- Department of Spine and Osteopathy Ward, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
Background: Anterior cervical decompression and fusion can effectively treat cervical spondylotic myelopathy (CSM). Accurately classifying patients with CSM who have undergone anterior cervical decompression and fusion is the premise of precision medicine. In this study, we used machine learning algorithms to classify patients and compare the postoperative efficacy of each classification.
Methods: A total of 616 patients with cervical spondylotic myelopathy who underwent anterior cervical decompression and fusion were enrolled. Unsupervised machine learning algorithms (UMLAs) were used to cluster subjects according to similar clinical characteristics. Then, the results of clustering were visualized. The surgical outcomes were used to verify the accuracy of machine learning clustering.
Results: We identified two clusters in these patients who had significantly different baseline clinical characteristics, preoperative complications, the severity of neurological symptoms, and the range of decompression required for surgery. UMLA divided the CSM patients into two clusters according to the severity of their illness. The repose to surgical treatment between the clusters was significantly different.
Conclusions: Our results showed that UMLA could be used to rationally classify a heterogeneous cohort of CSM patients effectively, and thus, it might be used as the basis for a data-driven platform for identifying the cluster of patients who can respond to a particular treatment method.
Introduction
The neurological symptoms of cervical spondylotic myelopathy (CSM) are mainly caused by spinal cord compression and can be relieved by surgical decompression (1,2). CSM was included in the umbrella term degenerative cervical myelopathy (DCM). DCM is a degenerative disease, and the process of disease progression is progressive and irreversible. Progressive compression of the cervical spinal cord by degeneration and spinal canal stenosis is the pathogenesis of DCM (3). Preventing symptom progression is one of the aims of surgery (4). After decompression of the cervical spinal cord, the pressure reduces, and some functions improve. Patients with more severe symptoms often show greater improvement in function (5,6). Anterior cervical decompression and fusion, including anterior cervical discectomy and fusion (ACDF) and anterior cervical corpectomy and fusion (ACCF), can effectively relieve cervical spinal cord compression and improve neurological symptoms (7). With the advancement in the medical field, precision medicine has greatly enhanced the effectiveness of medication and reduced the incidence of patient complications. Accurately identifying the problem and classifying the patient has become very important (8). With the development of artificial intelligence, machine learning has been applied to disease diagnosis, classification, and treatment, such as heart failure and child-specific dermatitis (8,9).
Supervised machine learning utilizes iterative algorithms and learns from a large and accurately labeled training dataset (10). Although it can accurately diagnose diseases, it is generally unable to infer “diagnostic reasoning” used in these algorithms. Unsupervised machine learning algorithms (UMLAs) cluster (or group) patients based on their characteristics rather than identifying the “truth” of diagnosis or prognosis (11). By clustering patients, it is possible to analyze the characteristics of similarly clustered individuals and determine the outcome of therapy.
Machine learning algorithms are widely used in clinical practice (12,13) but are seldom used to evaluate the efficacy of cervical spine surgery. It is important to effectively classify patients undergoing cervical surgery and identify their potential risks (14). Unsupervised machine learning algorithm (UMLA) can be used to cluster patients based on their disease characteristics and accurately and rationally classify a heterogeneous cohort effectively (15). We rationally classify a heterogeneous cohort of patients by UMLA and form a basis for a data-driven platform that can identify the subgroup of patients who might respond to a particular treatment. Eventually, the management of CSM patients including treatment decision making can be improved. In this study, we collected the clinical data of patients with CSM who underwent anterior cervical decompression and fusion. UMLA was performed to classify patients into two clusters based on the characteristics of the perioperative clinical data of the patients. Finally, we compared the differences in the clinical characteristics, the efficacy of the surgery, and postoperative complications between the clusters and verified the accuracy of the clustering performed.
Materials and methods
Patients
This study was authorized by the Ethics Committee of The First Affiliated Hospital of Guangxi Medical University. Informed consent forms were signed by the volunteers who participated in this study.
We collected the clinical data of the CSM patients who were hospitalized and underwent anterior cervical decompression and fusion (ACDF and ACCF) from June 2012 to June 2021 in the Department of Spinal Osteopathology, The First Affiliated Hospital of Guangxi Medical University. In total, 616 patients were enrolled in this study. We collected data on 31 perioperative variables, including 13 preoperative variables and 18 operative and postoperative variables. The 13 preoperative variables were gender, age, body mass index (BMI), the American Spinal Cord Injury Association (ASIA) score, the visual analog scale (VAS), stability of the cervical spine, segments of the spinal operation, and history of diabetes, osteoporosis, hypertension (HP), cardiovascular heart disease (CHD), cerebrovascular disease (CVD), and hepatic and renal function disorder (HRFD). The 18 operative and postoperative variables included respiratory failure, peptic ulcer postoperation, dysphagia, pneumonia, hoarseness, mental disorder, axial pane, leakage of cerebrospinal fluid, esophagostoma, wound hematomas, sense of girdle, wound infection, JOA improvement rate >25%, blood transfusion, operation time (OT), bleeding volume (BV), postoperative drainage volume (PDV), and the length of hospital stay (LOS). The inclusion criteria were as follows: (I) The inpatient was diagnosed with CSM. (II) The inpatient underwent anterior cervical decompression and fusion, including anterior cervical discectomy and fusion (ACDF) and anterior cervical corpectomy and fusion (ACDF). (III) There were symptoms, signs, and imaging manifestations of cervical spinal cord compression in hospitalized patients. (IV) All patients who completed the preoperative examination. (V) The anterior cervical decompression and fusion operation was completed. The exclusion criteria were as follows: (I) Inpatients with tumor, tuberculosis, and space-occupying lesions in the cervical medulla were excluded. (II) Inpatients with traumatic cervical spinal cord injury. (III) Inpatients with cervical spondylosis who received other approaches, such as a posterior approach or a combined (anterior and posterior) approach. (IV) Inpatients without complete perioperative clinical data.
