- 1Institute of Hygiene, Military Medical Academy, Belgrade, Serbia
- 2Faculty of Medicine of the Military Medical Academy, University of Defense, Belgrade, Serbia
- 3Clinic for Neurosurgery, Military Medical Academy, Belgrade, Serbia
- 4Institute of Hygiene and Medical Ecology, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 5School of Public Health and Health Management and Institute of Social Medicine, Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 6Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 7Department for Peripheral Nerve Surgery, Functional Neurosurgery and Pain Management Surgery, Clinic for Neurosurgery, University Clinical Center of Serbia, Belgrade, Serbia
Objective: Although the studies have shown the beneficial effects of diet, nutrition, and supplementation as an independent treatment modality, their roles are underestimated in the treatment of peripheral nerve injuries. This is in great part due to the development of efficient nerve repair techniques, combined with physical treatment and stimulation. To achieve the best possible functional recovery diet, nutrition, and supplementation should be implemented within a multidisciplinary approach. The aim of the study is to provide insight into the potentially beneficial effects of diet, nutrients, and supplementation, in the limitation of nerve damage and augmentation of the functional recovery after surgery in a review of human and animal studies.
Methods: The data relating to the diet, nutrients, and supplementation effects on peripheral nerve injuries and their treatment was extracted from the previously published literature.
Results: General balanced diet as well as obesity influence the initial nerve features prior to the injury. In the period following the injury, neuroprotective agents demonstrated beneficial effects prior to surgery, and immediately after the injury, while those potentiating nerve regeneration may be used after the surgical repair to complement the physical treatment and stimulation for improved functional recovery.
Conclusions: Standardized diet, nutrition, and supplementation recommendations and protocols may be of great importance for better nerve regeneration and functional recovery as a part of the multidisciplinary approach to achieve the best possible results in surgically treated patients with peripheral nerve injuries in the future.
1. Introduction
The development of peripheral nerve (PN) surgery after traumatic injuries had reached the limits of functional recovery through contemporary nerve repair techniques (1). The majority of studies dealing with surgical treatment are limited to the surgical perspective only, less commonly combined with physical treatment, and rarely with stimulation, while the medical and other treatment is usually reserved for those not being candidates for surgery (2).
The understanding of the Schwann cells response and the brain plasticity in PN injuries, together with modern nerve repair strategies had led to enviable functional recovery (3, 4). However, these results are in large part improved by mandatory physical treatment and stimulation (5). Diet, nutrients, and supplements impact on the other hand is observed as a sole treatment modality, usually in animal models with crush injury (6). Although the results are encouraging and the conclusions are extremely positive, human studies are lacking.
Complementary supplementation and nutrition are one more point where one can augment the outcome of repair; however, there are no guidelines, recommendations, or review studies to give nerve specialists another card to play with (7).
To understand the real-life impact of diet, nutrients, and supplementation on functional recovery in humans with injured nerves, we are not allowed to deprive the surgical treatment. Nevertheless, the two modalities are rarely combined on purpose. The augmentation of the functional recovery after a reconstructive surgical procedure, through the adjusted diet and nutrition, with additional supplementation is a perspective (8).
This review aimed to provide insight into the effects of diet, nutrients, and supplements related to PN preservation and regeneration after traumatic injury, as well as to imply the significance of outcome augmentation, in addition to the surgical repair in patients with PN injuries, through a review of animal models studies.
2. Methods
The data relating to the supplementation, nutrition and diet effects from the studies on PN injuries was extracted. Studies published in scientific literature included in the databases PubMed, Google Scholar, Science Direct, and Web of Science were evaluated. No limitations in terms of study design were applied. Both human and animal studies were included. Special attention was taken to the implications of outcome augmentation after surgical treatment.
To identify the nutritional factors impacting the recovery of the PN after injury, we have performed an initial search, to identify any review studies on diet, nutrients, and supplements' roles in PN injuries. This led to the identification of these factors for further literature search.
Articles of interest were found through the searches of PubMed, Science Direct, and Google Scholar databases using the keywords peripheral nerve OR brachial plexus OR peripheral nervous system AND injury OR trauma in combination with the common terms [e.g., “diet,” “nutrient(s),” “supplement”, etc.] and the identified factors keywords (e.g., “Vitamin D,” “alcohol,” etc.).
The analysis of cited references led to the inclusion of even more studies, which were omitted from the search results.
2.1. Inclusion criteria
• Nerve injury, including peripheral and cranial nerves,
• Either crush or transection injury, and
• Studies in patients or animal models.
2.2. Exclusion criteria
• Review papers and
• Combination with pharmacological agents (drugs).
3. Review and discussion
Out of the 42 identified publications, we included 34 relevant animal model experiments. Four reviews were excluded, and one human randomized controlled trial. Also, three animal studies were excluded for the combined use of targeted substances with pharmacological agents (drugs). The details of the studies, with the element in scope, the effect and the proposed mechanism are listed in Table 1.
