- 1Department of Urology, Cardinal Tien Hospital, New Taipei City, Taiwan
- 2Department of Urology, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 3Department of Urology, China Medical University and Hospital, Taichung, Taiwan
- 4Department of Urology, China Medical University Hsinchu Hospital, Hsinchu, Taiwan
- 5School of Medicine, China Medical University, Taichung, Taiwan
- 6Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 7Department of Urology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 8Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 9Division of Urology, Department of Surgery, Taipei Tzu Chi Hospital, The Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan
- 10School of Medicine, Buddhist Tzu Chi University, Hualien, Taiwan
- 11Department of Urology, Taiwan Adventist Hospital, Taipei, Taiwan
- 12Division of Urology, Department of Surgery, Far-Eastern Memorial Hospital, New Taipei, Taiwan
- 13Division of Urology, Department of Surgery, Taipei City Hospital Ren-Ai Branch, Taipei, Taiwan
- 14Department of Urology, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 15College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 16Department of Urology, MacKay Memorial Hospital, Taipei, Taiwan
- 17Mackay Medical College, Taipei, Taiwan
- 18Institute of Biomedical Informatics, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 19Division of Urology, Department of Surgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
- 20Department of Urology, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation and Tzu Chi University, Hualien, Taiwan
- 21Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 22Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 23TMU Research Center of Urology and Kidney, Taipei Medical University, Taipei, Taiwan
- 24Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Chia-Yi, Taiwan
- 25Chang Gung University of Science and Technology, Chia-Yi, Taiwan
- 26Department of Medicine, Chang Gung University, Taoyuan, Taiwan
- 27Department of Life Science, College of Science, National Taiwan Normal University, Taipei, Taiwan
- 28Department of Urology, Fu Jen Catholic University Hospital, Fu Jen Catholic University, New Taipei City, Taiwan
- 29School of Medicine, Fu-Jen Catholic University, New Taipei City, Taiwan
Purpose: Taiwan has a high incidence of upper tract urothelial carcinoma (UTUC). This study aimed to compare the surgical outcomes following transperitoneal hand-assisted laparoscopic nephroureterectomy (TP-HALNU) and transperitoneal pure laparoscopic nephroureterectomy (TP-LNU) from the Taiwan nationwide UTUC collaboration database using different parameters, including surgical volumes.
Materials and methods: The nationwide UTUC collaboration database includes 14 hospitals in Taiwan from the Taiwan Cancer Registry. We retrospectively reviewed the records of 622 patients who underwent laparoscopic nephroureterectomy between July 1988 and September 2020. In total, 322 patients who received TP-LNU or TP-HALNU were included in the final analysis. Clinical and pathological data and oncological outcomes were compared.
Results: Of the 322 patients, 181 and 141 received TP-LNU and TP-HALNU, respectively. There were no differences in clinical and histopathological data between the two groups. No differences were observed in perioperative and postoperative complications. There were no significant differences in oncological outcomes between the two surgical approaches. In the multivariate analysis, the cohort showed that age ≥70 years, positive pathological lymph node metastasis, tumors located in the upper ureter, and male sex were predictive factors associated with an increased risk of adverse oncological outcomes. A surgical volume of ≥20 cases showed a trend toward favorable outcomes on cancer-specific survival [hazard ratio (HR) 0.154, p = 0.052] and marginal benefit for overall survival (HR 0.326, p = 0.019) in the multivariate analysis.
Conclusion: Although different approaches to transperitoneal laparoscopic nephroureterectomy showed no significant differences in surgical outcomes, age, sex, lymph node metastasis, and tumor in the upper ureter in the following period were predictive factors for oncological outcomes. Higher surgical volume did not impact disease-free survival and bladder recurrence-free survival but was associated with improved overall survival and cancer-specific survival. Exploration of unknown influencing factors is warranted.
Introduction
Urothelial carcinoma (UC) is the fourth most common malignancy worldwide, with upper tract UC (UTUC) accounting for 5%–10% of these cases (1, 2). The gold standard for UTUC management is radical nephroureterectomy (NU), with the removal of the ipsilateral bladder cuff (2, 3). This procedure has historically been performed via an open approach [open NU (ONU)]; however, concerns regarding associated perioperative morbidity have led to the widespread adoption of minimally invasive surgery, laparoscopic NU (LNU), and robot-assisted NU (4–7).
LNU was first reported in 1991 (8). Its widespread adoption was initially limited by technical challenges and concerns about tumor cell dissemination via pneumoperitoneum (9), and it was later demonstrated that there was no difference in the risk of local recurrence between ONU and LNU (10). Minimally invasive NU results in shorter hospital stays, less blood loss, fewer complications, decreased use of postoperative pain medications, and superior patient satisfaction (5, 6, 9, 11–13). Most importantly, several multicenter retrospective studies, randomized trials, and systematic reviews have evaluated the oncological outcomes of ONU vs. LNU and have demonstrated equivalent oncological outcomes (4–7, 11, 13–16). It is not surprising, therefore, that for the exclusion of invasive tumors, larger tumors, or metastatic tumors, the use of LNU has increased in recent years (17).
However, the increased operation time, steep learning curve, and need for highly experienced laparoscopic surgeons have limited its widespread use. Hand-assisted laparoscopy (HAL) has provided a new minimally invasive alternative for patients with UTUC (18). HAL uses a unique approach that combines the finest aspects of open and laparoscopic surgery and en bloc specimen retrieval, thus maintaining the oncological principles used in open surgery (19).
