
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 18 October 2022
Sec. Surgical Oncology
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.933168
Background and Objectives: This study aims to perform a comprehensive clinical analysis of patients with primary malignant pituitary tumors (PMPT) that involves incidence, demographics, treatments, long-term survival, and death causes.
Materials and methods: Patients with PMPT were identified from registries of the Surveillance, Epidemiology, and End Results (SEER) database. Frequencies and average annual age-adjusted rate (AAR) were calculated for incidence trend analyses using Join-point regression. Univariate and multivariate Cox regression analyses were conducted to identify potential prognostic factors associated with patients' survival outcomes. Using the Kaplan–Meier method and log-rank test, survival curves were plotted and compared, respectively. Propensity score matching (PSM) was performed to balance baseline characteristics.
Results: The AAR for PMPT was 0.233 (95%CI: 0.205–0.264) per 1,000,000 using nine SEER registries from 1975 to 2017. The incidence trend has declined over years but without significance (–1.04% per year, P = 0.10). Besides, older age may indicate a higher incidence rate for both pediatric and adult patients. From 18 SEER registries, a total of 501 PMPT patients were also identified. Univariate and multivariate Cox regression showed age, sex, tumor extent, and marital status were independent prognostic factors for malignant pituitary tumors. Via PSM, we found that patients who received surgery, radiotherapy, and chemotherapy did not demonstrate significantly different survival than those who did not.
Conclusion: This study first conducts a comprehensive clinical analysis of patients with PMPT and provides guide effects on future study designs. More studies should be conducted to focus on its characteristics and therapy.
Pituitary tumors were the second malignant disease that accounted for 10%–15% of intracranial neoplasms. With the wide application of radiological techniques such as computing tomography (CT) and magnetic resonance imaging (MRI), pituitary adenomas found by accident has been increasing. It was previously reported to have an estimated overall prevalence of 16.7% (1). The most common tumor located in the pituitary is adenoma, which can be often classified according to the size, invasion, differentiation, and endocrinal nature of the tumor in clinical practice. Most adenomas showed no invasive behavior, while other malignant types including adenocarcinoma, germinoma, chordoma, etc. occurred very rarely. Previously, very few studies focused on the primary malignant tumors located in the pituitary gland, which may demonstrate different clinical characteristics and survival outcomes.
According to the functional status, pituitary tumors can be divided into functioning and non-functioning. Among functioning cases, there can be a series of clinical symptoms such as acromegaly, Cushing disease, etc. For them, surgical resection is recommended as a first-line treatment (2–4). However, the space-occupying effect may play a more critical role in the management of non-functional tumors for the lack of endocrine functions. Thus, fewer patients need clinical intervention, though the prevalence rate of non-functional pituitary tumors remains high (5). Besides, pituitary adenomas can be grouped into microadenomas (diameter ≤ 10 mm) and macroadenomas (diameter > 10 mm) according to their size. For microadenomas, especially asymptomatic cases, keeping follow-up or receiving conservative treatment can be chosen. Furthermore, based on imaging findings, biological behavior, and pathological features, pituitary tumors can be divided into invasive and non-invasive. The invasive type is difficult to resect completely and the drug treatment had very limited effects.
As one kind of complex endocrine tumor, pituitary tumors seriously affect the quality of life and prognosis of patients because of their endocrinal nature and/or space-occupying effect. For PMPT, the invasive behavior made it more complex and diverse. In the present study, using the large cancer database with clinical information, the Surveillance, Epidemiology, and End Results (SEER) program proposed by the National Cancer Institute of the United States, we analyzed the incidence, demographics, treatments, long-term survival, and death causes of patients with primary malignant tumors located at the pituitary gland, including adenocarcinoma, invasive adenoma, germinoma, and chordoma. We aimed to first provide an overview of the malignant pituitary tumor in the past decades.
Using SEER*Stat software (version 8.3.8; https://seer.cancer.gov/seerstat/), the population-based registries from the National Cancer Institute's SEER database were retrieved and identified for this retrospective study, which covers approximately 30% of cancer patients in the United States with clinical information of incidence, demographic characteristics, survival, and death. Patients diagnosed with primary malignant tumors located in the pituitary gland were regarded as our study population. Due to the anonymity of the SEER database, the informed consent was waived and this study was approved to be exempted research by the Ethics Committee of West China Hospital. In this study, PMPT was defined as primary tumors with malignant behavior located in the pituitary gland, mainly including adenocarcinoma, invasive adenoma, germinoma, and chordoma.