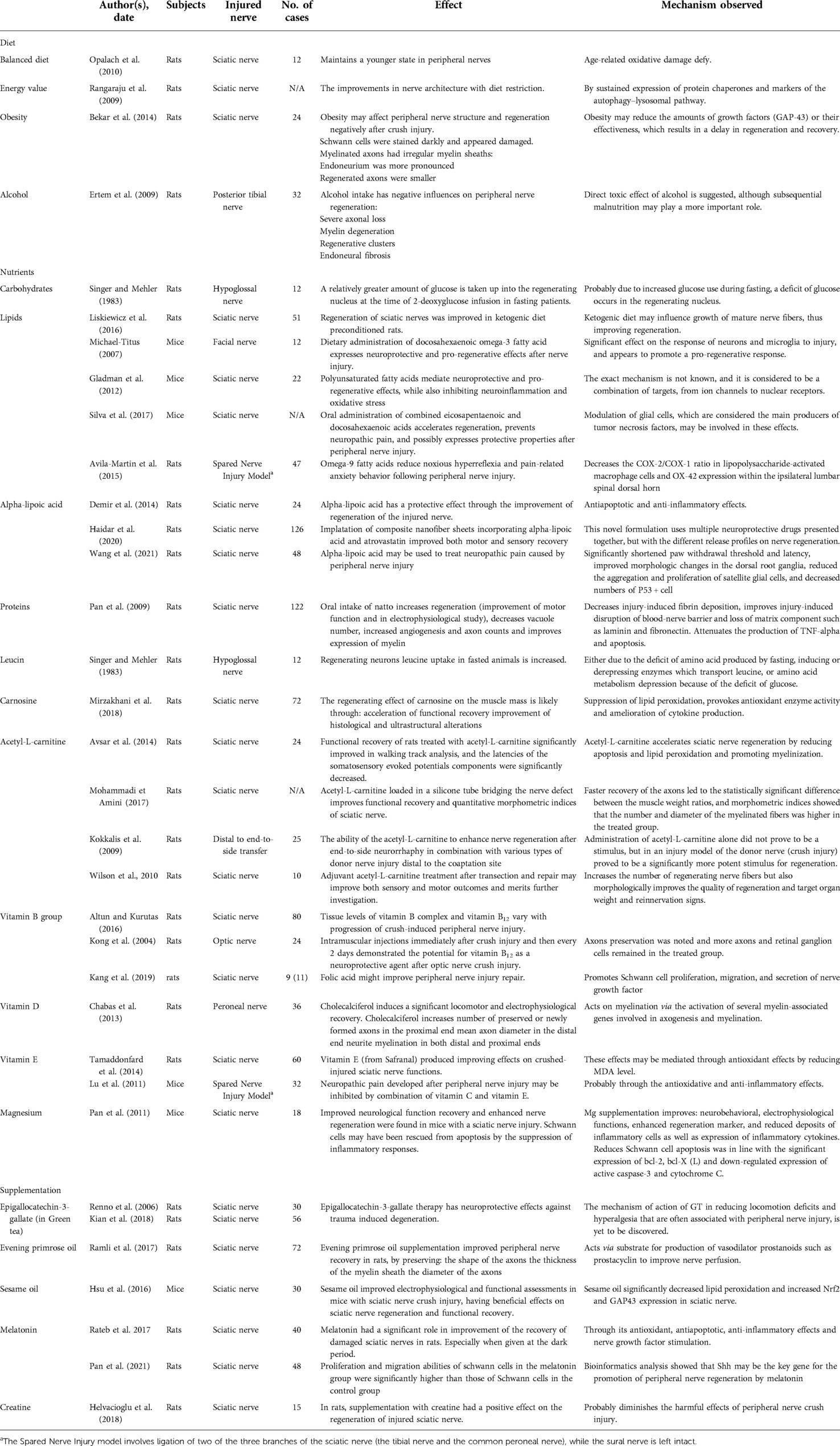
Table 1. Diet, nutrients, and supplements playing a role in peripheral nerve injuries with their effects and proposed mechanisms.
3.1. Diet
3.1.1. Balanced diet
The lifelong alleviated diet was previously referred to as the attenuation of lipid peroxidation, inflammation, and immune cell infiltration, thus acting neuroprotective, preventing age-related damage to the nerves (9, 10).
The dietary components may have effects on PN alone or in combination. The synergistic effect may be enhanced with the consumption of nutrients and bioactive components together, also supported by the production of endogenous neurotrophic factors that increase environmental nerve repair (6).
3.1.2. Energy value
Studies on the brain, spinal cord, and nerve regeneration have previously related low-calorie diet and hunger to improved regeneration (11). Limitation of energy intake in these patients is however complicated, and advising the patients to limit their energy intake is at least controversial.
3.1.3. Obesity
Obesity may negatively affect PN regeneration after injury, through the reduction of growth factors amounts or their effectiveness, resulting in the delay of the regeneration and recovery.
The study of morphological features revealed significant differences in nerve structure and regeneration caused by the negative effects on axon number, myelin thickness, nerve area, the amplitude of compound action potential, and reduction in the number of growth factors in the sciatic nerve-injured rats due to fat-diet induced obesity (12).
