fanchini M, et al. Long-term mortality in obese subjects undergoing malabsorptive surgery (biliopancreatic diversion and biliointestinal bypass) versus medical treatment. Int J Obesity. (2018) 43:1147–53. doi: 10.1038/s41366-018-0244-5
32. Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. Br Med J. (2013) 347:f5934. doi: 10.1136/bmj.f5934
33. Alalwan AA, Friedman J, Park H, Segal R, Brumback BA, Hartzema AG. US National trends in bariatric surgery: a decade of study. Surgery. (2021) 170:13–7. doi: 10.1016/j.surg.2021.02.002
34. Ghiassi S, Morton JM. Safety and efficacy of bariatric and metabolic surgery. Curr Obes Rep. (2020) 9:159–64. doi: 10.1007/s13679-020-00377-y
35. Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab. (2015) 21:369–78. doi: 10.1016/j.cmet.2015.01.001
36. Ji Y, Lee H, Kaura S, Yip J, Sun H, Guan L, et al. Effect of bariatric surgery on metabolic diseases and underlying mechanisms. Biomolecules. (2021) 11:1582. doi: 10.3390/biom11111582
37. Sandoval DA. Mechanisms for the metabolic success of bariatric surgery. J Neuroendocrinol. (2019) 31:e12708. doi: 10.1111/jne.12708
38. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes–3-year outcomes. N Engl J Med. (2014) 370:2002–13. doi: 10.1056/NEJMoa1401329
39. Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, et al. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. (2021) 397:1830–41. doi: 10.1016/S0140-6736(21)00591-2
40. Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab. (2015) 17:198–201. doi: 10.1111/dom.12405
41. Arterburn D, Wellman R, Emiliano A, Smith SR, Odegaard AO, Murali S, et al. Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORnet cohort study. Ann Intern Med. (2018) 169:741. doi: 10.7326/M17-2786
42. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults. JAMA. (2020) 324:879–87. doi: 10.1001/jama.2020.12567
43. Consortium LA of BS (LABS), Flum DR, Belle SH, King WC, Wahed AS, Berk P, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. (2009) 361:445–54. doi: 10.1056/NEJMoa0901836
44. Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr. (2014) 168:47–53. doi: 10.1001/jamapediatrics.2013.4296
45. Kindel TL, Higgins RM, Lak K, Gould J, Kreuziger LB, Mohammed A, et al. Bariatric surgery in patients with advanced heart failure: a proposed multi-disciplinary pathway for surgical care in medically complex patients. Surgery. (2021) 170:659–63. doi: 10.1016/j.surg.2021.04.036
46. Punchai S, Hanipah ZN, Sharma G, Aminian A, Steckner K, Cywinski J, et al. Laparoscopic sleeve gastrectomy in heart failure patients with left ventricular assist device. Obes Surg. (2019) 29:1122–9. doi: 10.1007/s11695-018-3570-8
47. Guggino J, Coumes S, Wion N, Reche F, Arvieux C, Borel A. Effectiveness and safety of bariatric surgery in patients with end-stage chronic kidney disease or kidney transplant. Obesity. (2020) 28:2290–304. doi: 10.1002/oby.23001
48. Spaggiari M, Cocco PD, Tulla K, Kaylan KB, Masrur MA, Hassan C, et al. Simultaneous robotic kidney transplantation and bariatric surgery for morbidly obese patients with end-stage renal failure. Am J Transplant. (2021) 21:1525–34. doi: 10.1111/ajt.16322
49. Sjöholm K, Carlsson LMS, Peltonen M, Taube M, et al. Response to Comment on Sjöholm et al. Association of bariatric surgery with cancer incidence in patients with obesity and diabetes: long-term results from the Swedish obese subjects study. Diabetes Care. (2022) 45:444–450. Diabetes Care. (2022) 45:e73–e73. doi: 10.2337/dci21-0065
50. Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery—systematic review and meta-analysis of national population-based cohort studies. Obes Surg. (2019) 29:1031–9. doi: 10.1007/s11695-018-3501-8
51. Casagrande DS, Rosa DD, Umpierre D, Sarmento RA, Rodrigues CG, Schaan BD. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg. (2014) 24:1499–509. doi: 10.1007/s11695-014-1276-0
