- 1Department of Thoracic Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Thoracic Surgery, Fujian Medical University Fujian Union Hospital, Fuzhou, China
- 3Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital / Guangdong Academy of Medical Sciences, Guangzhou, China
Objective: Anatomical segmentectomy has been proven to be a viable surgical treatment for small-size peripheral lung nodules. Three-dimensional (3D) reconstruction computed tomography (CT) has been proposed as an effective approach to overcome the challenges of encountering pulmonary anatomical variations when performing segmentectomy. Therefore, to further investigate the usefulness of preoperative 3D reconstruction CT in segmentectomy, we will conduct this prospective, multicenter randomized controlled DRIVATS study to compare the use of 3D reconstruction CT with standard chest CT in video-assisted segmentectomy (ClinicalTrials.gov ID: NCT04004494).
Methods: This study began in July 2019 and a total of 190 patients will be accrued from three clinical centers within 4 years. The main inclusion criteria are patients with a single peripheral nodule 0.8–2 cm with at least one of the following requirements: (i) histology of adenocarcinoma in situ; (ii) nodule has ≥50% ground-glass appearance on CT; (iii) radiologic surveillance confirms a long doubling time (≥400 days). Surgical procedures include segmental resection of the lesion and mediastinal lymph node sampling (subsegmental resection or combined subsegmental resection will not be included in this study). The primary endpoint is operative time. The secondary endpoints include incidence of change of surgical plan, intraoperative blood loss, conversion rate, operative accident event, incidence of postoperative complications, postoperative hospital stay, length of hospitalization, duration of chest tube placement, postoperative 30-day mortality, dissection of lymph nodes, overall survival, disease-free survival, preoperative lung function, and postoperative lung function.
Discussion: This multicenter DRIVATS study aims to verify the usefulness of preoperative 3D reconstruction CT compared with standard chest CT in segmentectomy. If successfully completed, this multicenter prospective study will provide a higher level of evidence for the use of 3D reconstruction CT in segmentectomy.
Introduction
Lung cancer is the leading cause of cancer-related death across the world (1). In 2020, lung cancer remained the leading cause of cancer death, with an estimated 1.8 million deaths (2). Non-small-cell lung cancer (NSCLC) is the predominant type of lung cancer, which takes up 85% of lung cancer (3). With the increasing usage of new screening technology such as low-dose computed tomography (CT), the detection of small pulmonary nodules, particularly small ground-glass opacity (GGO) nodules, has increased (4). There is an increasing interest in pursuing anatomical segmentectomy for these presumably low-risk tumors, especially for patients with impaired pulmonary function reserve. A series of studies conducted have validated the feasibility of segmentectomy in the treatment of these small-size nodules, which could achieve a long-term oncological effect comparable to that achieved with standard lobectomy (5, 6).
Maintaining an accurate surgical plane and a secure surgical margin is of great importance for a successful segmentectomy of a small and relatively deep pulmonary nodule, which makes it more challenging than lobectomy and identifies the need for a thorough understanding of the segmental anatomy. Vascular and bronchial variations of lung segments are inevitable. Anatomical variations of pulmonary vessels can cause serious problems such as unwanted bleeding in patients undergoing video-assisted thoracic surgery (VATS) (7–9) and some variation patterns of pulmonary vessels have been summarized in previous studies (10–13). Careful preoperative evaluation and surgical techniques are needed to prevent intraoperative damage due to unexpected anatomic anomalies. The technique of three-dimensional (3D) reconstruction CT has received a lot of attention as it can provide accurate information about the nodule segmental location as well as its relationship with intersegmental lines (14).
Some new 3D imaging software programs using 2D CT data have been developed and have shown some advantages in planning accurate segmental resections (15–18). A series of retrospective studies have been conducted, which have shown that this 3D reconstruction CT technique has some benefits in a clear delineation of the anatomy of the pulmonary vessel branches and adequate identification of anatomic variations (17, 19–22). Compared to patients who received preoperative standard chest CT, significantly shorter operative time and less intraoperative blood loss were observed in patients receiving preoperative 3D reconstruction CT (22, 23). Most of the studies that evaluated the benefits of preoperative 3D reconstruction CT were retrospective studies, with only one quasi-randomized clinical trial (23). However, in this quasi-randomized clinical trial, the number of patients included in the two groups was limited. Taken together, these results highlight the need for further investigation with preoperative 3D reconstruction CT in segmentectomy.