LNU and hand-assisted LNU (HALNU) had comparable oncological and better perioperative and postoperative outcomes than ONU, but HALNU may be inferior to LNU or ONU in terms of recurrence-free survival and intravesical recurrence-free survival rates (20, 21). However, these studies are heterogeneous as they encompassed transperitoneal or retroperitoneal approaches. Some studies showed different oncological results with either approach (22). Considering the limited number of laparoscopic retroperitoneal nephroureterectomy cases in Taiwan, we conducted a study focusing on the surgical outcomes following transperitoneal HALNU (TP-HALNU) or transperitoneal pure LNU (TP-LNU) using the Taiwan nationwide UTUC collaboration database.
In Taiwan, the training of urology residents includes laparoscopic surgery in individual secondary or tertiary referral hospitals. Residents must pass their own training programs through direct observation of procedural skills. However, hands-on laparoscopic procedures have not been tested in the national licensure examination. Laparoscopic procedures are not restricted to subspecialists. Surgical volumes and methods of individualized centers may considerably vary. Several studies have revealed that the learning curve may vary among individual surgeons, and a consensus should be reached for the minimum number of cases to achieve proficiency (23). Surgical volumes also affect outcomes (24). The learning thresholds with fewer intraoperative and perioperative complications varied in the literature (25, 26). A recent study found longer operation time and a trend toward more complications with <20 cases (27). In addition, a higher surgical volume (≥20 cases) of laparoscopic hysterectomy was inversely related to the conversion rate to laparotomy (28). Although the length of the learning curve has individual differences, low surgical volumes may impact surgical outcomes. Hence, the surgical volume (≥20 cases or not) was taken into evaluation in this Taiwan UTUC collaboration study.
Materials and methods
The Taiwan Cancer Registry (TCR), a population-based cancer registry, is a nationwide cancer registry. Hospitals with >50-bed capacity that provide outpatient and hospitalized cancer care were recruited to participate in reporting all newly diagnosed malignant neoplasms to the registry. The TCR was queried for registered patients diagnosed with malignant neoplasms of the renal pelvis and ureter between July 1988 and October 2020. The involved hospitals are listed in Supplementary Table S1.
All patients with a primary diagnosis of renal pelvic or ureteral neoplasm who underwent NU were identified using the International Classification of Diseases Ninth Revision diagnostic and procedure codes. Exclusion criteria included patients who did not undergo NU, who did not receive laparoscopic or HAL surgery, who had previous or synchronous bladder UC, who received neoadjuvant or adjuvant chemotherapy, and who underwent retroperitoneal laparoscopic surgery, as well as histological type other than UC, bilateral neoplasm, or graft neoplasm.
We retrospectively reviewed patient records from the Urology Research Study Group database of 14 participating Taiwanese hospitals. The database included patients with UTUC recorded between July 1988 and September 2020, of whom 322 patients who underwent LNU between June 2002 and August 2019 were selected for this study. This study was approved by the institutional review boards of our hospitals (CTH107-3-5-035 and 06-X34-105).
The demographic data included age and sex. The distribution of patients between the two surgical approaches was recorded. Tumor characteristics, including tumor size, tumor location, laterality, multiplicity, histological characteristics, surgical margin, and pathological staging, were recorded. Outcome assessments included complications, mortality, and a disease-free period. Complications included surgical complications recorded according to the Clavien–Dindo classification and postoperative complications, including ileus and ventral hernia. We also divided patients by the surgical volume of hospitals, with the higher volume group comprising all patients from hospitals with ≥20 nephroureterectomy cases and the lower volume group comprising patients from hospitals with <20 cases. Survival outcome parameters were defined as all-cause death, cancer-specific survival (CSS) as death due to UTUC, disease-free survival (DFS) as cancer recurrence or metastasis, and bladder recurrence-free survival (BRFS) as UC recurrence in the bladder.
Statistical analyses were performed using SPSS Statistics (IBM, Armonk, NY, USA, version 24). The Cox proportional hazard model was selected to assess the effect of the surgical approach on prognostic outcomes, alone and after adjusting for potential confounders. All parameters were analyzed using univariate analysis, and those of specific interest were included in the multivariate analysis. All statistical assessments were two-tailed and considered statistically significant at p < 0.05.
Results
In total, 663 patients who underwent LNU were included in this study. The patients were divided into four different surgical approach groups: TP-LNU, TP-HALNU, retroperitoneal LNU, and retroperitoneal HALNU, with 181, 141, 41, and 300 patients in each group, respectively (Figure 1). The transperitoneal group was enrolled, and 322 patients were enrolled in the final analyses.
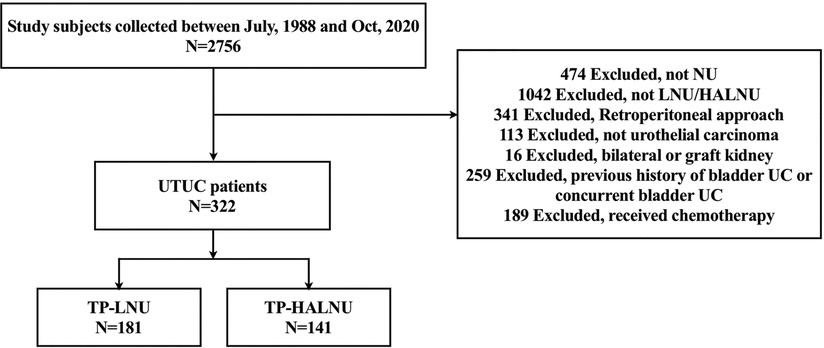
Figure 1. Study flowchart. UTC, upper tract urothelial carcinoma; UC, urothelial carcinoma; NU, nephroureterectomy; LNU, laparoscopic nephroureterectomy; HALNU, hand-assisted laparoscopic nephroureterectomy; TP-LNU, transperitoneal laparoscopic nephroureterectomy; TP-HALNU, transperitoneal hand-assisted laparoscopic nephroureterectomy.