Normalization of data and unsupervised machine learning
We used R software 4.0.3 to perform unsupervised machine learning. The data of anterior cervical decompression and fusion patients were normalized using the Scale function in the “factoextra” package (16). The Fpc package was used for determining the optimal clustering number (K value) by the Silhouette coefficient (SC) (17,18). Then, we used the K-means cluster algorithm to cluster the patients (13,18,19). The result of the K-means cluster was visualized using a clustergram and a radargram.
Statistical analysis
SPSS V.22.0 was used for performing statistical analyses. The clinical measurement data of the patients were represented as the median (P25, P75) and the mean (SD). We performed a chi-squared test, Mann–Whitney U-test, and Student's t-test to compare the differences. The differences were considered to be statistically significant at p < 0.05.
Results
Result of unsupervised machine learning algorithms
The K-means clustering algorithm was used to identify and classify the characteristic clinical data of the patients. It is a common unsupervised algorithm in machine learning. It can categorize the data of unknown labels into different groups based on their characteristics. Each group of data is also called a “cluster,” and the center point of each cluster is called a “centroid.” After normalizing the clinical data using the Scale function, we used the K-means clustering algorithm. The basic process is as follows: (I) The K initial centroids (that may not be sample points) are randomly selected, the nearest centroid for each sample point is found, and the sample points and centroids are grouped into the same cluster, thus generating K clusters. (II) When all sample points are divided, for each cluster, the new centroid (the average coordinate value of all points in the same cluster) is recalculated. (III) Iterations are performed until the position of the centroid becomes constant. In this study, we determine the K value using the SC
class="mb15">In this formula, a(i) represents the average distance between the sample point and all other points in the same cluster and b(i) represents the average distance between the sample point and all points in the next nearest cluster (20). The K-means clustering algorithm pursues that, for each cluster, the difference within the cluster is small, while the difference between clusters is large, and the Silhouette coefficient is the key indicator to describe the difference inside and outside the cluster. According to the formula, the value of SC can range from −1 to 1. When SC is closer to 1, the clustering effect is better; when SC is closer to −1, the clustering effect worsens. The peak of the curve is the best value for the Silhouette coefficient (Y-axis) such that the best K value is equal to 2 (X-axis) (Figure 1). Two clusters are optimal for the K-means clustering algorithm. The clinical data were sufficiently clustered into cluster 1 and cluster 2 (Figure 2). The results of the K-means clustering algorithm for the clinical data are presented in Table 1. The heterogeneity and homogeneity of the clinical characteristics of the two clusters were determined using a Venn diagram (Figure 3).
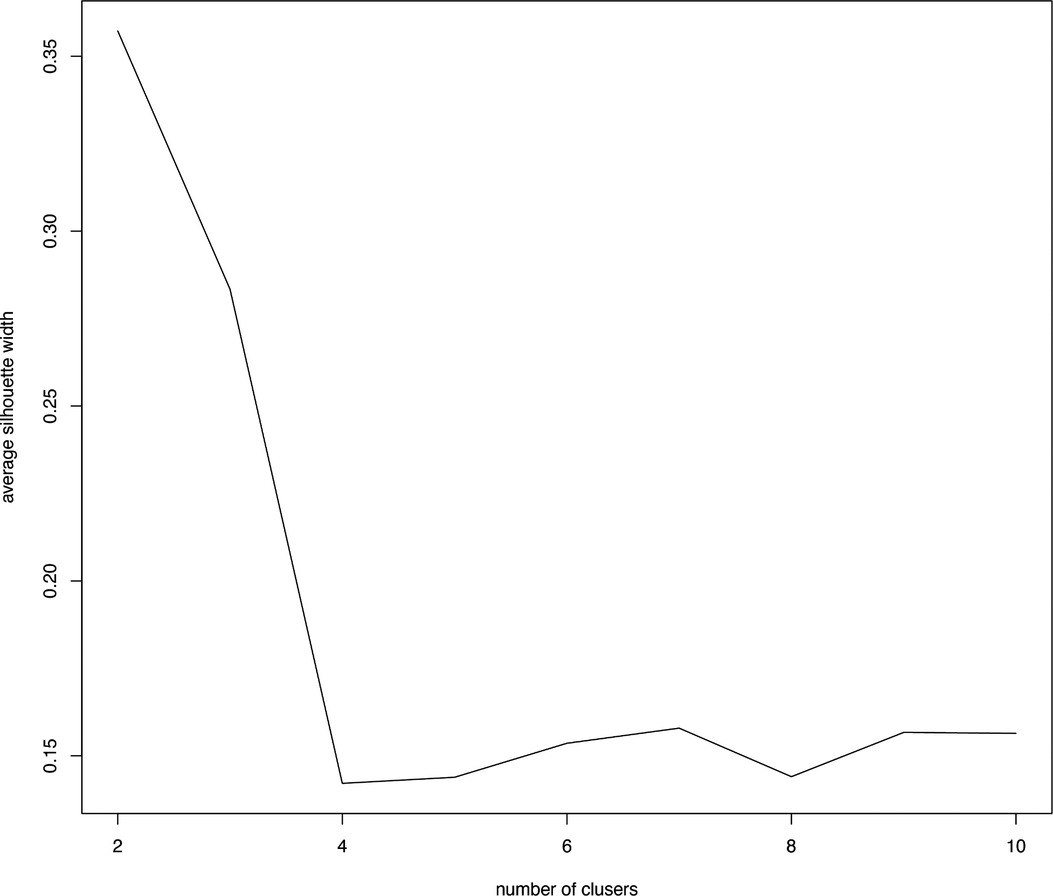
Figure 1. Optimal clustering number of the K-means clustering algorithm was determined by Silhouette coefficient (SC). The peak of the curve is the best value for the Silhouette coefficient (Y-axis); the best number of clusters was equal to 2 (X--axis).