52. Zhang K, Luo Y, Dai H, Deng Z. Effects of bariatric surgery on cancer risk: evidence from meta-analysis. Obes Surg. (2020) 30:1265–72. doi: 10.1007/s11695-019-04368-4
53. Schauer DP, Feigelson HS, Koebnick C, Caan B, Weinmann S, Leonard AC, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. (2019) 269:95–101. doi: 10.1097/SLA.0000000000002525
54. Rustgi VK, Li Y, Gupta K, Minacapelli CD, Bhurwal A, Catalano C, et al. Bariatric surgery reduces cancer risk in adults with nonalcoholic fatty liver disease and severe obesity. Gastroenterology. (2021) 161:171–184.e10. doi: 10.1053/j.gastro.2021.03.021
55. Sjöholm K, Carlsson LMS, Svensson P-A, Andersson-Assarsson JC, Kristensson F, Jacobson P, et al. Association of bariatric surgery with cancer incidence in patients with obesity and diabetes: long-term results from the Swedish obese subjects study. Diabetes Care. (2021) 45:444–50. doi: 10.2337/dc21-1335
56. Pothiawala S, Qureshi AA, Li Y, Han J. Obesity and the incidence of skin cancer in US Caucasians. Cancer Cause Control. (2012) 23:717–26. doi: 10.1007/s10552-012-9941-x
57. Taube M, Peltonen M, Sjöholm K, Anveden Å, Andersson-Assarsson JC, Jacobson P, et al. Association of bariatric surgery with skin cancer incidence in adults with obesity. JAMA Dermatol. (2020) 156:38–43. doi: 10.1001/jamadermatol.2019.3240
58. Rawla P, Thandra KC, Sunkara T. Pancreatic cancer and obesity: epidemiology, mechanism, and preventive strategies. Clin J Gastroenterol. (2019) 12:285–91. doi: 10.1007/s12328-019-00953-3
59. Almazeedi S, El-Abd R, Al-Khamis A, Albatineh AN, Al-Sabah S. Role of bariatric surgery in reducing the risk of colorectal cancer: a meta-analysis. Br J Surg. (2020) 107:348–54. doi: 10.1002/bjs.11494
60. Bailly L, Fabre R, Pradier C, Iannelli A. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surg. (2020) 155:395–402. doi: 10.1001/jamasurg.2020.0089
61. Taube M, Peltonen M, Sjöholm K, Palmqvist R, Andersson-Assarsson JC, Jacobson P, et al. Long-term incidence of colorectal cancer after bariatric surgery or usual care in the Swedish obese subjects study. PLoS ONE. (2021) 16:e0248550. doi: 10.1371/journal.pone.0248550
62. Tao W, Artama M, Euler-Chelpin M, Hull M, Ljung R, Lynge E, et al. Colon and rectal cancer risk after bariatric surgery in a multicountry Nordic cohort study. Int J Cancer. (2020) 147:728–35. doi: 10.1002/ijc.32770
63. Mackenzie H, Markar SR, Askari A, Faiz O, Hull M, Purkayastha S, et al. Obesity surgery and risk of cancer. Br J Surg. (2018) 105:1650–7. doi: 10.1002/bjs.10914
64. Charalampakis V, Tahrani AA, Helmy A, Gupta JK, Singhal R. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur J Obstet Gynecol Reprod Biol. (2016) 207:220–6. doi: 10.1016/j.ejogrb.2016.10.001
65. Balescu I, Bacalbasa N, Copaescu C. The effect of bariatric surgery on premalignant endometrial pathology in morbidly obese patients. Chirurgia-Bucharest. (2019) 114:704. doi: 10.21614/chirurgia.114.6.704
66. Argenta P, Kassing M, Truskinovsky A, Svendsen C. Bariatric surgery and endometrial pathology in asymptomatic morbidly obese women: a prospective, pilot study. BJOG Int J Obstet Gynaecol. (2013) 120:795–800. doi: 10.1111/1471-0528.12100
67. Ishihara BP, Farah D, Fonseca MCM, Nazario A. The risk of developing breast, ovarian, and endometrial cancer in obese women submitted to bariatric surgery: a meta-analysis. Surg Obes Relat Dis. (2020) 16:1596–602. doi: 10.1016/j.soard.2020.06.008
68. Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of breast cancer development? A systematic review. Obes Surg. (2017) 27:3014–20. doi: 10.1007/s11695-017-2901-5
69. Lovrics O, Butt J, Lee Y, Lovrics P, Boudreau V, Anvari M, et al. The effect of bariatric surgery on breast cancer incidence and characteristics: a meta-analysis and systematic review. Am J Surg. (2021) 222:715–22. doi: 10.1016/j.amjsurg.2021.03.016