Patient demographic and disease-specific characteristics are shown in Table 1. No significant difference in age was noted between these two groups, and the hand-assisted group had more male patients. There were no significant differences in tumor size, laterality, or multiplicity. We noted no significant difference in perioperative complications according to the Clavien–Dindo classification and postoperative complications of ileus and ventral herniation. TP-HALNU showed a higher overall mortality rate (44.7% vs. 26%) in the TP-LNU group but not in surgery-related mortality. Patients with TP-HALNU had a higher percentage of bladder UC cases after NU. The follow-up period for each approach was 30.26 months for TP-LNU and 62.06 months for TP-HALNU.
Detailed tumor characteristics were also recorded, which showed no significant differences in tumor location, surgical margin status, or pathological staging (Table 2). TP-HALNU had more cases of high-grade tumors, less pathologically positive lymph nodes, and more bladder UC after NU.
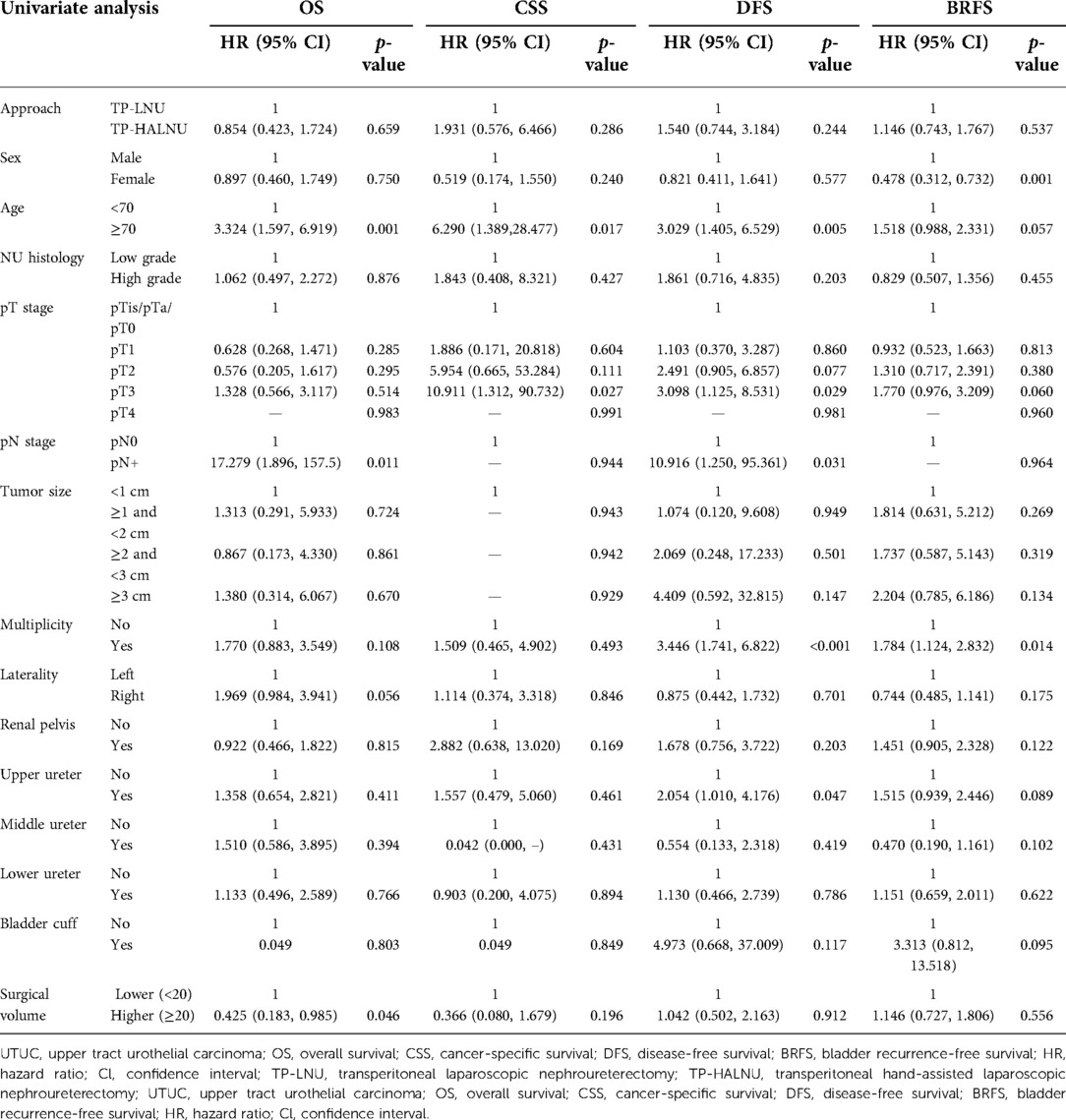
In the univariate survival analysis, surgical approach, histologic grade, tumor size, tumor laterality, and tumor location in the renal pelvis, middle ureter, lower ureter, and bladder cuff were independent predictors of survival outcomes (Table 3). The female sex was associated with shorter BRFS, with a hazard ratio (HR) of 0.478 (p = 0.001), but was not associated with other survival parameters. Age >70 years was independently associated with poorer overall survival (OS), CSS, and DFS [HR 3.324 (p = 0.001), 6.290 (p = 0.017), and 3.029 (p = 0.005), respectively]. Advanced pathological T staging and positive pathological N staging were associated with survival outcomes, with pT3 staging showing HR of 10.911 (p = 0.027) and 3.098 (p = 0.029) for CSS and DFS, and pN+ staging showing HR of 17.279 (p = 0.011) and 10.961 (p = 0.031) for OS and DFS, respectively. Tumor multiplicity was associated with DFS and BRFS, with HR of 3.446 (p < 0.001) and 1.784 (p = 0.014), respectively. Analysis of tumor location showed that only tumors located in the upper ureter were associated with DFS (HR 2.054, p = 0.047). Higher surgical volume (>20 cases) was associated with OS, with an HR of 0.425 (p = 0.046).