The results of previous studies justify a further prospective evaluation of the use of preoperative 3D reconstruction CT in segmental resections. Therefore, we designed and conducted this multicenter, randomized controlled DRIVATS study to compare the usefulness of 3D reconstruction CT and standard chest CT in preoperative planning of video-assisted segmentectomy (ClinicalTrials.gov ID: NCT04004494).
Materials and methods
Study design
The DRIVATS study is a prospective, multicenter, randomized controlled clinical trial to compare the usefulness of 3D reconstruction CT and standard chest CT in preoperative planning of video-assisted segmentectomy. Three high-volume medical centers in China are participating in this study (Ruijin Hospital, Shanghai Jiao Tong University School of Medicine; Fujian Medical University Union Hospital; and Guangdong Provincial People's Hospital). Ethics approval has been obtained from Ruijin Hospital Ethics Committee on March 21, 2019 (approval number, 2019-19). Other two clinical centers have also obtained ethics approval from their local ethics committee (approval number, 2019YF032-01 and GDREC2019522H) and a copy has been forwarded to the Central Coordinating Centre. All patients are required to sign an informed consent form after personal counseling by an independent research coordinator. This study was initiated in July 2019. With an estimated inclusion period of 4 years, the primary endpoint is anticipated to be achieved in December 2023.
Eligibility criteria
The inclusion criteria are as follows:
• Age older than 18 years.
• Pulmonary nodules or GGO found in chest CT examination and conform with indications for segmentectomy:
• Peripheral nodule 0.8–2 cm with at least one of the following requirements:
I. Histology of adenocarcinoma in situ,
II. Nodule has ≥50% ground-glass appearance on CT, and
III. Radiologic surveillance confirms a long doubling time (≥400 days).
• Adequate cardiac function, respiratory function, liver function, and renal function for anesthesia and VATS segmentectomy.
• American Society of Anesthesiologists (ASA) score: Grade I–III.
• Patients who can coordinate the treatment and research and sign the informed consent.
The exclusion criteria are as follows:
• Patients with significant medical condition which is thought unlikely to tolerate the surgery. For example, cardiac disease, and significant liver and renal function disorder.
• Patients with psychiatric diseases who may not comply with the protocol.
• Patients with a history of chest trauma or surgery on the ipsilateral chest, which may cause pleural adhesion.
Endpoints
The primary endpoint of this study is operative time.
The secondary endpoints are listed as follows:
• Incidence of change of surgical plan: change of surgical plan will be recorded when the actual resected bronchus and vessels are different from those decided in the preoperative surgical plan;
• Intraoperative blood loss;
• Postoperative hospital stay;
• Duration of chest tube placement;
• Length of hospitalization;
• Incidence of postoperative complications, which mainly include pneumonia, arrhythmia, incision infection, vocal cord paralysis, and trachea cannula;
• Conversion rate, defined as the rate of conversion to open surgery in the operation;
• Operative accident event, defined as the accident event that happened in operative, for example, a segmentectomy is converted to a lobectomy;
• Postoperative 30-day mortality;
• Dissection of lymph nodes, which includes overall lymph node count, number of stations dissected, and number of lymph nodes in each lymph node station;
• Five-year overall survival (OS) rate;
• Five-year disease-free survival rate;
• Preoperative lung function, which includes forced expiratory volume at 1 s (FEV1) in liter and maximal voluntary ventilation (MVV) in liter; and
• Postoperative lung function, which includes FEV1 in liter and MVV in liter;
Other prespecified outcome measures include total hospitalization expenditures and anatomical variations. Anatomical variations are defined as the rates of different anatomical variations of segmental bronchus and pulmonary vessels in the included patients.