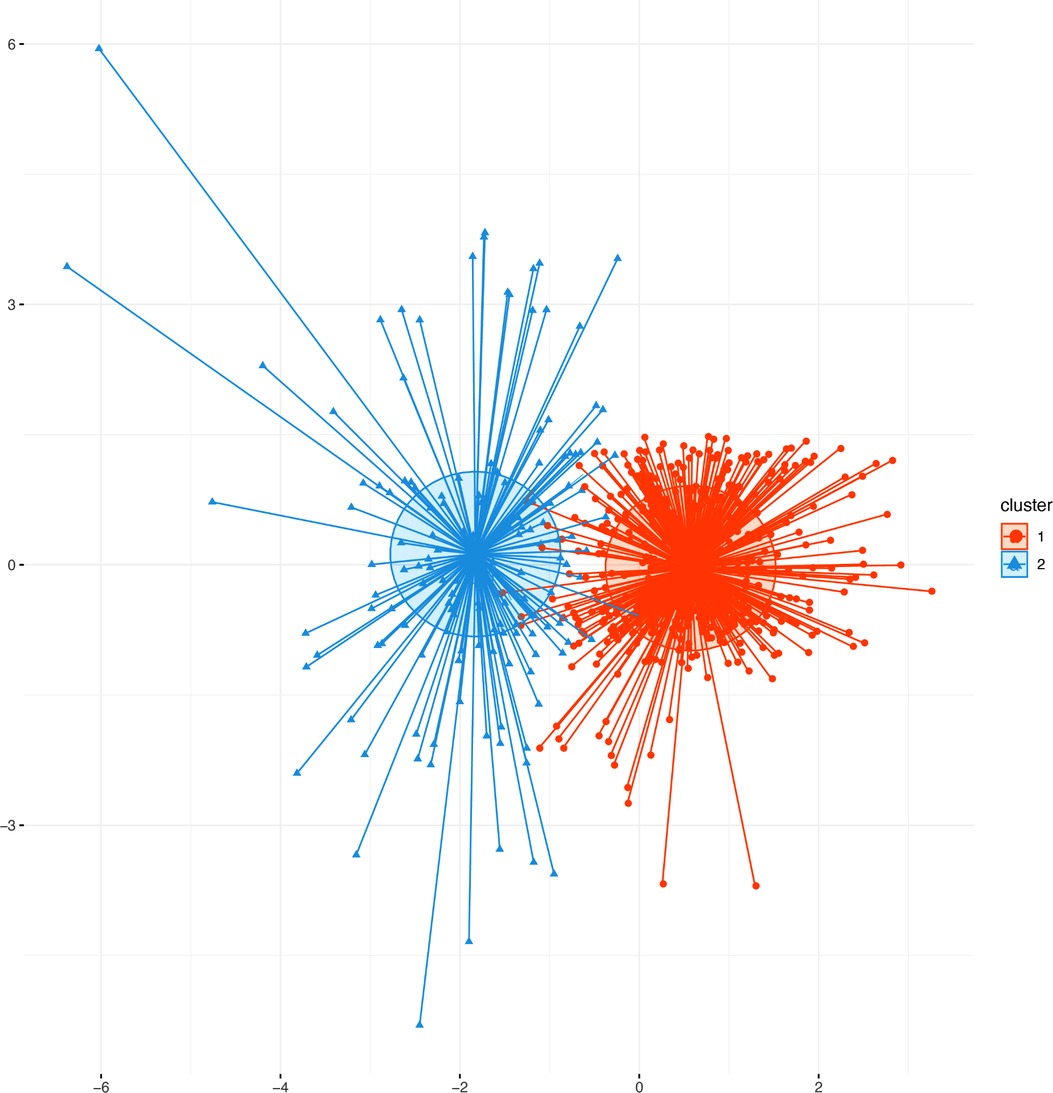
Figure 2. Scatter plots of patients’ clinical data. Scatter points on the graph represent each patient, and the K-means clustering algorithm divides patients into two clusters. The orange scatter represents cluster 1, and the blue scatter represents cluster 2.
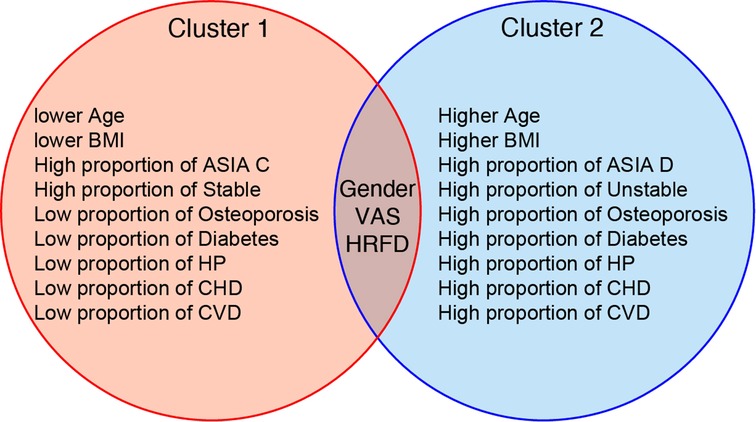
Figure 3. Typical clinical characteristics and features of two clusters. The blue circle represents cluster 1, more severe in condition, as opposed to the red circles. The Venn diagram summarizes the results of the unsupervised machine learning algorithm (UMLA). The results of UMLA are clinically explicable. BMI: body mass index; ASIA: American Spinal Cord Injury Association; HP: hypertension; CHD: cardiovascular heart disease; CVD: cerebrovascular disease; VAS: visual analog scale; HRFD: hepatic and renal function disorder.