3.1.4. Alcohol
Neuropathy due to alcohol abuse is a known entity (13). Painful peripheral neuropathy resulting from the excessive and chronic use of alcohol occurs due to an unknown pathophysiological mechanism (14). Apart from malnutrition and nutrient deficiency occurring due to malabsorption, the direct neurotoxic effect was shown to be an independent factor in the development of the disease (15).
The same factors influence the regeneration of the nerve after injury. The study on rat models has demonstrated the negative impact of alcohol in rats with transected nerves (16).
These effects were previously evaluated in the Danish study by Behse and Buchtal who have compared the groups with alcoholic neuropathy and malnutrition neuropathy (17). In this study, Danish beer was the predominant alcoholic beverage, fortified with thiamine and Vitamin B6 at that time, and resulted in an absence of malnutrition, further development of symptoms and even led to weight gain. Alcoholic neuropathy group symptoms were related to pain, while the malnutrition group experienced progressive weakness, casting a decent shadow on the direct neurotoxic effect of alcohol per se (18).
3.2. Macronutrients
3.2.1. Carbohydrates and lipids
The type and quantity of lipids may influence the regeneration of the PN by several mechanisms including pro-regenerative and neuroprotective, as well as pro-inflammatory and neurodegenerative effects when intake exceeds reasonable amounts.
A ketogenic diet had been attributed to a neuroprotective effect on PN, although more clinical trials are needed to prove the effects (19).
There is no specific recommendation, but the lipid content type and appropriate n − 6/n − 3 ratio seem to make a significant influence on nerve regeneration. Omega-3 and omega-9 polyunsaturated fatty acid's positive effects were demonstrated.
3.2.1.1. Omega-3 fatty acids
Experimental studies support the use of polyunsaturated fatty acids as a promising pharmacological approach in PN injuries.
Polyunsaturated fatty acids, such as eicosapentaenoic and docosahexaenoic acids, mediate neuroprotective and pro-regenerative effects, while also inhibiting neuroinflammation and oxidative stress. The exact mechanism is not known, and it is considered to be a combination of targets, from ion channels to nuclear receptors (20).
In mice, oral administration of combined eicosapentaenoic and docosahexaenoic acids had regenerative and possibly protective properties after PN injury (21).
3.2.1.2. Omega-9 fatty acids
CIS-monounsaturated omega-9 fatty acid—oleic acid, administered in combination with albumin or as 2-hydroxyoleic acid, promotes antinociception and anxiolytic effects following both central and PN injury. Although these results are generally positive, the impact on regeneration is questionable. Motor function improvement and spasticity reduction were observed in patients with spinal cord injury, implicating the possibility of a positive effect on PN (22).
3.2.1.3. Alpha-lipoic acid
In rats with sciatic nerve injury, alpha-lipoic acid has a protective effect through the improvement of regeneration of the injured nerve by its antiapoptotic and anti-inflammatory effects (23), while the implantation of composite nanofiber sheets incorporating alpha-lipoic acid and atorvastatin contributed to the recovery of the motor and sensory function and nerve regeneration (24). As a treatment for neuropathic pain caused by PN injury potentially is considered alpha-lipoic acid, which requires further verification (25).
3.2.2. Proteins and amino acids
The consumption of protein resources with high biological quality, especially including essential amino acids must, be maintained at a certain level to meet organism requirements (6).
The specific role in augmentation is reflected by the effects of natto (extracts of fermented soybeans) on the improvement of motor function and in electrophysiological results, through a complex nerve preservation mechanism (26).
3.2.2.1. Leucin
The study of Singer and Mehler, also tried to explain the increased leucine uptake in fasted animals. The two options included (1) deficit of amino acids produced by fasting, inducing or derepressing enzymes, which transport leucine, and (2) amino acid metabolism depression because of the deficit of glucose (resulting in a deficit of metabolic products of amino acids metabolism which could accelerate uptake) (27).
3.2.2.2. Carnosine
The gastrocnemius muscle mass reduction from a sciatic nerve crush injury may be improved near to its normal value with carnosine supplementation. The beneficial effects may be associated with the acceleration of functional recovery and the improvement of histological and ultrastructural alterations through the mechanisms of suppression of lipid peroxidation, provoking of antioxidant enzyme activity and amelioration of cytokine production (28).
3.2.3. Acetyl-L-carnitine
The modified amino acid is one of the most researched nutrients in peripheral nerve injuries and their surgical treatment. Several studies carried out in animals were published on the various applications of acetyl-L-carnitine for nerve preservation (29), regeneration (30), and augmentation of surgical treatment by both topical (31) and systemic administration (32), even in the delayed fashion (33).
The results of all these studies demonstrated beneficial effects on regeneration; however, the neuroprotective effects were not as pronounced, especially when the acetyl-L-carnitine was given in a delayed fashion (7 days after injury). A recent study evaluated individual and combined effects of erythropoietin and acetyl-L-carnitine; however, regardless of the positive impact from both, combined improved efficacy was not found (34).
The only human study related to nutritional therapy failed to stress the importance of diet nutrition and supplementation in nerve regeneration, although in a chronic entrapment, not the injury. In this double-blinded, randomized, placebo-controlled study, which included adult patients with severe carpal tunnel syndrome acetyl-L-carnitine did not improve nerve regeneration (35).