Randomization
Stratified blocked randomization was used for the randomization of the eligible patients. The randomization sequence was generated by using the Statistical Analysis System software version 9.4 (SAS Institute Inc., Cary, United States). Randomization was stratified by the surgeons. For each surgeon, a unique randomization sequence was generated by using the random permuted-block design (with blocks of varying sizes) to randomize patients in a 1:1 ratio to one of the two groups, 3D reconstruction CT or the standard chest CT. This method ensures that an approximately equal number of patients will be allocated to each treatment group.
Treatment methods
Three-dimensional reconstruction CT and standard chest CT were conducted preoperatively to assess the location of the lung nodule. In both groups, contrast-enhanced CT was performed in the supine position and full limb extension with 1 mm slice thickness. In the 3D reconstruction CT group, contrast-enhanced CT imaging data were imported into a 3D image-processing software for the 3D reconstruction of the pulmonary lobes, vessels, and bronchial trees. An example of the 3D reconstruction CT is illustrated in Figure 1.
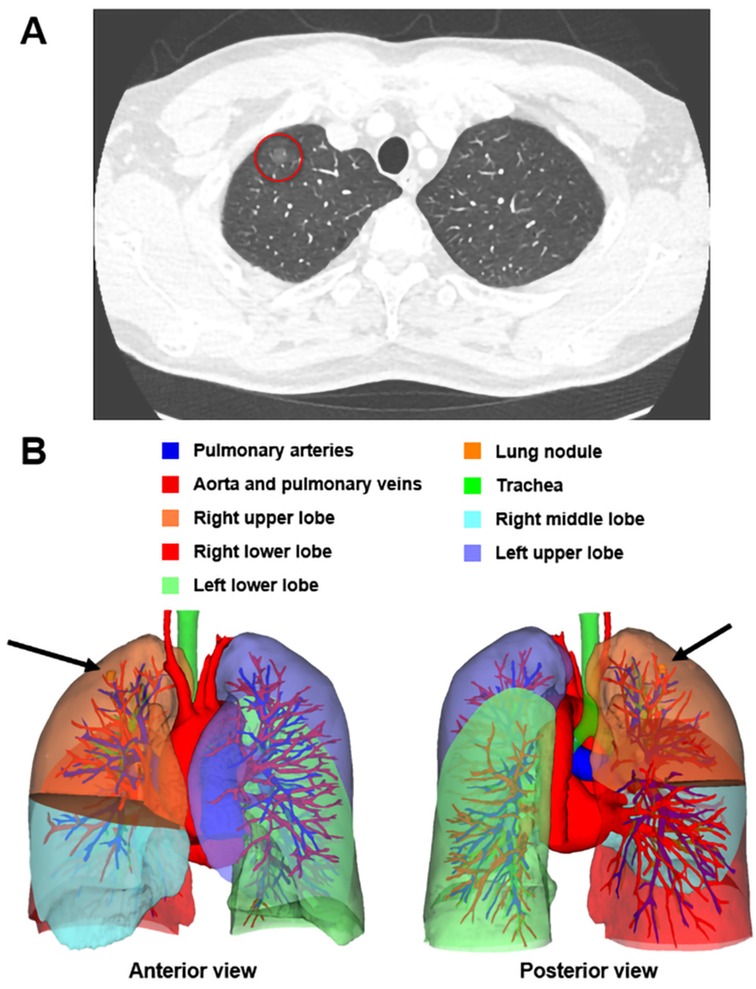
Figure 1. A 47-year-old male patient was admitted into our hospital after his chest CT scan revealed a GGO (A, red circle) in his right lung in his routine physical examination. The preoperative 3D reconstruction CT located the nodule in the peripheral region of the apical segment of the right upper lobe, measuring 7.6 mm × 7.3 mm × 7.8 mm in size (B, black arrow). This man received an apical segmentectomy and the pathology of this nodule was AIS. AIS, adenocarcinoma in situ; CT, computed tomography; GGO, ground-glass opacity; 3D, three-dimensional.
