- Intervention Treatment Department, The Second Hospital of Dalian Medical University, Dalian, China
Objective: Transarterial chemoembolization with CalliSpheres® Microspheres (CSM-TACE) presents favorable efficacy and tolerable safety in several cancers, while its application in head and neck cancer (HNC) is unclear. Thus, the current pilot study aims to evaluate the efficacy and safety of CSM-TACE in treating HNC.
Methods: A total of 15 HNC patients receiving CSM-TACE at the Second Affiliated Hospital of Dalian Medical University from March 2017 to December 2021 were enrolled in this study. Moreover, treatment information, treatment response, progression-free survival (PFS), overall survival (OS), changes in liver and renal function indices, and adverse events were recorded.
Results: There were nine patients receiving CSM-TACE as first-line treatment and six patients receiving CSM-TACE as second- or above-line treatment; meanwhile, there were seven, seven, and one patient undergoing one time, two times, and three times of CSM-TACE, respectively. Furthermore, the objective response rate (ORR) and the disease control rate (DCR) were 60.0% and 100%, respectively, at the first month; meanwhile, the ORR and the DCR were 53.3% and 73.3%, respectively, at the second month. Moreover, the 1-year PFS rate was 34.1%, and the 1-year OS rate was 38.9%. Additionally, no change in liver function indices (namely, total protein, albumin, total bilirubin, alanine aminotransferase, and aspartate aminotransferase) or in renal function indices (namely, creatinine and blood urea nitrogen) was found before and 1 month after treatment (all P > 0.05). Meanwhile, no severe adverse events were found during and after CSM-TACE.
Conclusion: CSM-TACE illustrates favorable treatment response and survival benefits as well as a tolerable safety profile in HNC patients.
Introduction
Head and neck cancer (HNC) affects the anatomical regions of the head and neck, such as oral and nasal cavity, and larynx (1, 2). It is viewed as one of the most common cancers worldwide, with the main risk factors being consumption of tobacco and alcohol as well as human papillomavirus infection (1, 3, 4). Meanwhile, HNC causes approximately 500,000 deaths annually, which accounts for nearly 3% of all cancerous deaths globally (1, 5). Over the past few decades, great advancements have been made in the treatment of unresectable HNC (radiation therapy, chemotherapy, immunotherapy, etc.); however, efficacy is limited and the safety profile is unfavorable (1, 2, 6–8). Considering that HNC causes a huge global burden on healthcare systems, the exploration of effective and safe treatment to improve the management of HNC has become the need of the hour (4, 9).
Conventional transarterial chemoembolization (cTACE) is able to kill tumor cells by embolizing the tumor blood–feeding artery and releasing chemotherapy drugs, and it has been widely applied in several cancers (10–12). For instance, cTACE provides good survival benefits and possesses a favorable safety profile in hepatocellular carcinoma (10); meanwhile, it has also been reported that cTACE elicits a favorable treatment response from lung cancer patients (11). Currently, drug-eluting beads TACE (DEB-TACE) is being proposed to achieve superior survival benefits in several cancers compared with cTACE, among which CalliSpheres® Microspheres (CSM) (the first microsphere product independently developed in China) can sustainedly and steadily release antitumor drugs in the tumor at high concentrations with favorable embolization efficacy (13–15). Moreover, past studies have proposed that CSM-TACE is effective and safe in treating several cancers (15–17), while its efficacy and safety in HNC are unclear.
Therefore, the current pilot study aims to explore the efficacy and safety of CSM-TACE in treating HNC patients.
Methods
Patients
This study consecutively recruited 15 HNC patients who were treated in the Second Affiliated Hospital of Dalian Medical University from March 2017 to April 2021. The inclusion criteria were: (i) those diagnosed as HNC by pathology, cytology, and imaging examinations; (ii) aged 18 years or older; (iii) about to receive CSM-TACE treatment based on clinical status and willingness; and (iv) available to be followed up regularly. The exclusion criteria were: (i) those allergic to the materials used in the study; (ii) having severe and uncorrectable coagulation abnormalities; (iii) complications with severe infections or other cancers; and (iv) women who had a positive pregnancy test or were breastfeeding. The informed consent forms were signed by the patients themselves. The study was approved by the Ethics Committee.