Characteristics of the patients by K-means clustering
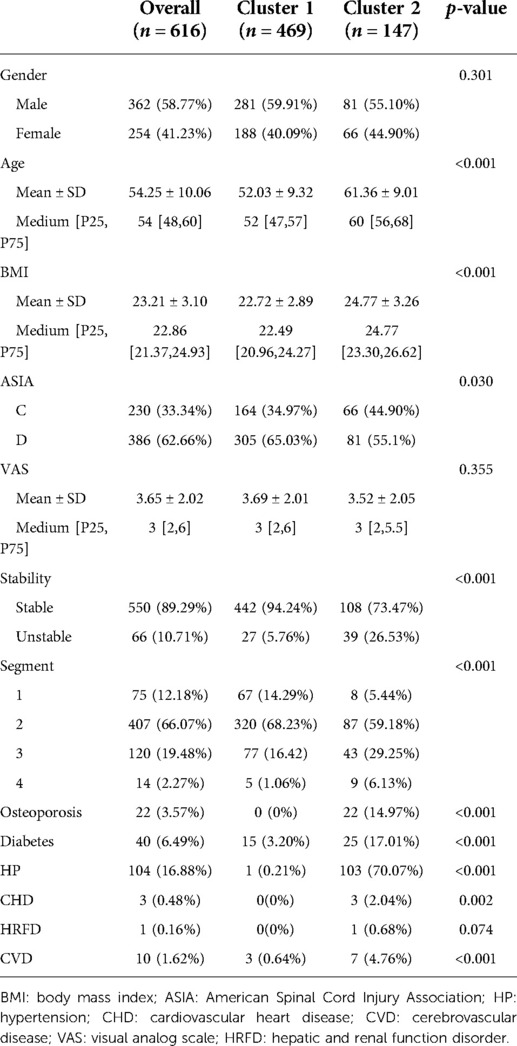
The 13 preoperative variables of the two clusters based on the K-means clustering algorithm are presented in Table 1. These variables included gender, age, BMI, the ASIA score, the VAS, stability of the cervical spine, segments of the spinal operation, and history of diabetes, osteoporosis, HP, CHD, CVD, and hepatic and renal function disorder (HRFD). The age and BMI of cluster 1 were significantly lower than those of cluster 2 (age: cluster 1/cluster 2 = 52.03 ± 9.32/61.36 ± 9.01, p < 0.001; BMI: cluster 1/cluster 2 = 22.72 ± 2.89/24.77 ± 3.26, p < 0.001). The ASIA score, stability of the cervical spine, and segments of the spinal operation were significantly different between cluster 1 and cluster 2 [ASIA score-C grade: cluster 1/cluster 2 = 164 (34.97%)/66 (44.90%), p = 0.030; instability of the cervical spine: cluster 1/cluster 2 = 442 (94.24%)/108 (73.47%), p < 0.001]. History of diabetes, osteoporosis, HP, CHD, and CVD were significantly different between cluster 1 and cluster 2 [diabetes: cluster 1/cluster 2 = 15 (3.20%)/25 (17.01%), p < 0.001; HP: cluster 1/cluster 2 = 1 (0.21%)/103 (70.07%), p < 0.001; CHD: cluster 1/cluster 2 = 0 (0%)/3 (2.04%), p = 0.002; CVD: cluster 1/cluster 2 = 3 (0.64%)/7 (4.76%), p < 0.001]. The preoperative variables are represented by a radargram in Figure 4.
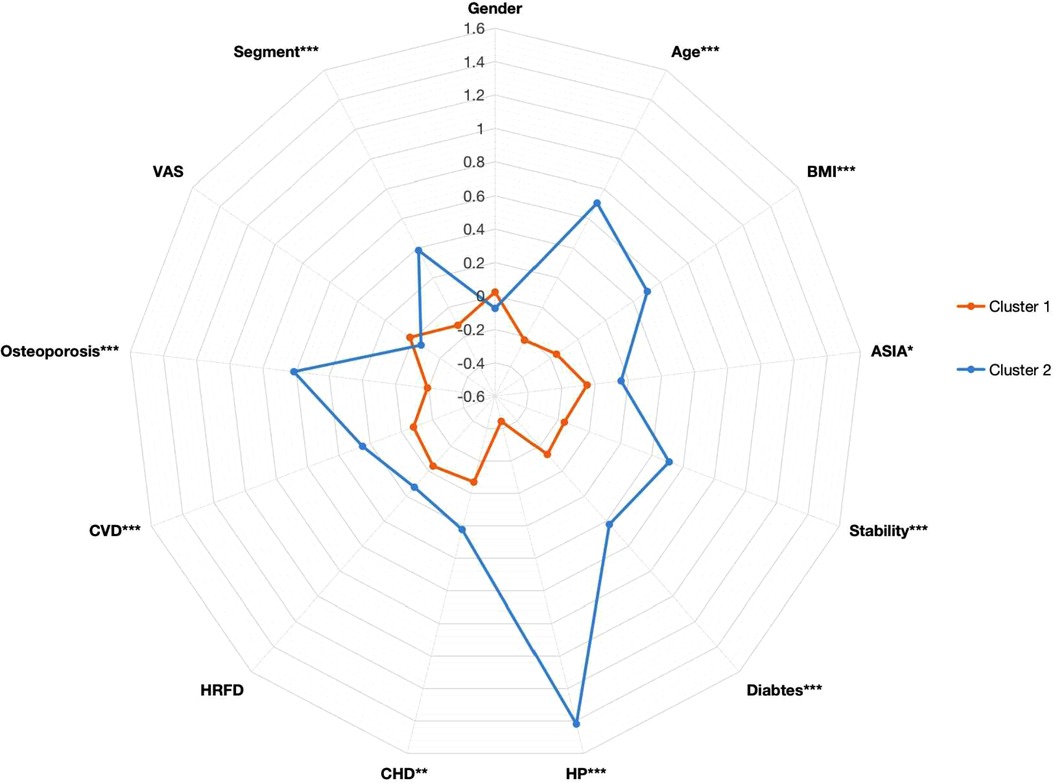
Figure 4. Radargram of 13 preoperative variables in cervical spondylotic myelopathy patients in two clusters. The K-means clustering algorithm normalized preoperative variables were compared between two clusters. Spoke lengths represent the average of each variable after the K-means clustering algorithm is normalized. Significance levels are presented with asterisks. BMI: body mass index; ASIA: American Spinal Cord Injury Association; HP: hypertension; CHD: cardiovascular heart disease; CVD: cerebrovascular disease; VAS: visual analog scale; HRFD: hepatic and renal function disorder. *p-value <0.05, **p-value <0.01, ***p-value <0.001.