3.3. Micronutrients
3.3.1. Vitamins B6 and B12
The beneficial effects of group B6 and B12 vitamins in peripheral neuropathies are well known. The possibility and impact of the improvement of nerve regeneration after injury are not sufficiently clarified. It was previously shown that the levels of the vitamin B complex are significantly lower immediately after the injury, with progression through time Supplementation of vitamin B complex in the acute period of PN injury deserves to be considered as an option, which may be useful for the acceleration of nerve regeneration (36). However, the mechanism of action in injured nerve regeneration is different, due to the different types of nerve lesions. Namely, the immediate damage, and the role of supplements is induction of regeneration, not protection (9).
Vitamin B12 (methylcobalamin) has an analgesic effect which may be explained by improving nerve conduction velocity and regeneration of injured nerve. Also, in neuropathic pain states, methylcobalamin inhibited the ectopic spontaneous discharges from peripheral sensory neurons (37).
The underlying pathophysiological mechanism is probably neuronal protection by promotion of regeneration of injured nerves (38) while antagonizing glutamate-induced neurotoxicity (37).
3.3.2. Folic acid
Supplementation with folic acid improved the organoleptic features of spinal axons in in vivo grafted PN segments of adult Sprague-Dawley rats. The same positive effects were noted in spinal cord contusion injuries, emphasizing that the folic acid supplementation should not be limited only to the embryonic period and prevention of neural-tube defects, but also to augmentation of functional recovery in surgically treated PN injuries (39).
In rats, folic acid might improve PN injury repair. Namely, Schwann cells’ proliferation and migration, as well as nerve growth factors’ secretion were promoted by folic acid (40).
3.3.3. Vitamin D
Vitamin D is known for its potential in immune response regulation in various diseases. In PN injury, the positive effect on myelination was demonstrated in rat models. This specific effect is intended to be used solely, although the use with the surgical repair might lead to a better functional recovery.
Chabas et al. on animal models have proven the potential of ergocalciferol (Vitamin D2) and cholecalciferol (Vitamin D3) in the augmentation of the spinal cord and PN regeneration. An analysis of the gene, which regulates Vitamin D3 in the ganglia of the back roots and Schwan's cells, was also performed.
Cholecalciferol is more effective than ergocalciferol and, when administered at a high dose (500 IJ/kg/day), cholecalciferol induces significant locomotor and electrophysiological recovery by increasing the number of preserved and newly formed axons at the proximal end, the mean diameter of the axon at the distal end, and induction of myelination at both distal and proximal ends.
A modified expression of several genes involved in axonogenesis and myelination was also found, after 24 h of vitamin D3 introduction, which leads to the conclusion that Vitamin D acts on myelinization by activating several associated genes (41).
3.3.4. Vitamin E
One study, analyzing the impact of vitamin E, found some improving effects on motor impairment, pain hypersensitivity, Wallerian degeneration, and muscular atrophy induced by a sciatic nerve crush injury. The effect is probably due to the inhibition of the oxidative stress pathway by reducing the malondialdehyde level.
Due to the high contents of Vitamin E, safranal, a major component of saffron, is recommended as a dietary supplement in patients with nerve injury (42).
It was also previously demonstrated that neuropathic pain developed after PN injury may be inhibited by the combination of vitamin C and vitamin E, probably through the antioxidative and anti-inflammatory effects (43).
3.3.5. Magnesium (Mg)
Magnesium supplements significantly improve functional recovery in various neurological disorders, especially in cerebrovascular disease through the decrease in systemic vascular resistance and improvement of cardiac function (44). Not as much data are available on PN injuries related effects, and even those studies involve filaments and wires, rather than supplementation (45, 46). In a study of the injury of the sciatic nerve in an animal model, the diet with high magnesium content significantly increased plasma and plasma magnesium concentrations. In addition, magnesium supplements improved neurobehavioral and electrophysiological functions, improved regeneration markers, and reduced inflammatory cell deposits, as well as the expression of inflammatory cytokines. Schwan cell cellular apoptosis was also reduced in accordance with significant expression of Bcl-2, Bcl-KSL and decreased expression of active Caspase-3 and cytochrome C. It was concluded that magnesium positively influences neurological regeneration and improves neural regeneration, while also preserving Schwan's cells of apoptosis by suppressing inflammatory response (47).
3.4. Supplementation
3.4.1. Green tea
The intake of green tea may assist nerve recovery after traumatic injuries. Although more studies should explore the cellular and molecular mechanisms, which mediate such effect, initial studies with a green tea polyphenol epigallocatechin gallate led to some promising conclusions (48).
3.4.1.1. Epigallocatechin gallate
Prior to the study on PN, a possible therapeutic effect in spinal cord injury was noticed through the improvement in the flat beam test. Furthermore, at an early stage of spinal cord injury, inflammatory cytokines were modulated and axonal sprouting was higher (49).
The study on sciatic nerve transection injury revealed biochemical, histopathological, and immunohistochemical evidence that epigallocatechin gallate therapy may have neuroprotective effects against injury-induced degeneration (50).