Data Collection
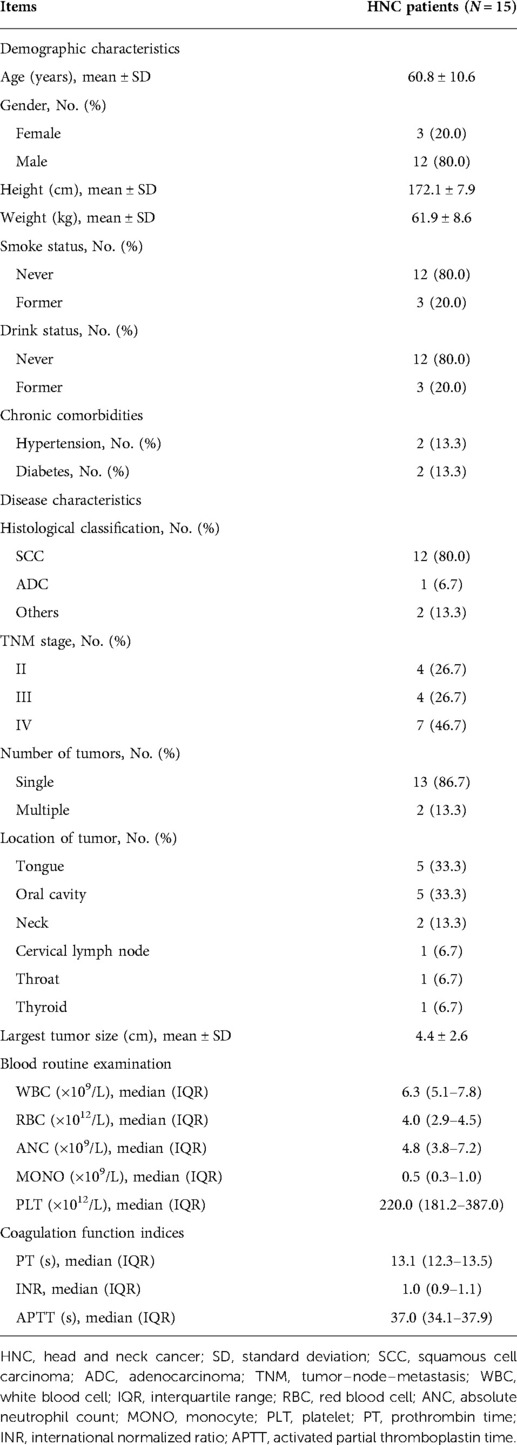
After recruitment and examination, the following clinical characteristics of the patients were recorded: (i) demographic characteristics: age, gender, height, weight, smoke status, and drink status; (ii) chronic comorbidities: hypertension and diabetes; (iii) disease characteristics: histological classification, tumor–node–metastasis (TNM) stage, number of tumors, location of tumor, and the largest tumor size; (iv) blood routine examination: white blood cell (WBC), red blood cell (RBC), absolute neutrophil count (ANC), monocyte (MONO), and platelet (PLT); (v) coagulation function indices: prothrombin time (PT), international normalized ratio (INR), and activated partial thromboplastin time (APTT); (vi) previous treatment history: history of surgery resection, history of chemotherapy, history of radiotherapy, and history of immunotherapy or targeted therapy; and (vii) current treatment: treatment line and times of CSM-TACE.