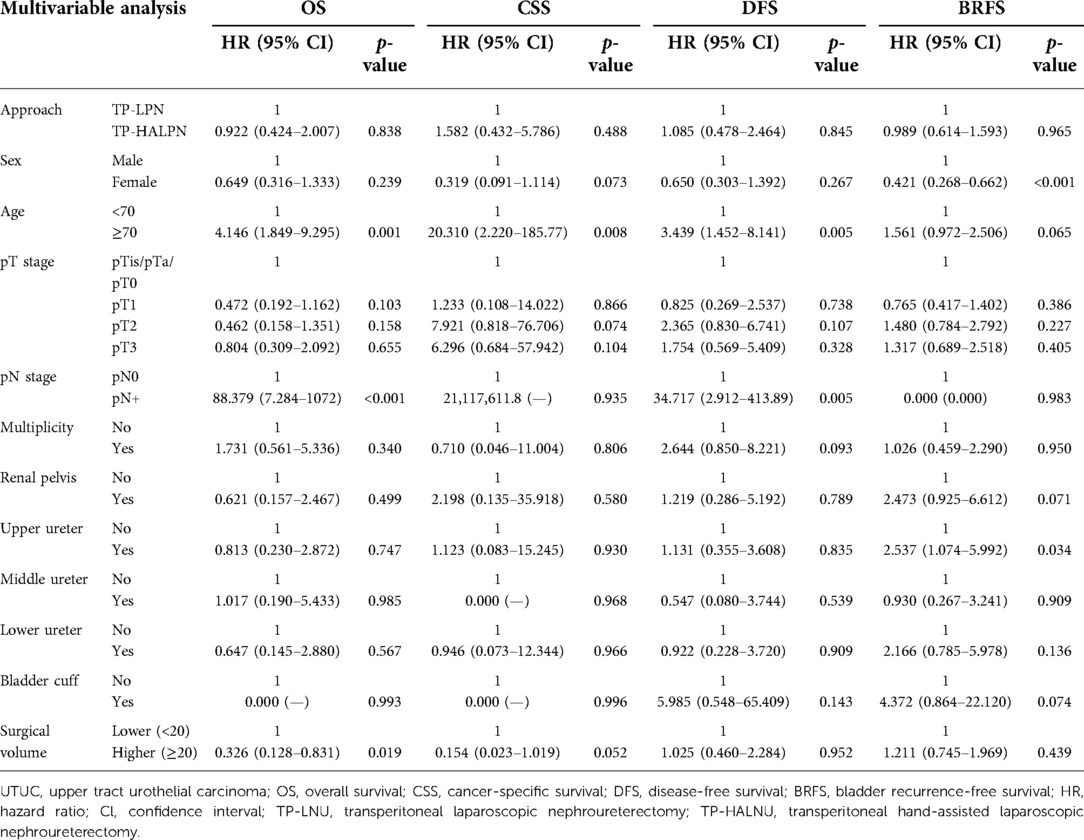
In the multivariate survival analysis, surgical approach, pathological T staging, tumor multiplicity, and tumor location in the renal pelvis, middle ureter, lower ureter, or bladder cuff showed no association with survival outcomes (Table 4). The female sex was associated with BRFS, with an HR of 0.421 (p < 0.001), but was not associated with other survival parameters. Age >70 years was associated with all four parameters except BRFS, with HR values of 4.146 (p = 0.001), 20.310 (p = 0.008), and 3.349 (p = 0.005), respectively. Positive pathological N staging was associated with OS and DFS, with HR values of 88.379 (p < 0.001) and 34.717 (p = 0.005), respectively. Analysis of tumor location showed that only tumors in the upper ureter were associated with BRFS, with an HR of 2.537 (p = 0.034). Higher surgical volume showed a trend toward better CSS (p = 0.052) and was associated with OS (HR 0.326, p = 0.019).
Discussion
The present study aimed to compare the surgical outcomes following TP-HALNU and TP-LNU from the Taiwan nationwide UTUC collaboration database for different parameters, including surgical volumes. We compared surgical outcomes, including OS, CSS, DFS, and BRFS, between the two surgical approaches in a nationwide database. Our study showed that the choice of surgical approach varies among urology departments or surgeon preferences, which shows a significant divergence between hospitals.
We noticed a significant difference in mortality between the two surgical approaches, with the TP-HALNU approach having an overall mortality rate of 44.7% compared to 26% in TP-LNU. This significant difference in overall mortality might be caused by the different follow-up periods (62 vs. 30 months). Although the two approaches showed significant differences in overall mortality, after the univariate and multivariate analyses for oncological outcomes, TP-LNU and TP-HALNU were not oncological predictors. Surgery-related mortality showed no difference, with a mortality rate of 0.7% vs. 0.6% between the two groups. The most common cause of death was listed as not UTUC-related. However, this was a cross-sectional, large-scale, retrospective cohort study using the UTUC collaboration database of the Taiwan Urology Association. Despite the presence of limitations, the results represent real-world data and demonstrate that either TP-LNU or TP-HALNU is feasible in the current setting in Taiwan. In addition, perioperative complications, according to the Clavien–Dindo classification, showed no significant difference between the two approaches.