Comparison of operative and postoperative variables among clusters
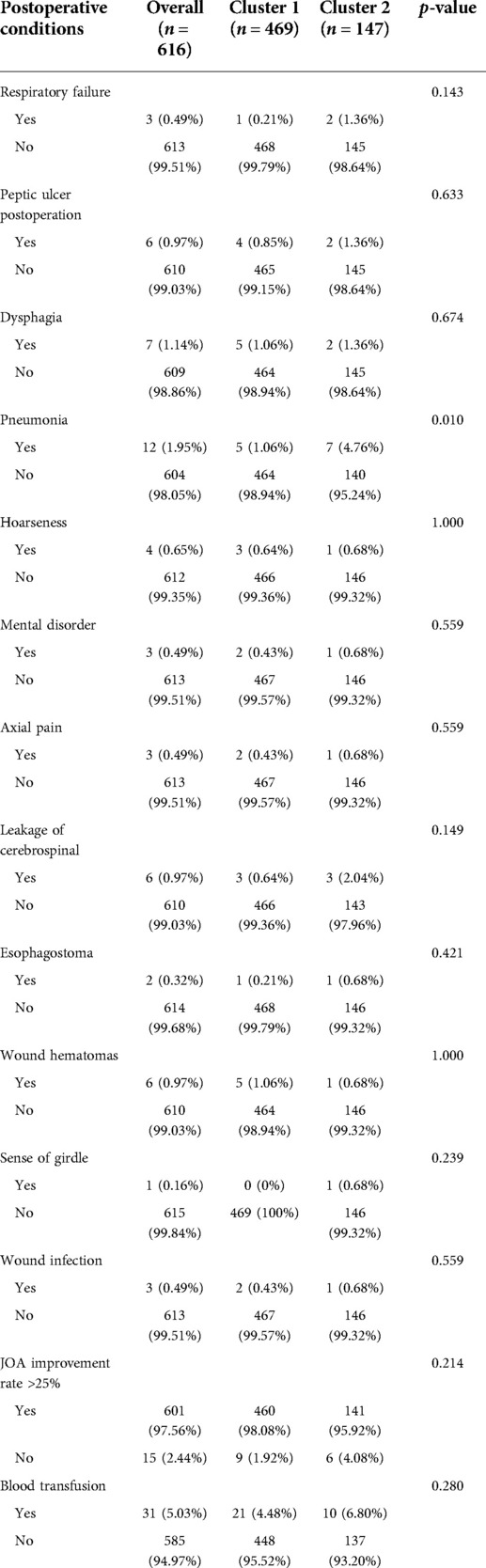
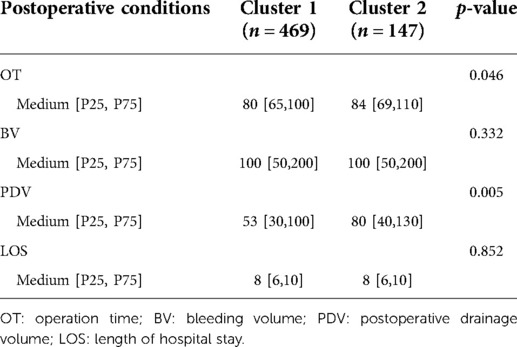
The differences in the 18 operative and postoperative variables between the clusters are presented in Tables 2 and 3. The incidence of postoperative pneumonia in cluster 2 was higher than that in cluster 1 (p < 0.05). The OT of cluster 1 was 80 [65, 100] min, and that of cluster 2 was 84 [69, 110] min (Figure 5A). The OT of cluster 1 was significantly shorter than that of cluster 2 (p = 0.046). The PDV of cluster 1 was 53 [30, 100] ml, and that of cluster 2 was 80 [40, 130] ml (Figure 5B). The PDV of cluster 1 was significantly lower than that of cluster 2 (p = 0.005). Other postoperative variables such as respiratory failure, peptic ulcer postoperation, dysphagia, hoarseness, mental disorder, axial pain, leakage of the cerebrospinal fluid, esophagostoma, wound hematomas, sense of girdle, wound infection, JOA improvement rate >25%, blood transfusion, BV, and the LOS were similar between the clusters (p > 0.05) (Supplementary Figures 1 and 2). The operative and postoperative variables are represented by a radargram in Figure 6.