Treatment
In this study, CalliSpheres® Microspheres (a diameter of 100–300 μm; Jiangsu Hengrui Medicine Co, Ltd., Jiangsu, China) loaded with oxaliplatin (80 mg), bevacizumab (100 mg), epirubicin (40 mg), or cisplatin (40–60 mg) were used as the chemoembolization reagent. CSM-TACE operations were performed in the digital subtraction angiography room. The detailed CSM-TACE procedures are as follows: after routine disinfection, the femoral artery was punctured by the Seldinger technique, and then angiography for the external carotid artery was performed to detect the tumor-supplying vessel. Next, superselective catheterization was performed according to the different areas in which lesions were located, such as the maxillary artery, lingual artery, ascending pharyngeal artery, and superficial temporal artery. Sequentially, the chemoembolization reagent was slowly injected through a microcatheter to the tumor-supplying vessel. The absorbable gelatin sponge microspheres were applied as complementary embolization materials if needed. The endpoint of embolization was stagnation of blood flow in the tumor-supplying vessel. Based on the treatment response at approximately 1 month after CSM-TACE, some patients received treatment to improve efficacy: six patients received cTACE or CSM-TACE; seven received arterial infusion chemotherapy.
Efficacy Evaluation
The treatment response was evaluated using computed tomography examination according to the modified response evaluation criteria in solid tumors at 1 month and 2 months after CSM-TACE operation (18), including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The proportion of patients achieving CR and PR was defined as the objective response rate (ORR), and the proportion of patients achieving CR, PR, and SD was defined as the disease control rate (DCR). In addition, all patients were followed up regularly, and the median follow-up period was 5.2 months. The last date of follow-up was December 20, 2021. Based on the follow-up, the progression-free survival (PFS) rate and the overall survival (OS) rate were calculated. PFS was defined as the duration from the CSM-TACE operation to disease progression or the patient's death; OS was defined as the duration from the CSM-TACE operation to the patient's death.
Safety Evaluation
Liver function indices and renal function indices were used to evaluate the safety of treatment, which was assessed before treatment and 1 month after treatment. The liver function indices included total protein (TP), albumin (ALB), total bilirubin (TBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). The renal function indices included creatinine (Cr) and blood urea nitrogen (BUN). In addition, adverse events during and after CSM-TACE were also recorded, such as allergy, pain, fever, vomiting, and spinal cord injury.
Statistics
All statistical analyses were conducted using SPSS Software, version 21.0 (IBM, San Jose, CA, USA), and all graphs were plotted using GraphPad Prism Software, version 6.01 (GraphPad Software Inc., San Diego, CA, USA). Data were expressed as the number of patients (%), mean ± standard deviation, or median (interquartile range). Kaplan–Meier curves were applied to show the PFS and OS of patients. Comparisons of biochemical indices before and after CSM-TACE treatment were analyzed using the Wilcoxon signed-rank test. The significance level of statistics was set as 0.05.
Results
Clinical Characteristics and Treatment Information
Among the 15 HNC patients, the mean age was 60.8 ± 10.6 years; out of these 15, there were 3 (20.0%) females and 12 (80.0%) males. With regard to histological classification, 12 (80.0%) patients had squamous cell carcinoma, 1 (6.7%) had adenocarcinoma, and 2 (13.3%) had other disease conditions. In terms of the TNM stage, there were four (26.7%) patients with stage II, four (26.7%) with stage III, and seven (46.7%) with stage IV. As far as the tumor location was concerned, five (33.3%) patients had tongue tumor, five (33.3%) had oral cavity tumor, two (13.3%) had neck tumor, one (6.7%) had cervical lymph node, one (6.7%) had throat tumor, and one (6.7%) had thyroid tumor; besides, the mean of the largest tumor size was 4.4 ± 2.6 cm. More clinical characteristics are detailed in Table 1.
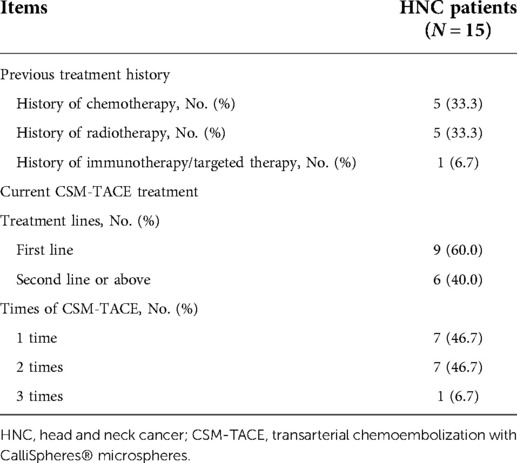
With regard to treatment history, five (33.3%) patients were treated by chemotherapy, five (33.3%) received radiotherapy, and one (6.7%) underwent immunotherapy/targeted therapy. In terms of current CSM-TACE treatment, nine (60.0%) patients received first-line CSM-TACE and six (40.0%) received second- or above-line CSM-TACE; meanwhile, seven (46.7%), seven (46.7%), and one (6.7%) patient received one time, two times, and three times of CSM-TACE, respectively (Table 2).