To investigate the long-term incidence of pituitary tumors in the past decades, the SEER Research Data with nine Registries (1975–2017) was used to estimate the frequencies and incidence rates by SEER*Stat software, which had been updated in November 2019. Moreover, the annual percent change (APC) was calculated using a Join-point regression analysis program (version 4.8.0.1; https://surveillance.cancer.gov/joinpoint/) to describe the incidence trend over time. In the Join-point analysis, the optimal fitting piecewise continuous log-linear model was identified, and the permutation test was used for finding the minimal number of join points that fit the data.
In order to describe pituitary malignant tumor patients' characteristics, we retrieved the SEER 18 Registries Custom Data (with additional treatment fields; November 2018 Sub; 1975–2016). These cases with indefinite primary tumors or unknown follow-up were excluded. The demographic and treatment characteristics included age (pediatric: <18 and adult: ≥18 years), race (white, black, and others, involving American Indian/AK Native, Asian/Pacific Islander), year of diagnosis for the primary pituitary tumor, tumor extent staging (localized, regional, distant and unknown), tumor size, histology (adenocarcinoma, invasive adenoma, germinoma, chordoma, and others/unknown), marital status (married, single, widowed, divorced/separated, unknown), surgery (yes and no), radiotherapy (yes and no), radiation sequence (no, after surgery, before surgery and received but unknown sequence), and chemotherapy (yes and no).
Furthermore, distributions of patients' characteristics were compared between different populations of different ages (pediatric and adult), and univariate Cox regression analyses for the corresponding subgroups were performed. Those variables with a P value < 0.05 for all patients were further entered into multivariate Cox regression. Then, survival curves were plotted for variables with a significant difference by the Kaplan–Meier method and were compared using the log-rank test.
To evaluate the effects of treatments on patients with pituitary tumors, the long-term survival outcome was compared between treatments before and after the adjustment by propensity score matching (PSM) analyses (using the nearest neighbor matching algorithm; ratio: 1:1, caliper value = 0.05).
The statistical analyses were performed using IBM SPSS 25.0 and R software (version 3.6.3; https://www.r-project.org). P value < 0.05 was considered statistically significant. Distributions of clinical characteristics between pediatric and adult patients were compared by Pearson's Chi-square test (or Fisher's exact test if necessary). In the Cox regression analyses, hazards ratio with a 95% confidence interval (95%CI) was calculated.
A total of 251 patients diagnosed with PMPT between 1975 and 2017 from the 9 SEER registries of Research Data were identified with an average annual age-adjusted rate (AAR) of 0.233 (95%CI: 0.205–0.264) per 1,000,000 individuals. As shown in Figure 1, the age-adjusted incidence rate seemed to become lower over time from 1975 to 2017 without any join point, though there was no significance for the changing trend with an average APC of −1.04% (95%CI: −2.2%−0.1%, P = 0.10) per year. Besides, the crude incidence rates at each range of ages were also calculated and presented in Figure 2, from which it could be presumed that the rate increasingly changed over age among both pediatric and adult populations, but it suddenly declined at the adult age. The old patients with age ≥60 years demonstrated largely higher incidence rates than those with younger people.