70. Upala S, Sanguankeo A. Bariatric surgery and risk of postoperative endometrial cancer: a systematic review and meta-analysis. Surg Obes Relat Dis. (2015) 11:949–55. doi: 10.1016/j.soard.2014.09.024
71. Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. (2018) 28:1433–40. doi: 10.1007/s11695-018-3151-x
72. Aminian A, Wilson R, Al-Kurd A, Tu C, Milinovich A, Kroh M, et al. Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA. (2022) 3:e229009. doi: 10.1001/jama.2022.9009
73. Zhou X, Yu J, Li L, Gloy VL, Nordmann A, Tiboni M, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. (2016) 26:2590–601. doi: 10.1007/s11695-016-2144-x
74. Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. (2016) 34:4225–30. doi: 10.1200/JCO.2016.69.4638
75. Jernigan AM, Maurer KA, Cooper K, Schauer PR, Rose PG, Michener CM. Referring survivors of endometrial cancer and complex atypical hyperplasia to bariatric specialists: a prospective cohort study. Am J Obstet Gynecol. (2015) 213:350.e1–e10. doi: 10.1016/j.ajog.2015.05.015
76. Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and meta-analysis. PLoS Med. (2020) 17:e1003206. doi: 10.1371/journal.pmed.1003206
77. Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. (2012) 126:176–9. doi: 10.1016/j.ygyno.2012.04.013
78. Neff R, McCann GA, Carpenter KM, Cohn DE, Noria S, Mikami D, et al. Is bariatric surgery an option for women with gynecologic cancer? Examining weight loss counseling practices and training among gynecologic oncology providers. Gynecol Oncol. (2014) 134:540–5. doi: 10.1016/j.ygyno.2014.06.006
79. Jernigan AM, Tergas AI, Satin AJ, Fader AN. Obesity management in gynecologic cancer survivors: provider practices and attitudes. Am J Obstet Gynecol. (2013) 208:408.e1–e8. doi: 10.1016/j.ajog.2013.02.002
80. Committee on Gynecologic Practice. Committee opinion No. 619. Obstet Gynecol. (2015) 125:274–8. doi: 10.1097/01.AOG.0000459870.06491.71
81. Mahdi H, Jernigan AM, Aljebori Q, Lockhart D, Moslemi-Kebria M. The impact of obesity on the 30-day morbidity and mortality after surgery for endometrial cancer. J Minim Invasive Gynecol. (2015) 22:94–102. doi: 10.1016/j.jmig.2014.07.014
82. Contreras N-A, Sabadell J, Verdaguer P, Julià C, Fernández-Montolí M-E. Fertility-sparing approaches in atypical endometrial hyperplasia and endometrial cancer patients: current evidence and future directions. Int J Mol Sci. (2022) 23:2531. doi: 10.3390/ijms23052531
83. Malik SM, Traub ML. Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World J Diabetes. (2012) 3:71. doi: 10.4239/wjd.v3.i4.71
84. Benito V, López-Tomassetti E, Esparza M, Arencibia O, Andújar M, Prieto M, et al. Bariatric surgery: does it play a role in fertility-preserving treatment among obese young women with endometrial cancer? J Minim Invasive Gynecol. (2015) 22:906–9. doi: 10.1016/j.jmig.2015.03.017
85. Sharma N, Ragupathy K. Morbidly obese patient with endometrial cancer treated by bariatric surgery to enable cancer treatment. BMJ Case Rep. (2021) 14:e243843. doi: 10.1136/bcr-2021-243843
86. Shafa A, Kumar A, Torres D, McKenzie TJ. Minimally invasive hysterectomy and bariatric surgery to improve endometrial cancer survivorship. Obstet Gynecol. (2019) 134:570–2. doi: 10.1097/AOG.0000000000003403
87. O’Malley DM, Bariani GM, Cassier PA, Marabelle A, Hansen AR, Acosta ADJ, et al. Pembrolizumab in patients with microsatellite instability – high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. (2022) 40:752–61. doi: 10.1200/JCO.21.01874
88. FDA approves pembrolizumab for advanced endometrial carcinoma (2022). https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-advanced-endometrial-carcinoma (accessed April 18, 2022).