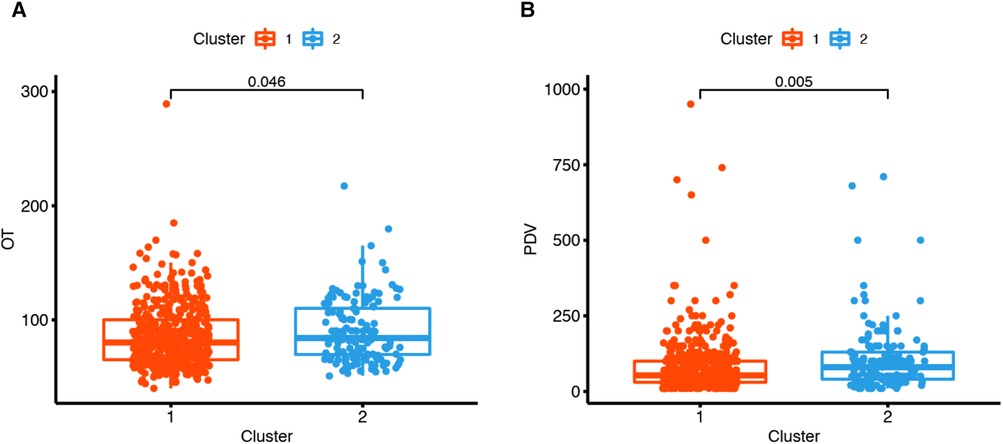
Figure 5. Two clustered boxplots of operative time and postoperative drainage volume. (A) The operation time (OT) of cluster 1 was 80[65, 100] min, and that of cluster 2 was 84[69, 110] min. The operation time of cluster 1 was significantly shorter than that of cluster 2 (p = 0.046). (B) The postoperative drainage volume (PDV) of cluster 1 was 53[30, 100] ml, and that of cluster 2 was 80[40, 130] ml. The postoperative drainage volume of cluster 1 was significantly less than that of cluster 2 (p = 0.005).

Figure 6. Radargram of 18 preoperative variables in cervical spondylotic myelopathy patients in two clusters. K-means clustering algorithm-normalized preoperative variables were compared between two clusters. Spoke lengths represent the average of each variable after the K-means clustering algorithm is normalized. Significance levels are presented with asterisks. OT: operation time; BV: bleeding volume; PDV: postoperative drainage volume; LOS: length of hospital stay. *p-value < 0.05, **p-value < 0.01, ***p-value < 0.001.
Discussion
Orientation and interpretability of unsupervised machine learning
In this study, machine learning algorithms could effectively classify patients with CSM who underwent anterior cervical decompression and fusion into two clusters based on 13 preoperative variables. The results of clustering are presented in Table 1. The results suggested that the UMLA accurately grouped patients according to the severity of their illness. This method was specifically designed to integrate clinical data, which is heterogeneous in an unsupervised manner (9). It can cluster patients with similar characteristics (21). The UMLA clusters patients based on their natural characteristics instead of a priori knowledge (22). Supervised machine learning algorithms were used in a study by Kwong et al. (23). In that study, random forest regression models were used to predict waitlist dropout among liver transplant candidates. Such methods provide criteria for categorizing patients, such as whether to drop out of the waitlist of liver transplant candidates. Unlike the above study, we focused on the intrinsic properties of the data, which allowed us to investigate the natural characteristics further and highlight the characteristics that were relevant to the hypothesis concerning medical research. This method provided a more meaningful description and distinction between patient clusters within the cohort (24). For example, cluster 1 and cluster 2 distinguished various attributes of clinical importance, such as age, BMI, and preoperative complications. The clinical attributes of the patients were more severe in cluster 2. The symptoms of CSM and the range of cervical spine lesions were efficiently clustered into the two clusters. Patients in cluster 2 had more severe neurological symptoms, more populations had cervical instability and osteoporosis, and more segments of the cervical spine vertebra needed operation during the surgery. In conclusion, unsupervised machine learning could classify patients into two clusters, according to the severity of the disease and their basic clinical attributes.
Outcomes of medical therapy based on the classification of unsupervised machine learning algorithms
Based on this classification, we further analyzed 18 operative and postoperative variables of the patients to evaluate the treatment outcomes of the patients in different clusters. Regarding the outcome of surgery, we focused on the postoperative variables axial pain, sense of girdle, and JOA improvement rate >25%. These three variables were similar between the clusters. Although the treatment outcomes between the groups were similar, cluster 2 patients were in a worse condition and had more comorbidities. Thus, the conditions of the patients with severe diseases (cluster 2) might improve considerably after surgical treatment, and the level of improvement of the symptoms might be the same as that of the patients with mild diseases (cluster 1). Recent studies supported the results of our study and demonstrated that patients undergoing anterior cervical decompression and fusion experience significant relief, and patients with more severe symptoms experience greater improvement (25,26). For example, Cole's study divided CSM patients into severe, moderate, and mild groups by severity of myelopathy. After surgical treatment, 30% of patients in the severe group and 58% of patients in the moderate group showed improvement in grip strength, while 9% of patients in the mild group showed improvement in grip strength (5). The results of this study suggested that surgical treatment might be equally effective in severely and mildly ill patients. The results showed the effectiveness of the unsupervised machine learning algorithm in classifying CSM based on their heterogeneous clinical characteristics. Local complications, such as hoarseness, leakage of the cerebrospinal fluid, esophagostoma, wound hematomas, and wound infection, were similar between the clusters. This indicated that not only does anterior cervical decompression and fusion improve symptoms in severely ill patients, but the incidence of surgical complications also does not increase with the severity of the illness (27).