Treatment Response
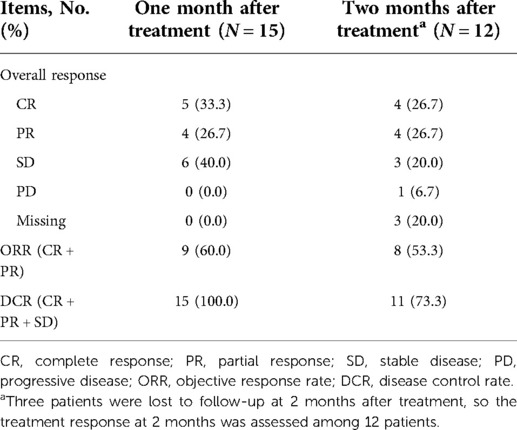
After one month of treatment, five (33.3%) patients achieved CR, four (26.7%) achieved PR, six (40.0%) realized SD, and none (0.0%) had PD; meanwhile, the ORR and DCR were 60.0% and 100%, respectively. In addition, 3 patients lost follow-up after 2 months of treatment, so the treatment response was assessed among 12 patients. The data showed that after 2 months of treatment, four (26.7%), four (26.7%), three (20.0%), and one (6.7%) patient achieved CR, PR, SD, and PD, respectively; besides, the ORR and DCR were 53.3% and 73.3%, respectively (Table 3).
Survival
In order to evaluate the long-term efficacy of CSM-TACE among HNC patients, PFS and OS rates were calculated; the 1-year PFS rate was 34.1% (Figure 1A) and the 1-year OS rate was 38.9% (Figure 1B).
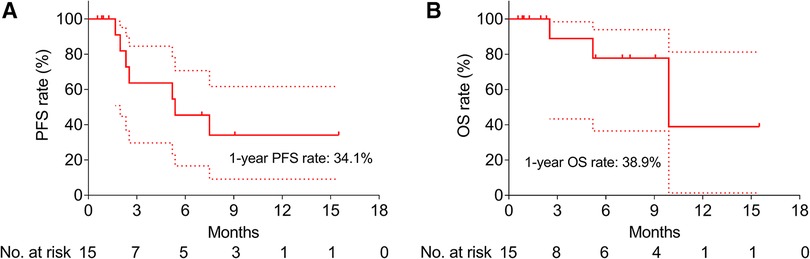
Figure 1. Survival of HNC patients after CSM-TACE. PFS (A) and OS (B) among HNC patients after CSM-TACE. PFS, progression-free survival; OS, overall survival; HNC, head and neck cancer; CSM-TACE, transarterial chemoembolization with CalliSpheres® Microspheres; dotted line, 95% confidence interval.