89. Wahid M, Akhter N, Jawed A, Dar SA, Mandal RK, Lohani M, et al. Pembrolizumab's non-cross resistance mechanism of action successfully overthrown ipilimumab. Crit Rev Oncol Hemat. (2017) 111:1–6. doi: 10.1016/j.critrevonc.2017.01.001
90. Makker V, Colombo N, Herráez AC, Santin AD, Colomba E, Miller DS, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. (2022) 386:437–48. doi: 10.1056/NEJMoa2108330
91. Slomovitz BM, Jiang Y, Yates MS, Soliman PT, Johnston T, Nowakowski M, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. (2015) 33:930–6. doi: 10.1200/JCO.2014.58.3401
92. Soliman PT, Westin SN, Iglesias DA, Fellman BM, Yuan Y, Zhang Q, et al. Everolimus, letrozole, and metformin in women with advanced or recurrent endometrioid endometrial cancer: a multi-center, single arm, phase II study. Clin Cancer Res. (2020) 26:581–7. doi: 10.1158/1078-0432.CCR-19-0471
93. Mirza MR, Bjørge L, Marmé F, Christensen RD, Gil-Martin M, Auranen A, et al. A randomised double-blind placebo-controlled phase II trial of palbociclib combined with letrozole (L) in patients (pts) with oestrogen receptor-positive (ER+) advanced/recurrent endometrial cancer (EC): NSGO-PALEO / ENGOT-EN3 trial. Ann Oncol. (2020) 31:S1160. doi: 10.1016/j.annonc.2020.08.2258
94. Porsche CE, Delproposto JB, Geletka L, O’Rourke R, Lumeng CN. Obesity results in adipose tissue T cell exhaustion. JCI Insight. (2021) 6:e139793. doi: 10.1172/jci.insight.139793
95. Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HCJ, Goldstein DR, et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. (2012) 188:1933–41. doi: 10.4049/jimmunol.1101098
96. Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. (2016) 17:7–19. doi: 10.1007/s10522-015-9578-8
97. Castoldi A, Souza Cd, Câmara NOS, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol. (2016) 6:637. doi: 10.3389/fimmu.2015.00637
98. McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. (2018) 19:310–22. doi: 10.1016/S1470-2045(18)30078-0
99. Jongbloed F, Meijers RWJ, IJzermans JNM, Klaassen RA, Dollé MET, Berg Svd, et al. Effects of bariatric surgery on telomere length and T-cell aging. Int J Obesity. (2019) 43:2189–99. doi: 10.1038/s41366-019-0351-y
100. Villarreal-Calderón JR, Cuéllar RX, Ramos-González MR, Rubio-Infante N, Castillo EC, Elizondo-Montemayor L, et al. Interplay between the adaptive immune system and insulin resistance in weight loss induced by bariatric surgery. Oxid Med Cell Longev. (2019) 2019:3940739. doi: 10.1155/2019/3940739
101. Wiewiora M, Mertas A, Gluck M, Nowowiejska-Wiewiora A, Czuba Z, Piecuch J. Effect of weight loss surgery on biomarkers of angiogenesis in obese patients. Obes Surg. (2020) 30:3417–25. doi: 10.1007/s11695-020-04580-7
102. Tsugane S, Inoue M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci. (2010) 101:1073–9. doi: 10.1111/j.1349-7006.2010.01521.x
103. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. (2012) 12:159–69. doi: 10.1038/nrc3215
104. Tian W, Teng F, Gao J, Gao C, Liu G, Zhang Y, et al. Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways. Cancer Biol Med. (2019) 16(1):55–70. doi: 10.20892/j.issn.2095-3941.2018.0157
105. Pinhel MdS, Nicoletti CF, Noronha NY, Oliveira Bd, Cortes-Oliveira C, Salgado W, et al. Mammalian target of rapamycin complex 2 signaling in obese women changes after bariatric surgery. Nutrition. (2018) 54:94–9. doi: 10.1016/j.nut.2018.02.016
106. Feng Y, Zhong C, Niu J, Zhang L, Zhao Y, Wang W, et al. Effects of sleeve gastrectomy on lipid and energy metabolism in ZDF rats via PI3K/AKT pathway. Am J Transl Res. (2018) 10:3713–22. PMID: 30662621, PMCID: PMC6291694
107. Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. (2016) 7:66–75. doi: 10.3945/an.115.010207
Keywords: bariatric surgery, cancer, obesity, endometrial cancer, metabolic surgery, malignancy
Citation: Ross RC, Akinde YM, Schauer PR, le Roux CW, Brennan D, Jernigan AM, Bueter M and Albaugh VL (2022) The role of bariatric and metabolic surgery in the development, diagnosis, and treatment of endometrial cancer. Front. Surg. 9:943544. doi: 10.3389/fsurg.2022.943544
Received: 13 May 2022; Accepted: 25 July 2022;
Published: 31 August 2022.
Edited by:
Gabriel Sandblom, Karolinska Institutet (KI), SwedenReviewed by:
Antonio Jose Torres, Complutense University of Madrid, Spain© 2022 Ross, Akinde, Schauer, le Roux, Brennan, Jernigan, Bueter and Albaugh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vance L. Albaugh dmFuY2UuYWxiYXVnaEBwYnJjLmVkdQ==
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.