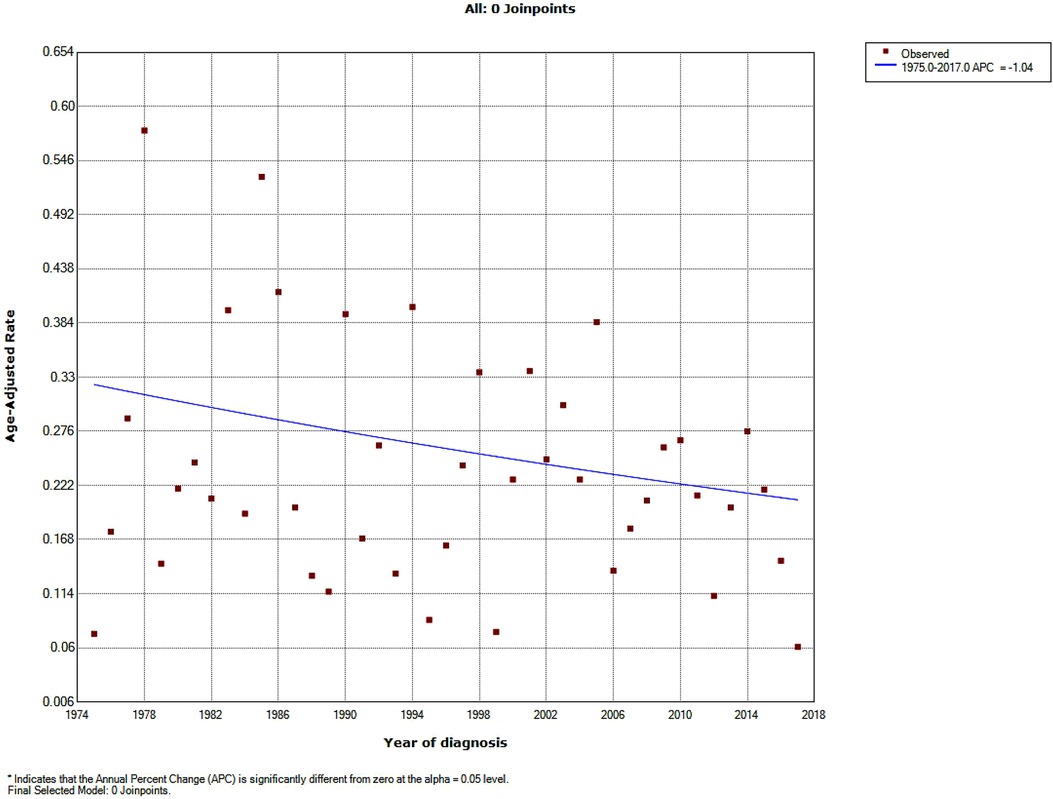
Figure 1. Incidence trend over years in patients with primary malignant pituitary tumor from the SEER research data with nine registries (1975–2017).
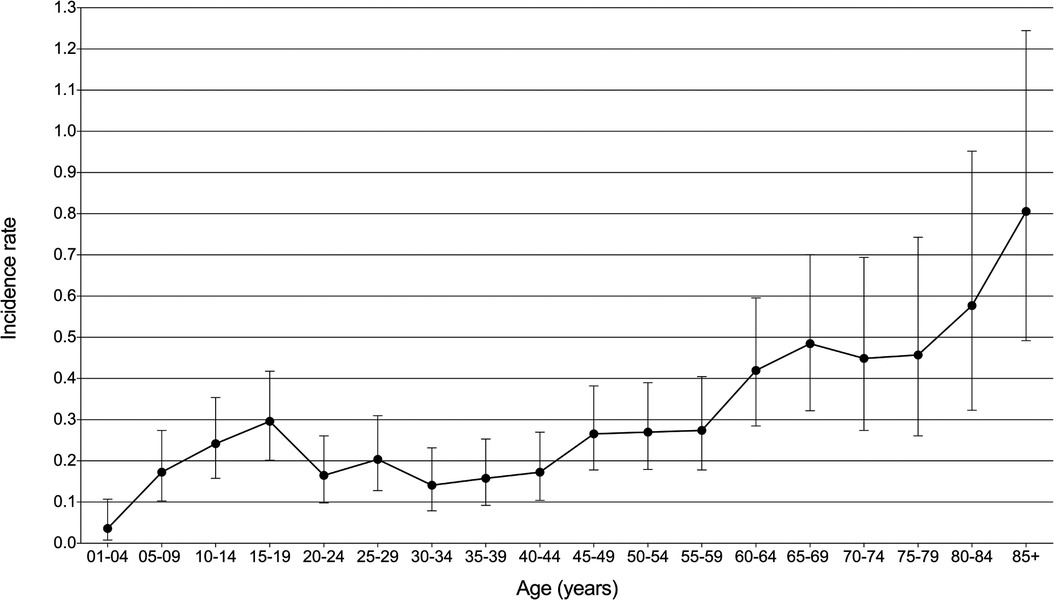
Figure 2. Incidence rate with 95% confidence interval at each age range in patients with primary malignant pituitary tumor from the SEER research data with nine registries (1975–2017).