In both groups, video-assisted thoracoscopic segmentectomy is performed with double-lumen endotracheal intubation and combined intravenous and inhalation anesthesia. The location and number of thoracoscopic ports are not limited, which depend on the habits of the surgeons. Surgical procedures include segmental resection of the lesion and mediastinal lymph node sampling (subsegmental resection or combined subsegmental resection are not included in this study). The distance from the dissection margin to the tumor edge must be no less than the maximum tumor diameter.
Appropriate N1 and N2 lymph node stations should be dissected for each patient. Hilar lymph node dissection must include ipsilateral node dissection of stations 10–13. The lymphatic drainage not only reaches the lymph nodes of the resected segment but also reaches the lymph nodes posterior to the resected segment (24). Therefore, the posterior node station 13 should be dissected, particularly when the tumor is located in the anterior segments. Node stations 12 or 13 are required for intraoperative frozen section analysis. For mediastinal lymph nodes sampling, the dissected N2 lymph nodes stations are dependent on the location of the lung nodule, based on the lobe-specific lymphatic drainage pattern (25). The mediastinal lymph node sampling will be carried out irrespective of the status of N1 lymph nodes.
Follow-up
All randomized patients are followed up for 5 years. Standard chest CT is evaluated at least every 6 months during the first 2 years and at least every 12 months for the duration of follow-up. When local recurrence or distance metastasis is suspected during follow-up, an additional visit will be scheduled, and contrast-enhanced chest CT or PET-CT will be performed.
Statistical analysis
According to Yao et al. (26), the operative time of segmentectomy with 3D image reconstruction was reported to be 121.5 min (range, 95–210 min). A retrospective study comparing 3D reconstruction CT and standard chest CT for segmentectomy reported the operative time to be 141.9 ± 29.1 and 160.9 ± 31.5 min, respectively (22). In our clinical center, we summarized the operative time of 124 cases of video-assisted segmentectomy with standard chest CT, which was 141.1 ± 40 min. Based on previous studies, the operative time of 3D reconstruction CT and standard chest CT for segmentectomy was assumed to be 120 and 140 min, respectively, with a difference of 20 min. With a power of 80%, a sample size of 86 will be required to detect a difference in operative time between 3D reconstruction CT group and standard chest CT group with a two-sided significance level of 5%. To allow for a 10% of dropout cases, the sample size was increased to 95 cases in each group. Data analysis will comply with the intention-to-treat principle. Data will be recorded and collected via standardized case report form.
Discussion
The DRIVATS study is a prospective, multicenter, randomized controlled clinical trial that aims to compare the usefulness of 3D reconstruction CT and standard chest CT in video-assisted segmentectomy. The flowchart of this study is shown in Figure 2. By conducting this clinical trial, we aim to provide evidence of the use of 3D reconstruction CT in the preoperative planning of lung segmentectomy.
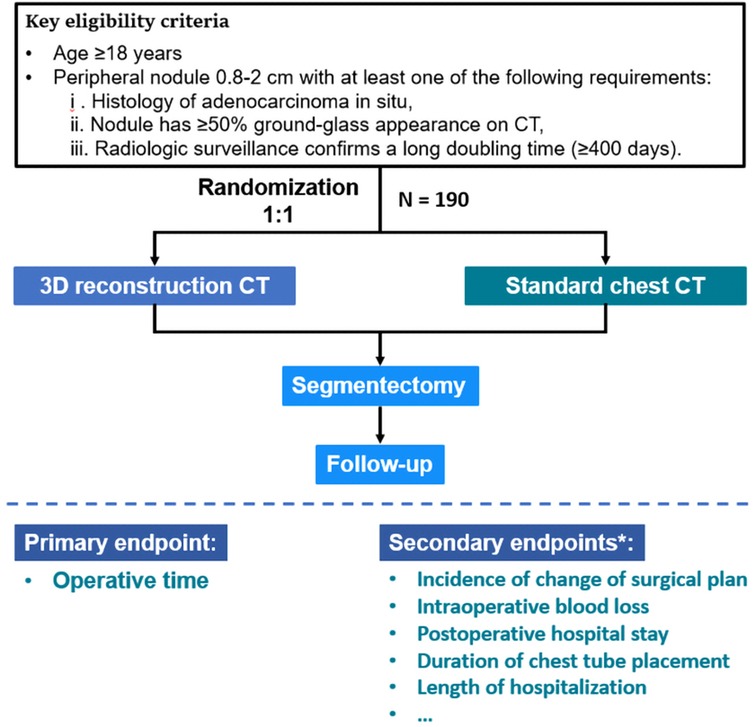
Figure 2. Trial design and flow chart of the DRIVATS study. *Details of the secondary endpoints are described in Materials and methods. CT, computed tomography; 3D, three-dimensional.