Several previous studies comparing HALNU with LNU or ONU showed comparable oncological outcomes and better perioperative and postoperative outcomes (20, 21, 29–32). However, HALNU may be inferior to LNU or ONU with respect to RFS and BRFS rates (20, 33) and may be associated with higher intravesical recurrence (32). The current study demonstrated no oncological outcome difference between TP-LNU and TP-HALNU in both univariate and multivariate analyses.
In this study, we concluded that age was an independent predictor of survival outcomes. The multivariate analysis showed an association between age and OS, CSS, and DFS, with an increased risk in patients aged ≥70 years. Another independent predictor was sex, which was a lower risk factor in female patients with BRFS. This finding is consistent with previous reports illustrating that male sex was strongly associated with intravesical recurrence in patients with UTUC who received radical NU (34–36). Other than BRFS, no significant association was noted between sex and survival outcomes. Another obvious independent predictor associated with oncological outcomes was positive pathological lymph node metastasis, which is an obvious risk factor associated with worse outcomes.
NU with bladder cuff excision is the gold standard treatment for UTUC (37–39), so tumors located at the bladder cuff present challenges for radical resection. There are several approaches to bladder cuff excision, including the open technique, transurethral incision of the ureteral orifice, intussusception technique, transvesical laparoscopic detachment, and laparoscopic stapling (40, 41). In our study, tumor location at the bladder cuff was not associated with oncological outcomes. However, this database did not provide detailed records of the bladder cuff excision methods or the margin status of the bladder cuff. This could be a possible confounding factor, although one large patient cohort in Taiwan concluded that the method by which the bladder cuff is removed does not affect cancer-specific outcomes (42).
Retroperitoneoscopic NU (RPNU), with or without hand assistance, is also a widely accepted treatment option for UTUC. Previous studies have shown that RPNU had comparable oncological outcomes compared with ONU (43), LNU (44), or HALNU (45), and may have better perioperative and postoperative outcomes than LNU (44). However, intestinal retraction is considerably easier with the transperitoneal approach. Besides, peritoneal tear during RPNU occurred in certain cases, even with experienced surgeons (46). The impact of peritoneal tear includes conversion to the transperitoneal approach, but the limited number of retroperitoneal LNU cases in the Taiwan UTUC database may confound the analysis. Thus, in this study, we compared TP-HALNU and TP-LNU and observed comparable outcomes. We suggest that either approach is safe and feasible, depending on surgeons’ preferences and experiences.
For surgical volume, we observed that higher surgical volume did not impact DFS or BRFS but was associated with a trend toward favorable CSS (HR 0.154, p = 0.052) and marginal benefit for OS (HR 0.326, p = 0.019). Several previous studies have reported fewer intraoperative and perioperative complications associated with the surgical learning curve, reporting different learning thresholds at 50 or 100 cases at a single center (25, 26). In the current study, lower surgical volume (<20 cases) did not significantly influence DFS or BRFS. However, higher surgical volume was associated with a trend toward improved CSS (p = 0.052). Although this result implies that surgical volume matters in oncological outcomes, it is difficult to conclude that lower surgical volume impacts OS by influencing the quality of oncological control in the results. A poor correlation between PFS and OS may occur when considering different tumor characteristics, recurrence patterns, subsequent heterogeneous treatment, and quality of care, which were not shown in this study (47).
From this study, we suggest that either method is safe and feasible, depending on the surgeon's preferences and experiences. Larger-scale and prospective studies are required, considering the surgical volume and learning curve.
Limitations
The current study had several limitations. First, the original database included retrospectively reviewed patients with a lack of detailed records on several parameters, including bladder cuff resection method, margin status of the bladder cuff, the extent of lymph node dissection, perioperative intravesical chemotherapy, and behavioral adjustments such as smoking discontinuation. All mortality data were retrieved from the National Cancer Registry, and patients without an assigned code for cause of death were grouped into “Unknown” in the mortality parameter. This could have caused a statistical bias for CCS. The current study enrolled 14 hospitals in which patients were not randomized for the two surgical approaches. The choice of approach, the extent of lymph node dissection, and the follow-up protocol were mainly decided by each surgeon's or urology department's preference, which may cause bias in comparing outcomes between different approaches.
Conclusion
No significant differences in oncological outcomes and postoperative complication rates were observed between the TP-LNU and TP-HALNU groups. Age, sex, and lymph node metastasis were independent predictors of oncological outcomes. Higher surgical volume did not impact DFS and BRFS but was associated with improved OS and CSS. Exploration of unknown influencing factors is warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by IRB of Cardinal Tien hospital (CTH107-3-5-035) and IRB of Taipei Tzu Chi General Hospital (06-X34-105). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
C-CK and B-JC contributed to the conception and design of the study. B-JC, and C-CK organized the database and performed the statistical analysis. C-CK wrote the first draft of the manuscript. H-SC and B-JC contributed to the manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Cardinal Tien Hospital and Fu-Jen Catholic University (grant number CTH110A-FJU-2229).