3.4.2. Sesame oil
Polyunsaturated (omega-6), and monounsaturated (omega-9) fatty acids account for more than 80% of the total fat contents of sesame oil. Together with the natural antioxidant sesamol and vitamin E, the content assures a neuroprotective effect.
In a study by Hsu et al. sesame oil improved electrophysiological and functional assessments in mice with sciatic nerve crush. The beneficial effects are based on significantly decreased lipid peroxidation and increased Nrf2 and GAP43 expression in the sciatic nerve (51).
3.4.3. Evening primrose oil
Supplementation with evening primrose oil might be significant in the therapy of PN injury. It was shown that evening primrose oil supplementation improved PN recovery in rats (52).
3.4.4. Melatonin
Melatonin may improve the proliferation and migration of Schwann cells via the Sonic Hedgehog signaling pathway after PN injury and in that way promote PN regeneration (53). Melatonin had a significant role in the improvement of the recovery of damaged sciatic nerves in rats, through its antioxidant, antiapoptotic, anti-inflammatory effects, and nerve growth factor stimulation. Giving melatonin during the dark period had better results than giving melatonin during the light period (54).
3.4.5. Creatine
In rats, supplementation with creatine had a positive effect on the regeneration of injured sciatic nerve, which was also verified by electronic microscopy (55).
3.5. Implications and limitations
Reviewed studies comprised different animal models and different options involving the dietary and nutritional interventions as well as the supplements used for the improvement of nerve preservation and regeneration after injury, as well as for the outcome augmentation following surgical repair.
Since the whole review is based on animal studies, we could not derive clear conclusions or recommendations for surgical treatment augmentation in the human population, but focused to give an insight into every possible dietary, nutritional or supplement-related influence.
Possible mechanisms of action are shown in Table 1 in detail and give a significant contribution to a deeper understanding of the general aspects of diet, nutrition, and supplementation effects on the peripheral nervous system.
To provide some initial waypoint, based on our review and the presumed mechanism of action, the specific target points for the introduction or activity of the individual nutrients and supplements were marked in Figure 1.
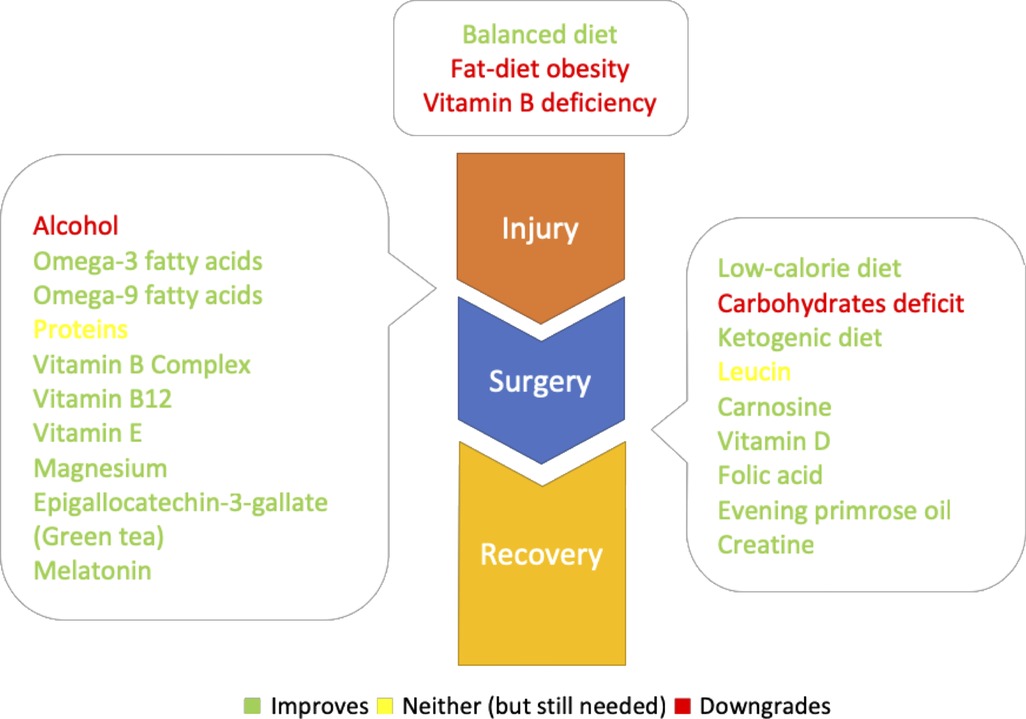
Figure 1. Diet, nutrients and supplements with their respective target points: up: predisposing factors; left: predominately neuroprotective (could be taken from the injury onset); right: predominately neuroregenerative (could be taken after the surgery).
4. Conclusions
Standardized diet, nutrition, and supplementation protocols may be of the greatest importance for better nerve regeneration and functional recovery, as a part of the multidisciplinary approach to achieve the best possible results in patients with PN injuries in the future.