From the SEER 18 Registries Custom Data (with additional treatment fields), a total of 501 patients diagnosed with PMPT between 1975 and 2016 finally met the inclusion criteria, involving 89 (17.8%) pediatric and 412 (82.2%) adult patients (Table 1). The univariate Cox regression analyses on all patients demonstrated that age (HR = 1.046, 95%CI: 1.038–1.053, P < 0.001; adult vs. pediatric, HR = 6.20, 95%CI: 3.28–11.70, P < 0.001), sex (female vs. male, HR = 0.69, 95%CI: 0.52–0.91, P = 0.008), tumor extent (regional vs. localized, HR = 2.26, 95%CI: 1.42–3.61, P = 0.001; distant vs. localized, HR = 5.36, 95%CI: 2.70–10.66, P < 0.001), histology (reference: adenocarcinoma; invasive adenoma: HR = 1.48, 95%CI: 0.54–4.03, P = 0.446; germinoma: HR = 0.33, 95%CI: 0.16–0.68, P = 0.003; chordoma: HR = 1.90, 95%CI: 1.03–3.50, P = 0.040; others/unknown: HR = 1.76, 95%CI: 1.06–2.91, P = 0.028), marital status (reference: married; single: HR = 0.38, 95%CI: 0.26–0.54, P < 0.001; widowed: HR = 2.47, 95%CI: 1.64–3.73, P < 0.001; divorced/separated: HR = 1.46, 95%CI: 0.90–2.39, P = 0.127), radiotherapy (yes vs. no, HR = 0.59, 95%CI: 0.44–0.79, P < 0.001), and chemotherapy (yes vs. no, HR = 0.50, 95%CI: 0.33–0.77, P = 0.002) were significantly associated with survival outcome. In addition, the cross-table analyses (as shown in Table 1) showed several characteristics were significantly differently distributed between pediatric and adult patients, including tumor extent (P = 0.003), histology (P < 0.001), marital status (P < 0.001), surgery (P = 0.017), radiotherapy (P < 0.001), radiation sequence (P = 0.007), and chemotherapy (P < 0.001).
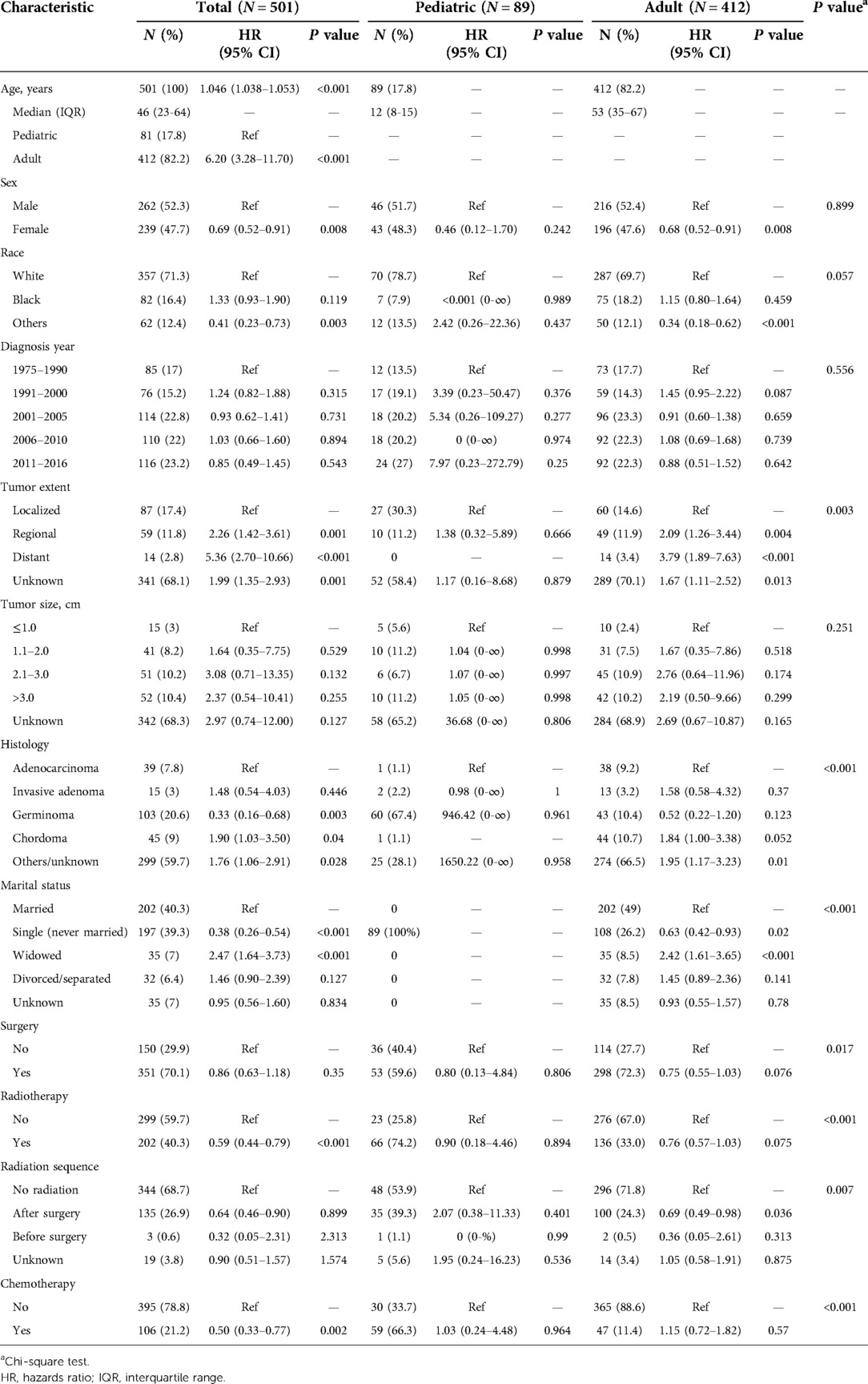