Acknowledgments
All members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration group: Allen W. Chiu, Bing-Juin Chiang, Chao-Hsiang Chang, Chao-Yuan Huang, Cheng-Huang Shen, Cheng-Kuang Yang, Cheng-Ling Lee, Chen-Hsun Ho, Che-Wei Chang, Chia-Chang Wu, Chieh-Chun Liao, Chien-Hui Ou, Chih-Chen Hsu, Chih-Chin Yu, Chih-Hung Lin, Chih-Ming Lu, Chih-Yin Yeh, Ching-Chia Li, Chi-Ping Huang, Chi-Rei Yang, Chi-Wen Lo, Chuan-Shu Chen, Chung-Hsin Chen, Chung-You Tsai, Chung-Yu Lin, Chun-Hou Liao, Chun-Kai Hsu, Fang-Yu Ku, Hann-Chorng Kuo, Han-Yu Weng, Hao-Han Chang, Hong-Chiang Chang, Hsiao-Jen Chung, Hsin-Chih Yeh, Hsu-Che Huang, Ian-Seng Cheong, I-Hsuan Alan Chen, Jen-Kai Fang, Jen-Shu Tseng, Jen-Tai Lin, Jian-Hua Hong, Jih-Sheng Chen, Jungle Chi-Hsiang Wu, Kai-Jie Yu, Keng-Kok Tan, Kuan-Hsun Huang, Kun-Lin Hsieh, Lian-Ching Yu, Lun-Hsiang Yuan, Luo HL, Marcelo Chen, Min-Hsin Yang, Pai-Yu Cheng, Po-Hung Lin, Richard Chen-Yu Wu, See-Tong Pang, Shin-Hong Chen, Shin-Mei Wong, Shiu-Dong Chung, Shi-Wei Huang, Shuo-Meng Wang, Shu-Yu,Wu, Steven Kuan-Hua Huang, Ta-Yao Tai, Thomas Y. Hsueh, Ting-En Tai, Victor Chia-Hsiang Lin, Wei-Chieh Chen, Wei-Ming Li, Wei-Yu Lin, Wen-Hsin Tseng, Wen-Jeng Wu, Wun-Rong Lin, Yao-Chou Tsai, Yen-Chuan Ou, Yeong-Chin Jou, Yeong-Shiau Pu, Yi-Chia Lin, Yi-Hsuan Wu, Yi-Huei Chang , Yi-sheng Lin, Yi-Sheng Tai, Yu-Khun Lee, Yuan-Hong Jiang, Yu-Che Hsieh, Yu-Chi Chen, Yu-Ching Wen, Yung-Tai Chen, Zhe-Rui Yang.
Conflict of interest
Reviewers H-YK and F-JH declared a shared affiliation with the authors C-YH and C-HC to the handling editor at the time of review. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.934355/full#supplementary-material.
References
1. Tawfiek ER, Bagley DH. Upper-tract transitional cell carcinoma. Urology. (1997) 50(3):321–9. doi: 10.1016/S0090-4295(97)00230-6
2. Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol. (2013) 189(4):1214–21. doi: 10.1016/j.juro.2012.05.079
3. Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, et al. European Guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. (2013) 63(6):1059–71. doi: 10.1016/j.eururo.2013.03.032
4. Berger A, Fergany A. Laparoscopic nephroureterectomy: oncologic outcomes and management of distal ureter; review of the literature. Adv Urol. (2009) 2009:826725. doi: 10.1155/2009/826725
5. Simone G, Papalia R, Guaglianone S, Ferriero M, Leonardo C, Forastiere E, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol. (2009) 56(3):520–6. doi: 10.1016/j.eururo.2009.06.013
6. Stewart GD, Humphries KJ, Cutress ML, Riddick AC, McNeill SA, Tolley DA. Long-Term comparative outcomes of open versus laparoscopic nephroureterectomy for upper urinary tract urothelial-cell carcinoma after a median follow-up of 13 years*. J Endourol. (2011) 25(8):1329–35. doi: 10.1089/end.2011.0223
7. Ribal MJ, Huguet J, Alcaraz A. Oncologic outcomes obtained after laparoscopic, robotic and/or single port nephroureterectomy for upper urinary tract tumours. World J Urol. (2013) 31(1):93–107. doi: 10.1007/s00345-012-0968-0
8. Clayman RV, Kavoussi LR, Figenshau RS, Chandhoke PS, Albala DM. Laparoscopic nephroureterectomy: initial clinical case report. J Laparoendosc Surg. (1991) 1(6):343–9. doi: 10.1089/lps.1991.1.343
9. Reymond MA, Schneider C, Hohenberger W, Kockerling F. The pneumoperitoneum and its role in tumor seeding. Dig Surg. (1998) 15(2):105–9. doi: 10.1159/000018602
10. Klingler HC, Lodde M, Pycha A, Remzi M, Janetschek G, Marberger M. Modified laparoscopic nephroureterectomy for treatment of upper urinary tract transitional cell cancer is not associated with an increased risk of tumour recurrence. Eur Urol. (2003) 44(4):442–7. doi: 10.1016/s0302-2838(03)00314-2
11. Favaretto RL, Shariat SF, Chade DC, Godoy G, Kaag M, Cronin AM, et al. Comparison between laparoscopic and open radical nephroureterectomy in a contemporary group of patients: are recurrence and disease-specific survival associated with surgical technique? Eur Urol. (2010) 58(5):645–51. doi: 10.1016/j.eururo.2010.08.005
12. Ni S, Tao W, Chen Q, Liu L, Jiang H, Hu H, et al. Laparoscopic versus open nephroureterectomy for the treatment of upper urinary tract urothelial carcinoma: a systematic review and cumulative analysis of comparative studies. Eur Urol. (2012) 61(6):1142–53. doi: 10.1016/j.eururo.2012.02.019
13. Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Use HJ. Costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. (2012) 187(4):1392–8. doi: 10.1016/j.juro.2011.11.089