The augmentation of functional recovery, as a part of the multidisciplinary approach, is far less controversial than the treatment of PN injuries based entirely on conservative options. Further human studies should focus on the augmentation of functional recovery, in addition to the surgical treatment of PN injuries, to clarify the definite underlying mechanisms and give clear recommendations and guidelines for better outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors have contributed significantly to the paper. ML and SM-R designed the study, SL and NB gathered the data, and LR revised the manuscript as an expert in peripheral nerve surgery. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Samardžić M, Rasulić L, Stanković L. Motor nerve transfers for restoration of upper arm function in adult brachial plexus injuries: basics, advantages, problems and strategies. Neurohirurgija. (2022) 1(1):9–16. doi: 10.55005/sjns.v1i1.6
2. Rasulic L. Current concept in adult peripheral nerve and brachial plexus surgery. J Brachial Plex Peripher Nerve Inj. (2017) 12(1):e7–14. doi: 10.1055/s-0037-1606841
3. Socolovsky M, Malessy M, Lopez D, Guedes F, Flores L. Current concepts in plasticity and nerve transfers: relationship between surgical techniques and outcomes. Neurosurg Focus. (2017) 42(3):E13. doi: 10.3171/2016.12.FOCUS16431
4. Barton M, John J, Clarke M, Wright A, Ekberg J. The Glia response after peripheral nerve injury: a comparison between Schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. Int J Mol Sci. (2017) 18(2):287. doi: 10.3390/ijms18020287
5. Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci. (2016) 43(3):336–50. doi: 10.1111/ejn.13005
6. Yildiran H, Macit MS, Ozata Uyar G. New approach to peripheral nerve injury: nutritional therapy. Nutr Neurosci. (2020) 23(10):744–55. doi: 10.1080/1028415X.2018.1554322
7. Steindler DA, Reynolds BA. Perspective: neuroregenerative nutrition. Adv Nutr. (2017) 8(4):546–57. doi: 10.3945/an.117.015388
8. Rasulić L, Lepić M, Savić A, Lepić T, Samardžić M. Peripheral nervous system surgery: travelling through no man's land to new horizons. Neurol India. (2019) 67(Supplement):S9-S15. doi: 10.4103/0028-3886.250732
9. Constantin A-M, Tache S. Stimulating factors for the regeneration of peripheral nerves. Clujul Med. (2012) 85(1):12–9.
10. Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. (2010) 13(1):65–74. doi: 10.1089/rej.2009.0892
11. Rangaraju S, Hankins D, Madorsky I, Madorsky E, Lee WH, Carter CS, et al. Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. (2009) 8(2):178–91. doi: 10.1111/j.1474-9726.2009.00460.x
12. Bekar E, Altunkaynak BZ, Balci K, Aslan G, Ayyildiz M, Kaplan S. Effects of high fat diet induced obesity on peripheral nerve regeneration and levels of GAP 43 and TGF-beta in rats. Biotech Histochem. (2014) 89(6):446–56. doi: 10.3109/10520295.2014.894575
13. Castelli G, Desai KM, Cantone RE. Peripheral neuropathy: evaluation and differential diagnosis. Am Fam Physician. (2020) 102(12):732–9.33320513
14. Chopra K, Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol. (2012) 73(3):348–62. doi: 10.1111/j.1365-2125.2011.04111.x
15. Bosch EP, Pelham RW, Rasool CG, Chatterjee A, Lash RW, Brown L, et al. Animal models of alcoholic neuropathy: morphologic, electrophysiologic, and biochemical findings. Muscle Nerve. (1979) 2(2):133–44. doi: 10.1002/mus.880020208
16. Ertem K, Ceylan F, Zorludemir S, Karakoc Y, Yologlu S. Impairment of peripheral nerve healing after nerve repair in rats chronically exposed to alcohol. Arch Med Res. (2009) 40(5):325–30. doi: 10.1016/j.arcmed.2009.05.006
17. Behse F, Buchthal F. Alcoholic neuropathy: clinical, electrophysiological, and biopsy findings. Ann Neurol. (1977) 2(2):95–110. doi: 10.1002/ana.410020203
18. Mellion M, Gilchrist JM, de la Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve. (2011) 43(3):309–16. doi: 10.1002/mus.21946
19. Liskiewicz A, Wlaszczuk A, Gendosz D, Larysz-Brysz M, Kapustka B, Laczynski M, et al. Sciatic nerve regeneration in rats subjected to ketogenic diet. Nutr Neurosci. (2016) 19(3):116–24. doi: 10.1179/1476830514Y.0000000163
20. Gladman SJ, Huang W, Lim SN, Dyall SC, Boddy S, Kang JX, et al. Improved outcome after peripheral nerve injury in mice with increased levels of endogenous omega-3 polyunsaturated fatty acids. J Neurosci. (2012) 32(2):563–71. doi: 10.1523/JNEUROSCI.3371-11.2012