Table 1. Univariate Cox regression analyses for overall survival in patients with primary malignant pituitary tumors.
Then, the significant variables in the univariate Cox regression analyses further entered into multivariate analysis, which demonstrated that age (adult vs. pediatric, HR = 3.18, 95%CI: 1.49–6.82, P = 0.003), sex (female vs. male, HR = 0.56, 95%CI: 0.41–0.74, P < 0.001), tumor extent (regional vs. localized, HR = 1.82, 95%CI: 1.12–2.95, P = 0.015; distant vs. localized, HR = 4.01, 95%CI: 1.96–8.21, P < 0.001) and marital status (reference: married; single: HR = 0.62, 95%CI: 0.41–0.93, P < 0.001; widowed: HR = 2.47, 95%CI: 1.62–3.75, P < 0.001; divorced/separated: HR = 1.72, 95%CI: 1.05–2.84, P = 0.033) were independent prognostic factors for pituitary tumor (Table 2).
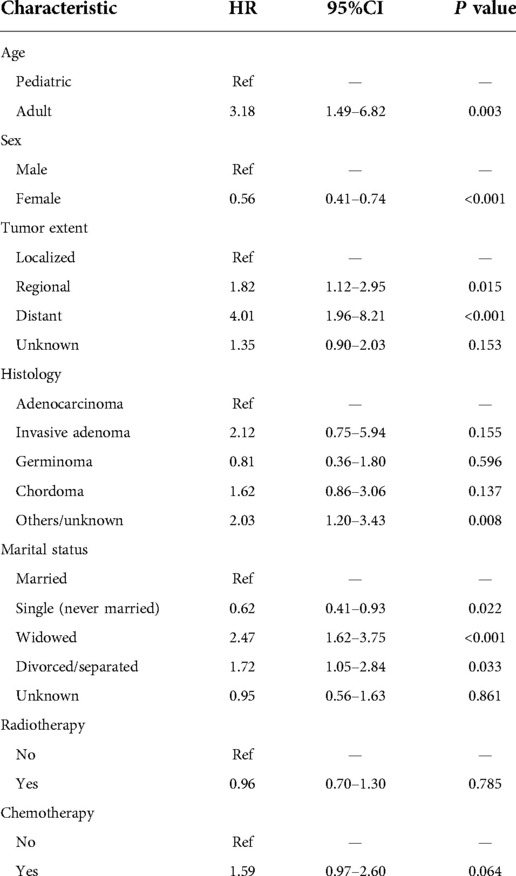
Table 2. Multivariate Cox regression analysis for overall survival in patients with primary malignant pituitary tumors.
For all enrolled patients, the mean follow-up was 102 months with a median survival time (MST) of 205 months (Figure 1). According to the results of multivariate Cox regression, the significant prognostic factors (age, tumor extent, and marital status) were used for plotting Kaplan–Meier survival curves. The pediatric patients (MST: 420 months) showed significantly better long-term survival than adults (MST: 145 months; Figure 3B, P < 0.001). Obviously, localized stage patients also demonstrated better survival than the regional stage, followed by the distant stage with the worst survival (Figure 3C, P < 0.001). As for marital status, the never-married patients had better survival outcomes than others, and the widowed people performed worst (Figure 3D, P < 0.001).