The OT and the postoperation drainage volume were higher in cluster 2. These differences were due to the condition of the patients, such as the surgical segments of the cervical spine, which were reflected in the clustering result. The surgical segments of patients in cluster 2 were larger (p < 0.001), and the proportion of patients with long-segment surgery (3 or 4 segments) was 35.47% in cluster 2 and 17.48% in cluster 1. Decompression of the cervical spinal cord or nerve root, reconstruction and stabilization of the cervical spine, and maintenance of the alignment of the spine are the aims of cervical spine surgery (28). Patients in cluster 2 had extensive cervical spinal cord compression. To achieve sufficient decompression, the number of segments requiring surgery increased, and the operative time and the postoperation volume of drainage also increased (Table 3 and Figures 5 and 6). Another reason for the higher postoperation volume of drainage in cluster 2 was that 14.97% of the patients in cluster 2 had osteoporosis. Osteoporosis causes osteopenia in the cervical vertebral body and increases bleeding on the bone graft surface during surgery; thus, patients have more postoperative drainage (29). These differences in the operative variables also verified the accuracy of UMLA in classifying patients with CSM who underwent anterior cervical decompression and fusion.
We did not construct a clinical model or an evaluation system for classifying the patients, as they require external validation and another novel algorithm. Instead, we assessed the ability of the unsupervised machine learning algorithm to cluster these patients and validate the effectiveness of the postoperative variables.
Our study had some limitations. First, the participants were from a single center. Second, this was a retrospective study, and thus, the data might have selection bias. Furthermore, the preferences and the experience of the surgeon might have influenced the results of the study.
Conclusion
This study showed the effectiveness of unsupervised machine learning as a novel method for classifying patients with CSM who have undergone anterior cervical decompression and fusion. Our results showed that the unsupervised machine learning algorithm could be used to rationally classify a heterogeneous cohort of patients and form a basis for a data-driven platform that can identify the subgroup of patients who might respond to a particular treatment. However, the feasibility and novelty of unsupervised machine learning algorithms and their value in making clinical decisions should be evaluated in prospective controlled trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
This study was approved by the Ethics Committee of The First Affiliated Hospital of Guangxi Medical University. Informed consent forms were signed by the volunteers who participated in this study.
Author contributions
CZ and SH participated conceptualization and methodology design of the study. TL, JC, TC, LC, XS, and JZ are in charge of data curation and investigation. JJ, HG, ZY, SW, and WC analyzed and visualized the data. CL and XZ performed writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
The National Natural Science Foundation of China sponsored this study (Grant/Award numbers 81860393, 81560359). Funding bodies had not participated in the design of the study, collection, interpretation, analysis of the data, or the writing of the manuscript.
Acknowledgments
The authors are very grateful to Prof. Xinli Zhan and Dr. Chong Liu (Spine and Osteopathy Ward, The First Affiliated Hospital of Guangxi Medical University) for support in all stages of this study.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.935656/full#supplementary-material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang A, Myers C, McDonald C, Alsoof D, Anderson G, Daniels A. Cervical myelopathy: diagnosis, contemporary treatment, and outcomes. Am J Med. (2022) 135(4):435–43. doi: 10.1016/j.amjmed.2021.11.007
2. Kalsi-Ryan S, Karadimas S, Fehlings M. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. (2013) 19(4):409–21. doi: 10.1177/1073858412467377
3. Ganau M, Holly L, Mizuno J, Fehlings M. Future directions and new technologies for the management of degenerative cervical myelopathy. Neurosurg Clin N Am. (2018) 29(1):185–93. doi: 10.1016/j.nec.2017.09.006
4. Badhiwala J, Hachem L, Merali Z, Witiw C, Nassiri F, Akbar M, et al. Predicting outcomes after surgical decompression for mild degenerative cervical myelopathy: moving beyond the mJOA to identify surgical candidates. Neurosurgery. (2020) 86(4):565–73. doi: 10.1093/neuros/nyz160
5. Cole T, Almefty K, Godzik J, Muma A, Hlubek R, Martinez-Del-Campo E, et al. Functional improvement in hand strength and dexterity after surgical treatment of cervical spondylotic myelopathy: a prospective quantitative study. J Neurosurg Spine. (2020) 32(6):1–7. doi: 10.3171/2019.10.SPINE19685
6. Khan I, Archer K, Wanner J, Bydon M, Pennings J, Sivaganesan A, et al. Trajectory of improvement in myelopathic symptoms from 3 to 12 months following surgery for degenerative cervical myelopathy. Neurosurgery. (2020) 86(6):763–8. doi: 10.1093/neuros/nyz325
7. Badhiwala J, Leung S, Ellenbogen Y, Akbar M, Martin A, Jiang F, et al. A comparison of the perioperative outcomes of anterior surgical techniques for the treatment of multilevel degenerative cervical myelopathy. J Neurosurg Spine. (2020) 33:1–8. doi: 10.3171/2020.4.SPINE191094
8. Cikes M, Sanchez-Martinez S, Claggett B, Duchateau N, Piella G, Butakoff C, et al. Machine learning-based phenogrouping in heart failure to identify responders to cardiac resynchronization therapy. Eur J Heart Fail. (2019) 21(1):74–85. doi: 10.1002/ejhf.1333