Pulmonary segmentectomy was first described in 1,939 for the bronchiectasis of lingula segment in the left upper lobe (27). Later, the feasibility of lung segmentectomy in the treatment of lung cancer was further investigated in several studies, which showed comparable results compared with lobectomy (28–30). In 1995, as the landmark clinical trial by the Lung Cancer Study Group (31) showed a higher death rate and local recurrence rate associated with limited resection, lobectomy was then considered the standard surgical procedure of choice for patients with peripheral T1N0 NSCLC. However, as more and more smaller lung nodules (≤2.0 cm) were detected, retrospective studies from single or several institutions showed that segmentectomy for tumors ≤2.0 cm also had excellent local control, with comparable survival with lobectomy, especially when performed by VATS (32–35). Recently, with the publication of the long-awaited prospective clinical trial JCOG0802, together with JCOG0804, which confirmed the noninferior results of relapse-free survival and OS brought by segmentectomy compared with lobectomy, segmentectomy has been established as a noninferior surgical procedure compared with lobectomy for patients with peripheral tumor ≤2 cm (5, 6).
When performing a segmentectomy, it is of great importance to achieve adequate margins and complete removal of the segment containing the tumor, which is not easy since the tumor is not always palpable. Thorough knowledge of segmental anatomy and tailored preoperative planning with the assessment of surgical margins are the essential prerequisite for a successful segmentectomy (36). Chest CT plays an indispensable role in the preoperative planning of any pulmonary surgery. However, it is hard for surgeons to have a thorough understanding of lung anatomy with only conventional CT images. The introduction of 3D reconstruction CT for preoperative assessment, which allows an intuitive recognition of the anatomy, has gained popularity amongst thoracic surgeons (16, 19, 20, 37). Pulmonary artery branches can be precisely identified in 3D reconstruction CT images, with an accuracy rate of over 95% based on previous studies (12, 20, 38). Additionally, preoperative 3D CT could significantly shorten the operative time of segmentectomy and lead to reduced intraoperative blood loss and postoperative complications compared with preoperative conventional 2D CT (22, 23).
Identification of anatomical anomalies is especially helpful for segmentectomy, which may increase the risks of complications during surgery. 3D imaging can be used for screening anatomical anomalies (7–9). Based on the 3D CT images, different patterns of anatomical variations have been identified and categorized, which could facilitate the creation of simplified models for use in preoperative planning for segmentectomy (10–13). In this DRIVATS study, we also aimed to prospectively collect the anatomical anomalies in the included patients. This anatomical data, along with all possible variations, will surely facilitate safe and accurate lung resections for thoracic surgeons, irrespective of the presence of preoperative 3D reconstruction CT.
Accurate pathologic nodal staging is especially important for sublobar resections like segmentectomy. False-negative results of lymph nodes have a negative impact on the prognosis of the patients (39). Based on a large cohort of NSCLC ≤3 cm, Naruke et al. (40) reported that the rates of lymph node stations 12 and 13 metastases were 12.4% and 8.4%, respectively. When the intrapulmonary lobar and segmental lymph nodes were not dissected, the false-negative rate for N staging would be 9.0% (41). In this DRIVATS study, intraoperative fast-frozen pathology of node stations 12 and 13 is mandatory which is a key surgical procedure to ensure the oncological effect of segmentectomy. Segmentectomy would not be suitable if metastasis to the adjacent segmental lymph nodes was confirmed by fast-frozen pathology.