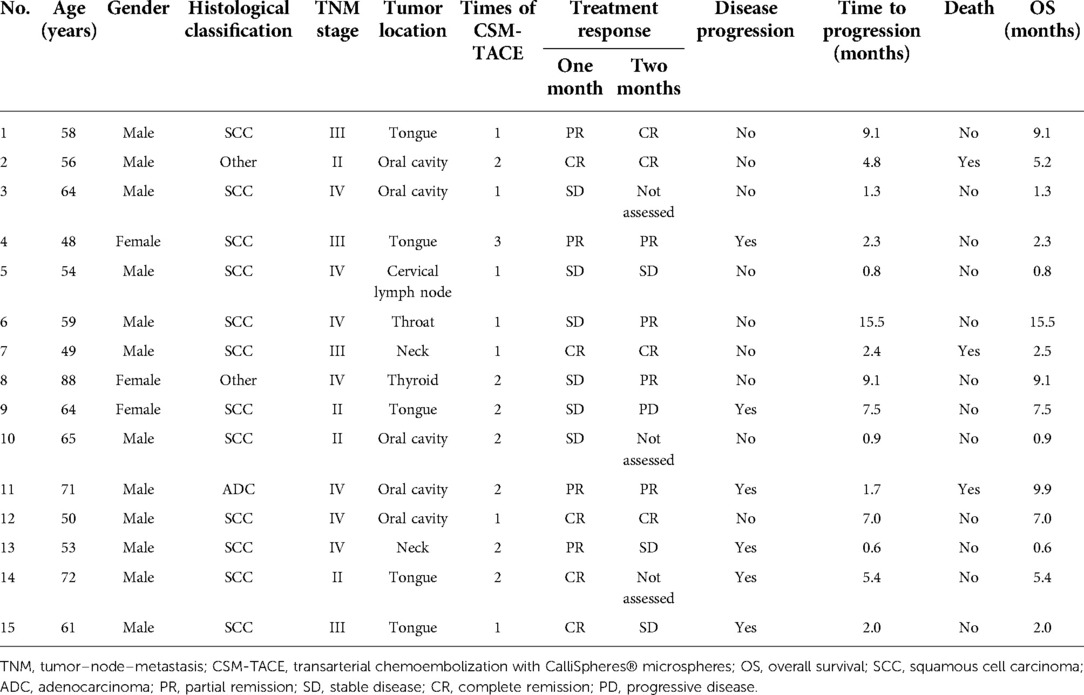
Moreover, PFS (P = 0.456) (Supplementary Figure 1A) and OS (P = 0.590) (Supplementary Figure 1B) did not vary between patients receiving CSM-TACE as first-line treatment and those receiving CSM-TACE as second-line or above-line treatment. Furthermore, no difference in PFS (P = 0.321) (Supplementary Figure 2A) and OS (P = 0.579) (Supplementary Figure 2B) was found in HNC patients having tumors in different locations. In addition, the key clinical characteristics and treatment outcomes of each patient are shown in Table 4 for a detailed presentation.
Liver, Renal Functions, and Adverse Events
No change in liver function indices (namely, TP, ALB, TBIL, ALT, and AST) and in renal function indices (namely, Cr and BUN) was found before and 1 month after treatment (all P > 0.05) (Table 5). In addition, there were no severe adverse events such as allergy, pain, fever, vomiting, and spinal cord injury.
Discussion
Radiotherapy plays an extremely important role in serving as the first-line treatment of both early and locally advanced patients. According to the recommendations given by National Comprehensive Cancer Network (NCCN) guidelines and Chinese Society of Clinical Oncology (CSCO) guidelines, applying radiotherapy as an early postoperative adjuvant therapy has been considered as the primary and nonsurgical choice for locally advanced patients, while chemotherapy has been recommended for locally advanced or advanced patients (19, 20). However, treatment response is still unsatisfactory among HNC patients after the current treatment regimen (21–25). For instance, the ORR reaches 46% among HNC patients after radiotherapy. However, radiation causes postradiation damage to the irradiated area, including changes in skin properties, local vascular toughness, and local soft tissue fibrosis. Irreversible tissue damage is often a result of radiotherapy, which affects subsequent treatment (26). Furthermore, HNC patients undergoing chemotherapy achieve an ORR of 29.9% (25); moreover, it has been reported that HNC patients achieve an ORR of 33% after immunotherapy (21). Thus, the exploration of new types of treatment has become necessary.
In this study, the ORR and DCR were 60.0% and 100% after 1 month of CSM-TACE, and they were 53.3% and 73.3% after 2 months of CSM-TACE. Compared with HNC treatment recommended by various guidelines (including National Comprehensive Cancer Network Clinical Practice Guidelines and Chinese Society of Clinical Oncology diagnosis and treatment guidelines for head and neck cancer 2018), HNC patients receiving CSM-TACE realized elevated ORR, DCR, and PFS than those receiving chemotherapy and immunotherapy (6, 19, 20). The possible reasons for this might be that: (1) CSM could effectively embolize tumor blood supply vessels to induce tumor necrosis and meanwhile sustainably release antitumor drugs to maintain locoregional high concentrations, which resulted in satisfactory tumor treatment response (27); and (2) the different composition of patients might affect treatment response.