21. Silva RV, Oliveira JT, Santos BLR, Dias FC, Martinez AMB, Lima CKF, et al. Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front Pharmacol. (2017) 8:723. doi: 10.3389/fphar.2017.00723
22. Avila-Martin G, Galan-Arriero I, Ferrer-Donato A, Busquets X, Gomez-Soriano J, Escribá PV, et al. Oral 2-hydroxyoleic acid inhibits reflex hypersensitivity and open-field-induced anxiety after spared nerve injury. Eur J Pain. (2015) 19(1):111–22. doi: 10.1002/ejp.528
23. Demir R, Yayla M, Akpinar E, Cakir M, Calikoglu C, Ozel L, et al. Protective effects of alpha-lipoic acid on experimental sciatic nerve crush injury in rats: assessed with functional, molecular and electromicroscopic analyses. Int J Neurosci. (2014) 124(12):935–43. doi: 10.3109/00207454.2014.902375
24. Haidar MK, Timur SS, Kazanci A, Turkoglu OF, Gursoy RN, Nemutlu E, et al. Composite nanofibers incorporating alpha lipoic acid and atorvastatin provide neuroprotection after peripheral nerve injury in rats. Eur J Pharm Biopharm. (2020) 153:1–13. doi: 10.1016/j.ejpb.2020.05.032
25. Wang J, Lou Z, Xi H, Li Z, Li L, Li Z, et al. Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model. Open Life Sci. (2021) 16(1):222–8. doi: 10.1515/biol-2021-0026
26. Pan HC, Cheng FC, Chen CJ, Lai SZ, Liu MJ, Chang MH, et al. Dietary supplement with fermented soybeans, natto, improved the neurobehavioral deficits after sciatic nerve injury in rats. Neurol Res. (2009) 31(5):441–52. doi: 10.1179/174313209X403878
27. Singer PA, Mehler S. Fasting increases glucose and leucine uptake during regeneration of the hypoglossal nerve in the rat. Neurosci Lett. (1983) 41(1–2):115–8. doi: 10.1016/0304-3940(83)90232-X
28. Mirzakhani N, Farshid AA, Tamaddonfard E, Imani M, Erfanparast A, Noroozinia F. Carnosine improves functional recovery and structural regeneration after sciatic nerve crush injury in rats. Life Sci. (2018) 215:22–30. doi: 10.1016/j.lfs.2018.10.043
29. Avsar Z, Avsar U, Aydin A, Yayla M, Ozturkkaragoz B, Un H, et al. L-carnitine alleviates sciatic nerve crush injury in rats: functional and electron microscopy assessments. Neural Regen Res. (2014) 9:1020–4. doi: 10.4103/1673-5374.133163
30. Wilson AD, Hart A, Wiberg M, Terenghi G. Acetyl-L-carnitine increases nerve regeneration and target organ reinnervation – a morphological study. J Plast Reconstr Aesthet Surg. (2010) 63(7):1186–95. doi: 10.1016/j.bjps.2009.05.039
31. Mohammadi R, Amini K. Topically-administered acetyl-L-carnitine increases sciatic nerve regeneration and improves functional recovery after tubulization of transected short nerve gaps. J Neurosurg Sci. (2017) 61(4):395–402. doi: 10.23736/S0390-5616.16.02845-9
32. Kokkalis ZT, Soucacos PN, Terzis JK. Effect of acetyl-L-carnitine on axonal sprouting following donor nerve injury distal to an end-to-side neurorrhaphy model. J Reconstr Microsurg. (2009) 25(8):483–95. doi: 10.1055/s-0029-1234027
33. Wilson AD, Hart A, Brännström T, Wiberg M, Terenghi G. Delayed acetyl-L-carnitine administration and its effect on sensory neuronal rescue after peripheral nerve injury. J Plast Reconstr Aesthet Surg. (2007) 60(2):114–8. doi: 10.1016/j.bjps.2006.04.017
34. Kencebay Manas C, Derin N, Arican RY, Tanriover G, Dilmac S, Ozcanli H. Comparison of the therapeutic effects of erythropoietin and acetyl-L-carnitine on sciatic nerve injury in rats. Neurol Res. (2022) 44(7):659–66. doi: 10.1080/01616412.2022.2029293
35. Curran MWT, Morhart MJ, Olson JL, Hachisuka A, Chan KM. Acetyl-L-carnitine to enhance nerve regeneration in carpal tunnel syndrome. Plast Reconstr Surg. (2019) 143(1):111e–20e. doi: 10.1097/PRS.0000000000005089
36. Altun I, Kurutas EB. Vitamin B complex and vitamin B12 levels after peripheral nerve injury. Neural Regen Res. (2016) 11(5):842–5. doi: 10.4103/1673-5374.177150
37. Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plast. (2013) 2013:424651. doi: 10.1155/2013/424651
38. Kong X, Sun X, Zhang J. The protective role of mecobalamin following optic nerve crush in adult rats. Yan Ke Xue Bao. (2004) 20(3):171–7.15499726
39. Iskandar BJ, Nelson A, Resnick D, Skene JH, Gao P, Johnson C, et al. Folic acid supplementation enhances repair of the adult central nervous system. Ann Neurol. (2004) 56(2):221–7. doi: 10.1002/ana.20174
40. Kang WB, Chen YJ, Lu DY, Yan JZ. Folic acid contributes to peripheral nerve injury repair by promoting Schwann cell proliferation, migration, and secretion of nerve growth factor. Neural Regen Res. (2019) 14(1):132–9. doi: 10.4103/1673-5374.243718