However, some practical and operational limitations exist when performing this multicenter clinical trial. First, the endpoints of our study may be susceptible to some confounding factors such as the surgical procedures and techniques, and the experience of the surgeons. We believe randomization and subsequent subgroup analysis may relieve this bias to some extent. Second, the different types of CT scanners as well as reconstruction techniques adopted by different medical centers may affect the study results. This may have an impact on the generalization of this study's conclusions.
In summary, the DRIVATS study is a prospective, multicenter, randomized controlled clinical trial that aims to compare the usefulness of 3D reconstruction CT and standard chest CT in video-assisted segmentectomy. The results of this study will facilitate the clinical application of segmentectomy and improve the accuracy and safety of segmentectomy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethics approval has been obtained from Ruijin Hospital Ethics Committee on March 21, 2019 (approval number, 2019-19).
Author contributions
ZN, KC, and RJ contributed to the design, collection of data, manuscript, and editing. BZ, XG, QN, and BJ contributed to the collection of data. WZ, CC, and HL contributed to the design of the work. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Key Research and Development Program of China (2021YFC2500903, 2021YFC2500905), National Natural Science Foundation of China (82072557, 81871882), Shanghai Municipal Commission of Health and Family Planning Outstanding Academic Leaders Training Program (2017BR055), and Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant (20172005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553(7689):446–54. doi: 10.1038/nature25183
4. Kudo Y, Matsubayashi J, Saji H, Akata S, Shimada Y, Kato Y, et al. Association between high-resolution computed tomography findings and the IASLC/ATS/ERS classification of small lung adenocarcinomas in Japanese patients. Lung Cancer. (2015) 90(1):47–54. doi: 10.1016/j.lungcan.2015.07.007
5. Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg. (2022) 163(1):289.e282–301.e282. doi: 10.1016/j.jtcvs.2020.09.146
6. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607l): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. (2022) 399(10335):1607–17. doi: 10.1016/S0140-6736(21)02333-3
7. Xu XF, Chen L, Wu WB, Zhu Q. Thoracoscopic right posterior segmentectomy of a patient with anomalous bronchus and pulmonary vein. Ann Thorac Surg. (2014) 98(6):e127–29. doi: 10.1016/j.athoracsur.2014.09.059
8. Hayashi K, Motoishi M, Horimoto K, Sawai S, Hanaoka J. Left upper division segmentectomy with a simultaneous displaced bronchus and pulmonary arteriovenous anomalies: a case report. J Cardiothorac Surg. (2018) 13(1):40. doi: 10.1186/s13019-018-0741-6
9. Yamada S, Suga A, Inoue Y, Iwazaki M. Importance of preoperative assessment of pulmonary venous anomaly for safe video-assisted lobectomy. Interact Cardiovasc Thorac Surg. (2010) 10(6):851–4. doi: 10.1510/icvts.2009.221804
10. Shimizu K, Nagashima T, Ohtaki Y, Obayashi K, Nakazawa S, Kamiyoshihara M, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg. (2016) 64(10):604–11. doi: 10.1007/s11748-016-0686-4
11. Zhang M, Mao N, Zhang K, Zhang M, Liu Y, Wang RF, et al. Analysis of the variation pattern in left upper division veins and establishment of simplified vein models for anatomical segmentectomy. Ann Transl Med. (2020) 8(22):1515. doi: 10.21037/atm-20-6925
12. Nagashima T, Shimizu K, Ohtaki Y, Obayashi K, Kakegawa S, Nakazawa S, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg. (2015) 63(6):354–60. doi: 10.1007/s11748-015-0531-1
13. Ishikawa Y, Iwano S, Usami N, Yokoi K. An anomalous segmental vein of the left upper lobe of the lung: preoperative identification by three-dimensional computed tomography pulmonary angiography. Interact Cardiovasc Thorac Surg. (2012) 15(3):512–3. doi: 10.1093/icvts/ivs205
14. Gavrielides MA, Zeng R, Myers KJ, Sahiner B, Petrick N. Benefit of overlapping reconstruction for improving the quantitative assessment of CT lung nodule volume. Acad Radiol. (2013) 20(2):173–80. doi: 10.1016/j.acra.2012.08.014
15. Chan EG, Landreneau JR, Schuchert MJ, Odell DD, Gu S, Pu J, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. (2015) 150(3):523–8. doi: 10.1016/j.jtcvs.2015.06.051
16. Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis. (2016) 8(Suppl 3):S295–301.