9. Bakker D, de Graaf M, Nierkens S, Delemarre E, Knol E, van Wijk F, et al. Unraveling heterogeneity in pediatric atopic dermatitis: identification of serum biomarker based patient clusters. J Allergy Clin Immunol. (2022) 149(1):125–34. doi: 10.1016/j.jaci.2021.06.029
10. Gulshan V, Peng L, Coram M, Stumpe M, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal Fundus photographs. JAMA. (2016) 316(22):2402–10. doi: 10.1001/jama.2016.17216
11. Sajda P. Machine learning for detection and diagnosis of disease. Annu Rev Biomed Eng. (2006) 8:537–65. doi: 10.1146/annurev.bioeng.8.061505.095802
12. Maddali M, Churpek M, Pham T, Rezoagli E, Zhuo H, Zhao W, et al. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: an observational, multicohort, retrospective analysis. Lancet Respir Med. (2022) 10(4):367–77. doi: 10.1016/S2213-2600(21)00461-6
13. Kobayashi M, Huttin O, Magnusson M, Ferreira J, Bozec E, Huby A, et al. Machine learning-derived echocardiographic phenotypes predict heart failure incidence in asymptomatic individuals. JACC Cardiovasc Imaging. (2022) 15(2):193–208. doi: 10.1016/j.jcmg.2021.07.004
14. Takenaka S, Kashii M, Iwasaki M, Makino T, Sakai Y, Kaito T. Risk factor analysis of surgery-related complications in primary cervical spine surgery for degenerative diseases using a surgeon-maintained database. Bone Joint J. (2021) 1:157–63. doi: 10.1302/0301-620X.103B1.BJJ-2020-1226.R1
15. Wang Z, Tang Z, Zhu Y, Pettigrew C, Soldan A, Gross A, et al. AD risk score for the early phases of disease based on unsupervised machine learning. Alzheimer's Dement. (2020) 16(11):1524–33. doi: 10.1002/alz.12140
16. Wu S, Liu S, Chen N, Zhang C, Zhang H, Guo X. Genome-wide identification of immune-related alternative splicing and splicing regulators involved in abdominal aortic aneurysm. Front Genet. (2022) 13:816035. doi: 10.3389/fgene.2022.816035
17. Sebastian A, Cistulli P, Cohen G, de Chazal P. Association of snoring characteristics with predominant site of collapse of upper airway in obstructive sleep apnea patients. Sleep. (2021) 44(12):1–9. doi: 10.1093/sleep/zsab176
18. Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diab Endocrinol. (2018) 6(5):361–9. doi: 10.1016/S2213-8587(18)30051-2
19. Brusco M, Shireman E, Steinley D. A comparison of latent class, K-means, and K-median methods for clustering dichotomous data. Psychol Methods. (2017) 22(3):563–80. doi: 10.1037/met0000095
20. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. (1984) 20:53–65. doi: 10.1016/0377-0427(87)90125-7
21. Benito-León J, Del Castillo M, Estirado A, Ghosh R, Dubey S, Serrano J. Using unsupervised machine learning to identify age- and sex-independent severity subgroups among patients with COVID-19: observational longitudinal study. J Med Internet Res. (2021) 23(5):e25988. doi: 10.2196/25988
22. Kalscheur M, Kipp R, Tattersall M, Mei C, Buhr K, DeMets D, et al. Machine learning algorithm predicts cardiac resynchronization therapy outcomes: lessons from the companion trial. Circ Arrhythm Electrophysiol. (2018) 11(1):e005499. doi: 10.1161/CIRCEP.117.005499
23. Kwong A, Hameed B, Syed S, Ho R, Mard H, Arshad S, et al. Machine learning to predict waitlist dropout among liver transplant candidates with hepatocellular carcinoma. Cancer Med. (2022) 11(6):1535–41. doi: 10.1002/cam4.4538
24. Sweatt A, Hedlin H, Balasubramanian V, Hsi A, Blum L, Robinson W, et al. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ Res. (2019) 124(6):904–19. doi: 10.1161/CIRCRESAHA.118.313911
25. Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. (2007) 17(4):315–22. doi: 10.1111/j.1552-6569.2007.00119.x
26. John S, Moorthy R, Sebastian T, Rajshekhar V. Evaluation of hand function in healthy individuals and patients undergoing uninstrumented central corpectomy for cervical spondylotic myelopathy using nine-hole peg test. Neurol India. (2017) 65(5):1025–30. doi: 10.4103/neuroindia.NI_12_17
27. Nguyen W, Chang K, Formanek B, Ghayoumi P, Buser Z, Wang J. Comparison of postoperative complications and reoperation rates following surgical management of cervical spondylotic myelopathy in the privately insured patient population. Clin Spine Surg. (2021) 34(9):E531–E6. doi: 10.1097/BSD.0000000000001216
28. Theodore N. Degenerative cervical spondylosis. N Engl J Med. (2020) 383(2):159–68. doi: 10.1056/NEJMra2003558
Keywords: machine learning, anterior cervical discectomy and fusion, anterior cervical corpectomy and fusion, cervical spondylotic myelopathy, cluster analysis
Citation: Zhou C, Huang S, Liang T, Jiang J, Chen J, Chen T, Chen L, Sun X, Zhu J, Wu S, Ye Z, Guo H, Chen W, Liu C and Zhan X (2022) Machine learning-based clustering in cervical spondylotic myelopathy patients to identify heterogeneous clinical characteristics. Front. Surg. 9:935656. doi: 10.3389/fsurg.2022.935656
Received: 4 May 2022; Accepted: 30 June 2022;
Published: 25 July 2022.
Edited by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Ziya Levent Gokaslan, Brown University, United StatesNikolaos Ch. Syrmos, Aristotle University of Thessaloniki, Greece
© 2022 Zhou, Huang, Liang, Jiang, Chen, Chen, Chen, Sun, Zhu, Wu, Ye, Guo, Chen, Liu and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong Liu bGNneHlrZHhAMTYzLmNvbQ== Xinli Zhan emhhbnhpbmxpQHN0dS5neG11LmVkdS5jbg==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
 Chenxing Zhou†
Chenxing Zhou† Tuo Liang
Tuo Liang Jie Jiang
Jie Jiang Jiarui Chen
Jiarui Chen Tianyou Chen
Tianyou Chen Wenkang Chen
Wenkang Chen Chong Liu
Chong Liu Xinli Zhan
Xinli Zhan