41. Chabas JF, Stephan D, Marqueste T, Garcia S, Lavaut MN, Nguyen C, et al. Cholecalciferol [vitamin D(3)] improves myelination and recovery after nerve injury. PLoS One. (2013) 8(5):e65034. doi: 10.1371/journal.pone.0065034
42. Tamaddonfard E, Farshid AA, Maroufi S, Kazemi-Shojaei S, Erfanparast A, Asri-Rezaei S, et al. Effects of safranal, a constituent of saffron, and vitamin E on nerve functions and histopathology following crush injury of sciatic nerve in rats. Phytomedicine. (2014) 21(5):717–23. doi: 10.1016/j.phymed.2013.10.031
43. Lu R, Kallenborn-Gerhardt W, Geisslinger G, Schmidtko A. Additive antinociceptive effects of a combination of vitamin C and vitamin E after peripheral nerve injury. PLoS One. (2011) 6(12):e29240. doi: 10.1371/journal.pone.0029240
44. Banjanin N, Belojevic G. Changes of blood pressure and hemodynamic parameters after oral magnesium supplementation in patients with essential hypertension-an intervention study. Nutrients. (2018) 10(5):581. doi: 10.3390/nu10050581
45. Hopkins TM, Little KJ, Vennemeyer JJ, Triozzi JL, Turgeon MK, Heilman AM, et al. Short and long gap peripheral nerve repair with magnesium metal filaments. J Biomed Mater Res A. (2017) 105(11):3148–58. doi: 10.1002/jbm.a.36176
46. Li BH, Yang K, Wang X. Biodegradable magnesium wire promotes regeneration of compressed sciatic nerves. Neural Regen Res. (2016) 11(12):2012–7. doi: 10.4103/1673-5374.197146
47. Pan HC, Sheu ML, Su HL, Chen YJ, Chen CJ, Yang DY, et al. Magnesium supplement promotes sciatic nerve regeneration and down-regulates inflammatory response. Magnes Res. (2011) 24(2):54–70. doi: 10.1684/mrh.2011.0280
48. Renno WM, Saleh F, Klepacek I, Al-Khaledi G, Ismael H, Asfar S. Green tea pain modulating effect in sciatic nerve chronic constriction injury rat model. Nutr Neurosci. (2006) 9(1–2):41–7. doi: 10.1080/10284150600576705
49. Machova Urdzikova L, Ruzicka J, Karova K, Kloudova A, Svobodova B, Amin A, et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology. (2017) 126:213–23. doi: 10.1016/j.neuropharm.2017.09.006
50. Kian K, Khalatbary AR, Ahmadvand H, Karimpour Malekshah A, Shams Z. Neuroprotective effects of (−)-epigallocatechin-3-gallate (EGCG) against peripheral nerve transection-induced apoptosis. Nutr Neurosci. (2019) 22(8):578–86. doi: 10.1080/1028415X.2017.1419542
51. Hsu CC, Huang HC, Wu PT, Tai TW, Jou IM. Sesame oil improves functional recovery by attenuating nerve oxidative stress in a mouse model of acute peripheral nerve injury: role of Nrf-2. J Nutr Biochem. (2016) 38:102–6. doi: 10.1016/j.jnutbio.2016.09.003
52. Ramli D, Aziz I, Mohamad M, Abdulahi D, Sanusi J. The changes in rats with sciatic nerve crush injury supplemented with evening primrose oil: behavioural, morphologic, and morphometric analysis. Evid Based Complement Alternat Med. (2017) 2017:3476407. doi: 10.1155/2017/3476407
53. Pan B, Jing L, Cao M, Hu Y, Gao X, Bu X, et al. Melatonin promotes Schwann cell proliferation and migration via the shh signalling pathway after peripheral nerve injury. Eur J Neurosci. (2021) 53(3):720–31. doi: 10.1111/ejn.14998
54. Rateb EE, Amin SN, El-Tablawy N, Rashed LA, El-Attar S. Effect of melatonin supplemented at the light or dark period on recovery of sciatic nerve injury in rats. EXCLI J. (2017) 16:138–50. doi: 10.17179/excli2016-763
Keywords: nutrition, supplements, peripheral nervous system, injury, surgery, regeneration, functional recovery
Citation: Lepić S, Lepić M, Banjanin N, Mandić-Rajčević S and Rasulić L (2022) A review of the diet, nutrients, and supplementation potential for the outcome augmentation in surgical treatment of peripheral nerve injuries. Front. Surg. 9:942739. doi: 10.3389/fsurg.2022.942739
Received: 12 May 2022; Accepted: 13 October 2022;
Published: 9 November 2022.
Edited by:
Shimon Rochkind, Tel Aviv University, IsraelReviewed by:
Stefano Ferraresi, Hospital Santa Maria della Misericordia of Rovigo, ItalyLiliana Lykowska-Szuber, Human Nutrition and Internal Medicine University Poznan, Poland
© 2022 Lepić, Lepić, Banjanin, Mandić-Rajčević and Rasulić. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas Rasulić bHVrYXMucmFzdWxpY0BnbWFpbC5jb20=
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Sanja Lepić
Sanja Lepić Milan Lepić
Milan Lepić Nikolina Banjanin
Nikolina Banjanin Stefan Mandić-Rajčević5
Stefan Mandić-Rajčević5 Lukas Rasulić
Lukas Rasulić