17. Yang Q, Xie B, Hu M, Sun X, Huang X, Guo M. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg. (2016) 23(2):183–9. doi: 10.1093/icvts/ivw085
18. Nakao M, Omura K, Hashimoto K, Ichinose J, Matsuura Y, Okumura S, et al. Novel three-dimensional image simulation for lung segmentectomy developed with surgeons’ perspective. Gen Thorac Cardiovasc Surg. (2021) 69(9):1360–5. doi: 10.1007/s11748-021-01666-6
19. Oizumi H, Kanauchi N, Kato H, Endoh M, Suzuki J, Fukaya K, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg. (2011) 141(3):678–82. doi: 10.1016/j.jtcvs.2010.08.027
20. Hagiwara M, Shimada Y, Kato Y, Nawa K, Makino Y, Furumoto H, et al. High-quality 3-dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short-term surgical outcomes in consecutive patients undergoing video-assisted thoracic surgery. Eur J Cardiothorac Surg. (2014) 46(6):e120–26. doi: 10.1093/ejcts/ezu375
21. Kato H, Oizumi H, Suzuki J, Hamada A, Watarai H, Sadahiro M. Thoracoscopic anatomical lung segmentectomy using 3D computed tomography simulation without tumour markings for non-palpable and non-visualized small lung nodules. Interact Cardiovasc Thorac Surg. (2017) 25(3):434–41. doi: 10.1093/icvts/ivx113
22. She XW, Gu YB, Xu C, Li C, Ding C, Chen J, et al. Three-dimensional (3D)-computed tomography bronchography and angiography combined with 3D-video-assisted thoracic surgery (VATS) versus conventional 2D-VATS anatomic pulmonary segmentectomy for the treatment of non-small cell lung cancer. Thorac Cancer. (2018) 9(2):305–9. doi: 10.1111/1759-7714.12585
23. Chen Y, Zhang J, Chen Q, Li T, Chen K, Yu Q, et al. Three-dimensional printing technology for localised thoracoscopic segmental resection for lung cancer: a quasi-randomised clinical trial. World J Surg Oncol. (2020) 18(1):223. doi: 10.1186/s12957-020-01998-2
24. Nomori H, Ohba Y, Shibata H, Shiraishi K, Mori T, Shiraishi S. Required area of lymph node sampling during segmentectomy for clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg. (2010) 139(1):38–42. doi: 10.1016/j.jtcvs.2009.04.003
25. Shapiro M, Kadakia S, Lim J, Breglio A, Wisnivesky JP, Kaufman A, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest. (2013) 144(5):1615–21. doi: 10.1378/chest.12-3069
26. Yao F, Wang J, Yao J, Hang F, Lei X, Cao Y. Three-dimensional image reconstruction with free open-source OsiriX software in video-assisted thoracoscopic lobectomy and segmentectomy. Int J Surg. (2017) 39:16–22. doi: 10.1016/j.ijsu.2017.01.079
27. Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis: the lingula segment of the left upper lobe. Ann Surg. (1939) 109(4):481–99. doi: 10.1097/00000658-193904000-00001
28. Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg. (1994) 107(4):1087–93, discussion 1093–4. doi: 10.1016/S0022-5223(12)70385-9
29. Read RC, Yoder G, Schaeffer RC. Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg. (1990) 49(3):391–8, discussion 399–400. doi: 10.1016/0003-4975(90)90242-X
30. Jensik RJ, Faber LP, Kittle CF. Segmental resection for bronchogenic carcinoma. Ann Thorac Surg. (1979) 28(5):475–83. doi: 10.1016/S0003-4975(10)63157-8
31. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung cancer study group. Ann Thorac Surg. (1995) 60(3):615–22, discussion 622–3. doi: 10.1016/0003-4975(95)00537-U
32. Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. (2006) 132(4):769–75. doi: 10.1016/j.jtcvs.2006.02.063
33. Shapiro M, Weiser TS, Wisnivesky JP, Chin C, Arustamyan M, Swanson SJ. Thoracoscopic segmentectomy compares favorably with thoracoscopic lobectomy for patients with small stage I lung cancer. J Thorac Cardiovasc Surg. (2009) 137(6):1388–93. doi: 10.1016/j.jtcvs.2009.02.009
34. Schuchert MJ, Pettiford BL, Pennathur A, Abbas G, Awais O, Close J, et al. Anatomic segmentectomy for stage I non-small-cell lung cancer: comparison of video-assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg. (2009) 138(6):1318.e1–25.e1. doi: 10.1016/j.jtcvs.2009.08.028
35. Atkins BZ, Harpole DH Jr, Mangum JH, Toloza EM, D'Amico TA, Burfeind WR Jr. Pulmonary segmentectomy by thoracotomy or thoracoscopy: reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg. (2007) 84(4):1107–12, discussion 1112–3. doi: 10.1016/j.athoracsur.2007.05.013
36. Nakazawa S, Shimizu K, Mogi A, Kuwano H. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg. (2018) 66(2):81–90. doi: 10.1007/s11748-017-0878-6
37. Shimizu K, Nakazawa S, Nagashima T, Kuwano H, Mogi A. 3D-CT anatomy for VATS segmentectomy. J Vis Surg. (2017) 3:88. doi: 10.21037/jovs.2017.05.10
38. Fukuhara K, Akashi A, Nakane S, Tomita E. Preoperative assessment of the pulmonary artery by three-dimensional computed tomography before video-assisted thoracic surgery lobectomy. Eur J Cardiothorac Surg. (2008) 34(4):875–77. doi: 10.1016/j.ejcts.2008.07.014
39. Osarogiagbon RU, Decker PA, Ballman K, Wigle D, Allen MS, Darling GE. Survival implications of variation in the thoroughness of pathologic lymph node examination in American College of Surgeons Oncology Group Z0030 (Alliance). Ann Thorac Surg. (2016) 102(2):363–69. doi: 10.1016/j.athoracsur.2016.03.095
40. Naruke T, Tsuchiya R, Kondo H, Nakayama H, Asamura H. Lymph node sampling in lung cancer: how should it be done? Eur J Cardiothorac Surg. (1999) 16(Suppl 1):S17–24. doi: 10.1016/S1010-7940(99)00178-5
Keywords: 3D reconstruction CT, segmentectomy, video-assisted thoracoscopic, pulmonary nodules, multicenter randomized controlled trial
Citation: Niu Z, Chen K, Jin R, Zheng B, Gong X, Nie Q, Jiang B, Zhong W, Chen C and Li H (2022) Three-dimensional computed tomography reconstruction in video-assisted thoracoscopic segmentectomy (DRIVATS): A prospective, multicenter randomized controlled trial. Front. Surg. 9:941582. doi: 10.3389/fsurg.2022.941582
Received: 11 May 2022; Accepted: 13 September 2022;
Published: 13 October 2022.
Edited by:
Alexander Kluge, Pius-Hospital Oldenburg, GermanyReviewed by:
Beatrice Aramini, University of Bologna, ItalyMehmet Ali Bedirhan, Yedikule Teaching Hospital, Turkey
Jun Suzuki, Yamagata University, Japan
© 2022 Niu, Chen, Jin, Zheng, Gong, Nie, Jiang, Zhong, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hecheng Li bGloZWNoZW5nMjAwMEBob3RtYWlsLmNvbQ== Chun Chen Y2hlbmNodW4wMjA5QGZqbXUuZWR1LmNu Wenzhao Zhong c3l6aG9uZ3dlbnpoYW9Ac2N1dC5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Thoracic Surgery, a section of the journal Frontiers in Surgery
 Zhenyi Niu
Zhenyi Niu Kai Chen
Kai Chen Runsen Jin
Runsen Jin Bin Zheng
Bin Zheng Xian Gong2
Xian Gong2 Benyuan Jiang
Benyuan Jiang Wenzhao Zhong
Wenzhao Zhong Chun Chen
Chun Chen Hecheng Li
Hecheng Li