Until now, the prognosis of HNC patients is still unfavorable (1–3). It has been proposed that the median PFS is 3.6 months among HNC patients receiving chemotherapy (25); meanwhile, the OS rate is 16% among HNC patients receiving immunotherapy (28). In this study, the 1-year PFS rate was 34.1% and the 1-year OS rate was 38.9% after CSM-TACE, which were numerically prolonged than chemotherapy and immunotherapy (22, 28). The potential reasons for this might be that: (1) CSM-TACE served as a terminal embolization, which could block the blood supply of tumor lesions to the greatest extent possible; meanwhile, CSM-TACE could fully plug tumor target vessels and minimize tumor blood supply due to the small size of CalliSpheres® Microspheres (15); (2) CalliSpheres® Microspheres could release drugs slowly but continuously, and therefore, the local concentration of chemotherapy drugs in tumor lesions could be reached over a long period of time, which led to prolonged survival (17); and (3) CSM-TACE was characterized by a long period of effective time, and thus, it achieved favorable survival rates among HNC patients (29).
With regard to the safety profile of CSM-TACE, a previous study illustrated that liver function indices (namely, TP, TBIL, ALT, and AST) were similar among hepatocellular carcinoma patients before and after 1–3 months of CSM-TACE; meanwhile, the main adverse events included pain, fever, nausea, and vomiting, which were all manageable and tolerable (30). Another interesting research proposed that only mild pain and fever were observed among locally advanced breast cancer patients after CSM-TACE (16). In this study, we recorded changes in liver and renal function indices before and after treatment, as well as adverse events during and after treatment. Surprisingly, the data showed that no changes in the liver and renal function indices occurred before and after CSM-TACE; meanwhile, the postoperative adverse events were only mild and tolerable pain. Besides, there was no focal necrosis with abscess and surrounding tissue ischemia necrosis. The potential reason for this might be that CSM could directly release antitumor drugs into the target tumor, which consequently reduced the systemic toxicity; hence, the safety profile of CSM-TACE was favorable among HNC patients.
Compared with the HNC treatment recommended by the existing authority guidelines, HNC patients receiving CSM-TACE achieved better ORR, DCR, and PFS than those receiving conventional treatments (including chemotherapy and immunotherapy) (21, 26, 28). Furthermore, with regard to the postoperative adverse events among HNC patients receiving CSM-TACE, no grade III and IV adverse events were found but only mild and tolerable pain; besides, there was no focal necrosis among patients with abscess and surrounding tissue ischemia necrosis.
CSM-TACE was a relatively novel treatment method for HNC patients, which led to a limited sample size; thus, we enrolled patients having tumors in six different locations in the HNC area. In addition, four patients with stage II tumors had a surgical contraindication or were unwilling to receive surgery; thus, a decision to perform CSM-TACE on them depended on their willingness. Moreover, such a decision to be taken for all the included 15 patients was codetermined by patients and doctors; as far as the patients were concerned, they were willing to receive CSM-TACE as a first-choice treatment; the doctors , on their part, wanted to ensure that patients benefited from CSM-TACE. Additionally, the primary tumor for the included patients was different; thus, the loaded chemotherapeutic drug was different, while no difference in treatment efficacy was found in CSM-TACE loaded with different drugs.
However, there were several limitations in the present study: (1) this was an exploratory pilot study with a small sample size, and thus, studies with larger sample sizes could be conducted in the future; (2) this was a single-arm study, and thus, randomized controlled trials could be conducted to further explore the efficacy and safety of CSM-TACE in HNC patients; (3) the efficacy of CSM-TACE with different diameters of CSM in HNC patients could be investigated subsequently; and (4) the efficacy of DEB-TACE with other microspheres in HNC patients could be explored in the future.