14. Capitanio U, Shariat SF, Isbarn H, Weizer A, Remzi M, Roscigno M, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol. (2009) 56(1):1–9. doi: 10.1016/j.eururo.2009.03.072
15. Waldert M, Remzi M, Klingler HC, Mueller L, Marberger M. The oncological results of laparoscopic nephroureterectomy for upper urinary tract transitional cell cancer are equal to those of open nephroureterectomy. BJU Int. (2009) 103(1):66–70. doi: 10.1111/j.1464-410X.2008.07950.x
16. Walton TJ, Novara G, Matsumoto K, Kassouf W, Fritsche HM, Artibani W, et al. Oncological outcomes after laparoscopic and open radical nephroureterectomy: results from an international cohort. BJU Int. (2011) 108(3):406–12. doi: 10.1111/j.1464-410X.2010.09826.x
17. Tinay I, Gelpi-Hammerschmidt F, Leow JJ, Allard CB, Rodriguez D, Wang Y, et al. Trends in utilisation, perioperative outcomes, and costs of nephroureterectomies in the management of upper tract urothelial carcinoma: a 10-year population-based analysis. BJU Int. (2016) 117(6):954–60. doi: 10.1111/bju.13375
18. Wolf JS Jr., Moon TD, Nakada SY. Hand assisted laparoscopic nephrectomy: comparison to standard laparoscopic nephrectomy. J Urol. (1998) 160(1):22–7. doi: 10.1016/S0022-5347(01)63016-7
19. Munver R, Del Pizzo JJ, Sosa RE. Hand-assisted laparoscopic nephroureterectomy for upper urinary-tract transitional-cell carcinoma. J Endourol. (2004) 18(4):351–8. doi: 10.1089/089277904323056898
20. Kitamura H, Maeda T, Tanaka T, Fukuta F, Kobayashi K, Nishiyama N, et al. Comparison of laparoscopic, hand-assisted, and open surgical nephroureterectomy. JSLS. (2014) 18(2):288–93. doi: 10.4293/108680813X13794522666842
21. Nouralizadeh A, Tabatabaei S, Basiri A, Simforoosh N, Soleimani M, Javanmard B, et al. Comparison of open versus laparoscopic versus hand-assisted laparoscopic nephroureterectomy: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A. (2018) 28(6):656–81. doi: 10.1089/lap.2017.0662
22. Kim TH, Suh YS, Jeon HG, Jeong BC, Seo SI, Jeon SS, et al. Transperitoneal radical nephroureterectomy is associated with worse disease progression than retroperitoneal radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Sci Rep. (2019) 9(1):6294. doi: 10.1038/s41598-019-42739-0
23. Abboudi H, Khan MS, Guru KA, Froghi S, de Win G, Van Poppel H, et al. Learning curves for urological procedures: a systematic review. BJU Int. (2014) 114(4):617–29. doi: 10.1111/bju.12315
24. Passerotti CC, Nguyen HT, Retik AB, Peters CA. Patterns and predictors of laparoscopic complications in pediatric urology: the role of ongoing surgical volume and access techniques. J Urol. (2008) 180(2):681–5. doi: 10.1016/j.juro.2008.04.042
25. Jha MS, Gupta N, Agrawal S, Ansari MS, Dubey D, Mandhani A, et al. Single-centre experience of laparoscopic nephrectomy: impact of learning curve on outcome. Indian J Urol. (2007) 23(3):253–6. doi: 10.4103/0970-1591.33719
26. Kanno T, Shichiri Y, Oida T, Kanamaru H, Takao N, Shimizu Y. Complications and the learning curve for a laparoscopic nephrectomy at a single institution. Int J Urol. (2006) 13(2):101–4. doi: 10.1111/j.1442-2042.2006.01239.x
27. Masoud M, Ibrahim A, Elemam A, Elatreisy A, Noureldin Y, Aubé M, et al. Learning curve of laparoscopic nephrectomy: a prospective pilot study. Afr J Urol. (2020) 26(1):24. doi: 10.1186/s12301-020-00024-x
28. Keurentjes JHM, Briët JM, de Bock GH, Mourits MJE. Surgical volume and conversion rate in laparoscopic hysterectomy: does volume matter? A multicenter retrospective cohort study. Surg Endosc. (2018) 32(2):1021–6. doi: 10.1007/s00464-017-5780-x
29. Landman J, Lev RY, Bhayani S, Alberts G, Rehman J, Pattaras JG, et al. Comparison of hand assisted and standard laparoscopic radical nephroureterectomy for the management of localized transitional cell carcinoma. J Urol. (2002) 167(6):2387–91. doi: 10.1016/S0022-5347(05)64990-7
30. Silberstein J, Parsons JK. Hand-assisted and total laparoscopic nephrectomy: a comparison. JSLS. (2009) 13(1):36–43. PMID: 19366539, PMCID: 3015892
31. Raman JD, Palese MA, Ng CK, Boorjian SA, Scherr DS, Del Pizzo JJ, et al. Hand-assisted laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma. JSLS. (2006) 10(4):432–8. PMID: 17575752, PMCID: 3015762
32. Yu CC, Chen CH, Hong JH, Ke HL, Li WM, Chung SD, et al. Comparison of oncological outcomes for hand-assisted and pure laparoscopic radical nephroureterectomy: results from the Taiwan upper tract urothelial cancer collaboration group. Surg Endosc. (2022) 36(6):4342–8. doi: 10.1007/s00464-021-08779-2
33. Kamihira O, Hattori R, Yamaguchi A, Kawa G, Ogawa O, Habuchi T, et al. Laparoscopic radical nephroureterectomy: a multicenter analysis in Japan. Eur Urol. (2009) 55(6):1397–407. doi: 10.1016/j.eururo.2009.03.003
34. Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Kobayashi H, et al. The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: a multi-institutional study. Urol Oncol. (2014) 32(1):48.e19–26. doi: 10.1016/j.urolonc.2013.07.003
35. Xylinas E, Kluth L, Passoni N, Trinh QD, Rieken M, Lee RK, et al. Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision-making tool. Eur Urol. (2014) 65(3):650–8. doi: 10.1016/j.eururo.2013.09.003
36. Wu YT, Luo HL, Wang HJ, Chen YT, Cheng YT, Chiang PH. Gender effect on the oncologic outcomes of upper urinary tract urothelial carcinoma in Taiwan. Int Urol Nephrol. (2020) 52(6):1043–8. doi: 10.1007/s11255-020-02396-z
37. Huben RP, Mounzer AM, Murphy GP. Tumor grade and stage as prognostic variables in upper tract urothelial tumors. Cancer. (1988) 62(9):2016–20. doi: 10.1002/1097-0142(19881101)62:9<2016::aid-cncr2820620924>3.0.co;2-g
38. Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. (1998) 52(4):594–601. doi: 10.1016/s0090-4295(98)00295-7
39. Kirkali Z, Tuzel E. Transitional cell carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol. (2003) 47(2):155–69. doi: 10.1016/s1040-8428(03)00079-9
40. Laguna MP, de la Rosette JJ. The endoscopic approach to the distal ureter in nephroureterectomy for upper urinary tract tumor. J Urol. (2001) 166(6):2017–22. doi: 10.1016/S0022-5347(05)65497-3
41. Steinberg JR, Matin SF. Laparoscopic radical nephroureterectomy: dilemma of the distal ureter. Curr Opin Urol. (2004) 14(2):61–5. doi: 10.1097/00042307-200403000-00003
42. Li WM, Shen JT, Li CC, Ke HL, Wei YC, Wu WJ, et al. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur Urol. (2010) 57(6):963–9. doi: 10.1016/j.eururo.2009.12.032
43. Chung SD, Huang CY, Chueh SC, Pu YS, Lai MK, Yu HJ, et al. Intermediate follow-up of hand-assisted retroperitoneoscopic nephroureterectomy for management of upper urinary tract urothelial carcinoma: comparison with open nephroureterectomy. Urology. (2007) 69(6):1030–4. doi: 10.1016/j.urology.2007.01.088
44. Chiang PH, Luo HL, Chen YT, Kang CH, Chuang YC, Lee WC. Is hand-assisted retroperitoneoscopic nephroureterectomy better than transurethral bladder cuff incision-assisted nephroureterectomy? J Endourol. (2011) 25(8):1307–13. doi: 10.1089/end.2011.0094
45. Chung SD, Chueh SC, Huang CY, Lai MK, Pu YS, Yu HJ, et al. Comparison between hand-assisted laparoscopic and retroperitoneoscopic nephroureterectomy for the management of upper urinary tract urothelial carcinoma: analysis of an intermediate follow-up period. Surg Laparosc Endosc Percutan Tech. (2008) 18(1):49–53. doi: 10.1097/SLE.0b013e318159e84b
46. Liapis D, de la Taille A, Ploussard G, Robert G, Bastien L, Hoznek A, et al. Analysis of complications from 600 retroperitoneoscopic procedures of the upper urinary tract during the last 10 years. World J Urol. (2008) 26(6):523. doi: 10.1007/s00345-008-0319-3
Keywords: urothelial carcinoma, upper urinary tract urothelial carcinoma, laparoscopic nephroureterectomy, hand-assisted laparoscopic nephroureterectomy, oncological outcome, surgical volume
Citation: Kuo C, Chen G, Chang C, Huang C, Chen C, Li C, Wu W, Yu C, Lo C, Chen Y, Chen S, Cheng P, Hsueh T, Chiu AW, Lin P, Tseng J, Lin J, Jiang Y, Wu C, Lin W, Huang H, Chiang H and Chiang B (2022) Surgical outcome predictor analysis following hand-assisted or pure laparoscopic transperitoneal nephroureterectomy using the Taiwan upper urinary tract urothelial carcinoma database. Front. Surg. 9:934355. doi: 10.3389/fsurg.2022.934355
Received: 2 May 2022; Accepted: 8 August 2022;
Published: 1 September 2022.
Edited by:
Yao-Chou Tsai, Taipei Medical University Hospital, TaiwanReviewed by:
Hung-Yang Kuo, National Taiwan University Hospital, TaiwanFu-Jen Hsueh, National Taiwan University Hospital, Taiwan
Atsushi Okada, Nagoya City University, Japan
© 2022 Kuo, Chen, Chang, Huang, Chen, Li, Wu, Yu, Lo, Chen, Chen, Cheng, Hsueh, Chiu, Lin, Tseng, Lin, Jiang, Wu, Lin, Huang, Chiang and Chiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing-Juin Chiang YmluZ2p1aW5jaGlhbmdAZ21haWwuY29t; Han-Sun Chiang MDUzODI0QG1haWwuZmp1LmVkdS50dw==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Chih-Chun Kuo
Chih-Chun Kuo Guang-Heng Chen3,4
Guang-Heng Chen3,4 Chao-Hsiang Chang
Chao-Hsiang Chang Chao-Yuan Huang
Chao-Yuan Huang Chung-Hsin Chen
Chung-Hsin Chen Chi-Wen Lo
Chi-Wen Lo Yung-Tai Chen
Yung-Tai Chen Pai-Yu Cheng
Pai-Yu Cheng Jen-Tai Lin
Jen-Tai Lin Wei-Yu Lin
Wei-Yu Lin Bing-Juin Chiang
Bing-Juin Chiang