In conclusion, CSM-TACE illustrates favorable treatment response and survival benefits, as well as a tolerable safety profile in HNC patients, which may provide a potential treatment choice for the management of HNC, while further validation by a larger sample size study is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Second Hospital of Dalian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LS and KW conceived and designed the study. FG and JG collected and analyzed the data, prepared the figures and tables, and also contributed to the writing of the manuscript. All authors wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.938305/full#supplementary-material.
References
2. Porcheri C, Mitsiadis TA. Notch in head and neck cancer. Adv Exp Med Biol. (2021) 1287:81–103. doi: 10.1007/978-3-030-55031-8_7
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Rahman QB, Iocca O, Kufta K, Shanti RM. Global burden of head and neck cancer. Oral Maxillofac Surg Clin North Am. (2020) 32:367–75. doi: 10.1016/j.coms.2020.04.002
5. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
6. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:873–98. doi: 10.6004/jnccn.2020.0031
7. Napolitano M, Schipilliti FM, Trudu L, Bertolini F. Immunotherapy in head and neck cancer: the great challenge of patient selection. Crit Rev Oncol Hematol. (2019) 144:102829. doi: 10.1016/j.critrevonc.2019.102829
8. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. (2016) 91:386–96. doi: 10.1016/j.mayocp.2015.12.017
9. Huang G, Pan ST. ROS-mediated therapeutic strategy in chemo-/radiotherapy of head and neck cancer. Oxid Med Cell Longev. (2020) 2020:5047987. doi: 10.1155/2020/5047987.32774675
10. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. (2018) 24:2074–81. doi: 10.1158/1078-0432.CCR-17-2899
11. Jin SQ, Zhao HY, Bai B, Ma CH, Cao HL. Transcatheter arterial chemoembolization improves clinical efficacy and life quality of patients with lung cancer and reduces adverse reactions. Am J Transl Res. (2021) 13:10396–403.34650708
12. Grozinsky-Glasberg S, Bloom AI, Lev-Cohain N, Klimov A, Besiso H, Gross DJ. The role of hepatic trans-arterial chemoembolization in metastatic medullary thyroid carcinoma: a specialist center experience and review of the literature. Eur J Endocrinol. (2017) 176:463–70. doi: 10.1530/EJE-16-0960
13. Han T, Yang X, Zhang Y, Li G, Liu L, Chen T, et al. The clinical safety and efficacy of conventional transcatheter arterial chemoembolization and drug-eluting beads-transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis. Biosci Trends. (2019) 13:374–81. doi: 10.5582/bst.2019.01153
14. Xu H, Min X, Ren Y, Yang L, Liu F. Comparative study of drug-eluting beads versus conventional transarterial chemoembolization for treating peculiar anatomical sites of gastric cancer liver metastasis. Med Sci Monit. (2020) 26:e922988. doi: 10.12659/MSM.922988
15. Liang B, Makamure J, Shu S, Zhang L, Sun T, Zheng C. Treatment response, survival, and safety of transarterial chemoembolization with CalliSpheres((R)) microspheres versus conventional transarterial chemoembolization in hepatocellular carcinoma: a meta-analysis. Front Oncol. (2021) 11:576232. doi: 10.3389/fonc.2021.576232
16. Wang Z, Niu H, Li Z, Zhang J, Sha L, Zeng Q, et al. Superselective arterial embolization with drug-loaded microspheres for the treatment of unresectable breast cancer. Gland Surg. (2019) 8:740–7. doi: 10.21037/gs.2019.12.06
17. Shang B, Li J, Wang X, Li D, Liang B, Wang Y, et al. Clinical effect of bronchial arterial infusion chemotherapy and CalliSpheres drug-eluting beads in patients with stage II–IV lung cancer: a prospective cohort study. Thorac Cancer. (2020) 11:2155–62. doi: 10.1111/1759-7714.13522
18. Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, Harmath CB, Lewandowski RJ, Salem R, et al. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology. (2015) 62:1111–21. doi: 10.1002/hep.27915
19. Caudell JJ, Gillison ML, Maghami E, Spencer S, Pfister DG, Adkins D, et al. NCCN guidelines(R) insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. (2022) 20:224–34. doi: 10.6004/jnccn.2022.0016
20. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for head and neck cancer 2018 (English version). Chin J Cancer Res. (2019) 31:84–98. doi: 10.21147/j.issn.1000-9604.2019.01.05
21. Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. (2019) 5:67–73. doi: 10.1001/jamaoncol.2018.4051
22. Fushimi C, Okamoto I, Matsuki T, Masubuchi T, Okada T, Sato H, et al. Salvage chemotherapy after nivolumab for recurrent or metastatic head and neck carcinoma. Anticancer Res. (2020) 40:5277–83. doi: 10.21873/anticanres.14532
23. McBride S, Sherman E, Tsai CJ, Baxi S, Aghalar J, Eng J, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. (2021) 39:30–7. doi: 10.1200/JCO.20.00290
24. Saba NF, Chen ZG, Haigentz M, Bossi P, Rinaldo A, Rodrigo JP, et al. Targeting the EGFR and immune pathways in squamous cell carcinoma of the head and neck (SCCHN): forging a new alliance. Mol Cancer Ther. (2019) 18:1909–15. doi: 10.1158/1535-7163.MCT-19-0214
25. Ye W, Liu R, Pan C, Jiang W, Zhang L, Guan Z, et al. Multicenter randomized phase 2 clinical trial of a recombinant human endostatin adenovirus in patients with advanced head and neck carcinoma. Mol Ther. (2014) 22:1221–9. doi: 10.1038/mt.2014.53
26. Tonse R, Ramamoorthy V, Rubens M, Saxena A, McGranaghan P, Veledar E, et al. Hospitalization rates from radiotherapy complications in the United States. Sci Rep. (2022) 12:4371. doi: 10.1038/s41598-022-08491-8
27. Han X, Chen Q, Sun Y, Han L, Sha X. Morphology, loadability, and releasing profiles of CalliSpheres Microspheres in delivering oxaliplatin: an in vitro study. Technol Cancer Res Treat. (2019) 18:1533033819877989. doi: 10.1177/1533033819877989
28. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. (2017) 35:1542–9. doi: 10.1200/JCO.2016.70.1524
29. Xiang H, Long L, Yao Y, Fang Z, Zhang Z, Zhang Y. CalliSpheres drug-eluting bead transcatheter arterial chemoembolization presents with better efficacy and equal safety compared to conventional TACE in treating patients with hepatocellular carcinoma. Technol Cancer Res Treat. (2019) 18:1533033819830751. doi: 10.1177/1533033819830751
30. Sun J, Zhou G, Xie X, Gu W, Huang J, Zhu D, et al. Efficacy and safety of drug-eluting beads transarterial chemoembolization by CalliSpheres((R)) in 275 hepatocellular carcinoma patients: results from the Chinese CalliSpheres((R)) transarterial chemoembolization in liver cancer (CTILC) study. Oncol Res. (2020) 28:75–94. doi: 10.3727/096504019X15662966719585
Keywords: transarterial chemoembolization with CalliSpheres® Microspheres, head and neck cancer, treatment response, survival, safety profile
Citation: Gao F, Gao J, Wang K and Song L (2022) Efficacy and safety of transarterial chemoembolization with CalliSpheres® Microspheres in head and neck cancer. Front. Surg. 9:938305. doi: 10.3389/fsurg.2022.938305
Received: 7 May 2022; Accepted: 19 July 2022;
Published: 25 August 2022.
Edited by:
Małgorzata Wierzbicka, Poznan University of Medical Sciences, PolandReviewed by:
Xiaohua Guo, Jinhua Central Hospital, ChinaJiansong Ji, Lishui Central Hospital, China
Baosheng Ren, The First Affiliated Hospital of Soochow University, China
© 2022 Gao, Gao, Wang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Song bGlleW9uZzkyODQ4Njc5MkAxNjMuY29t Kuiyang Wang a2FueW9uZzY0OTQ0OTQ1OEAxNjMuY29t
†These authors have contributed equally to this work.
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Fei Gao†
Fei Gao† Lei Song
Lei Song