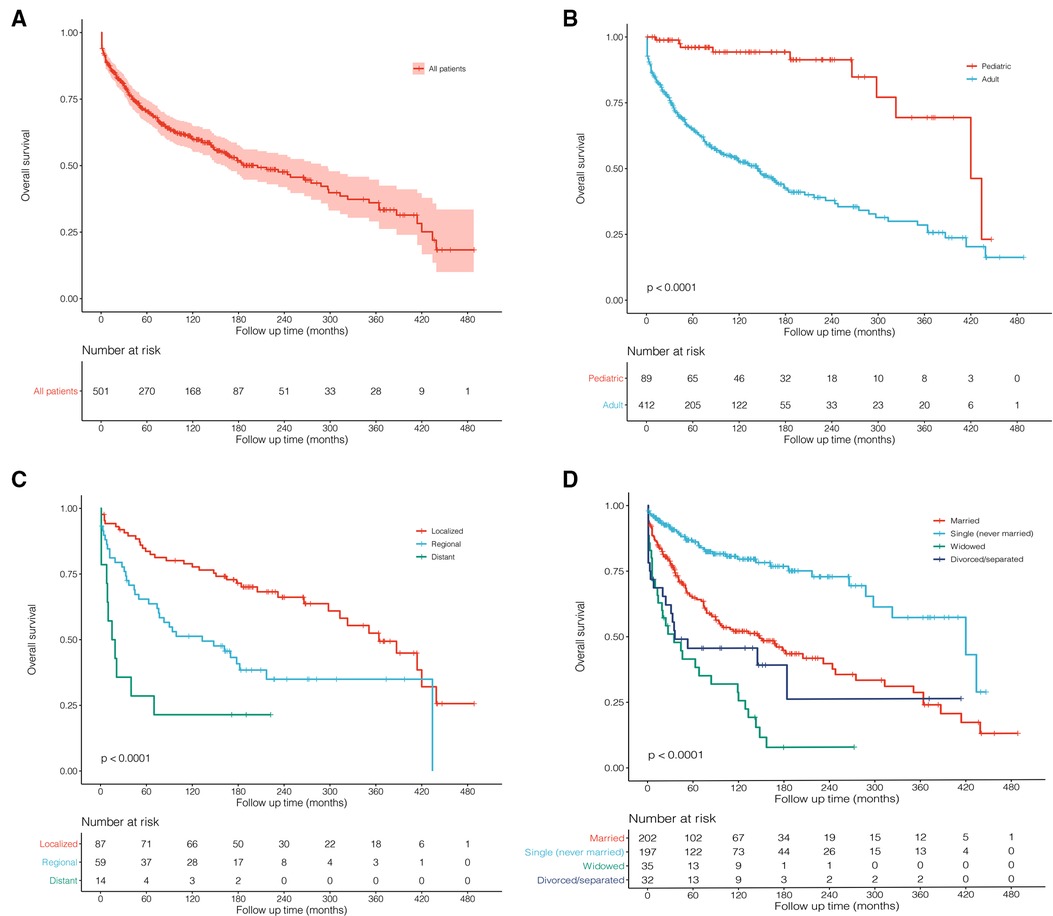
Figure 3. Kaplan–Meier survival curves and log-rank tests for possible prognostic factors. (A) All 501 patients were diagnosed with primary malignant pituitary tumors between 1975 and 2016 from the SEER 18 Registries Custom Data. (B) Age: pediatric and adult. (C) Tumor extent: localized, regional, and distant. (D) Marital status: married, single (never married), widowed, and divorced/separated.
Table 3 describes treatment patterns for patients with PMPT. The most common therapy for them was surgery plus radiotherapy (MST: 297 months) and only surgery (MST: 143 months). To evaluate the prognostic effects of these treatments, PSM analyses were performed to balance the remaining characteristics. Surgical resection did not benefit the survival outcome of pituitary tumors (Figure 4A, P = 0.350; Figure 4B, P = 0.250). Before matching, patients undergoing surgery, radiotherapy, and chemotherapy showed better survival than those who did not (Figure 4C, P < 0.001; Figure 4E, P < 0.001). However, the significant differences faded away after PSM (Figure 4D, P = 0.081; Figure 4F, P = 0.120).
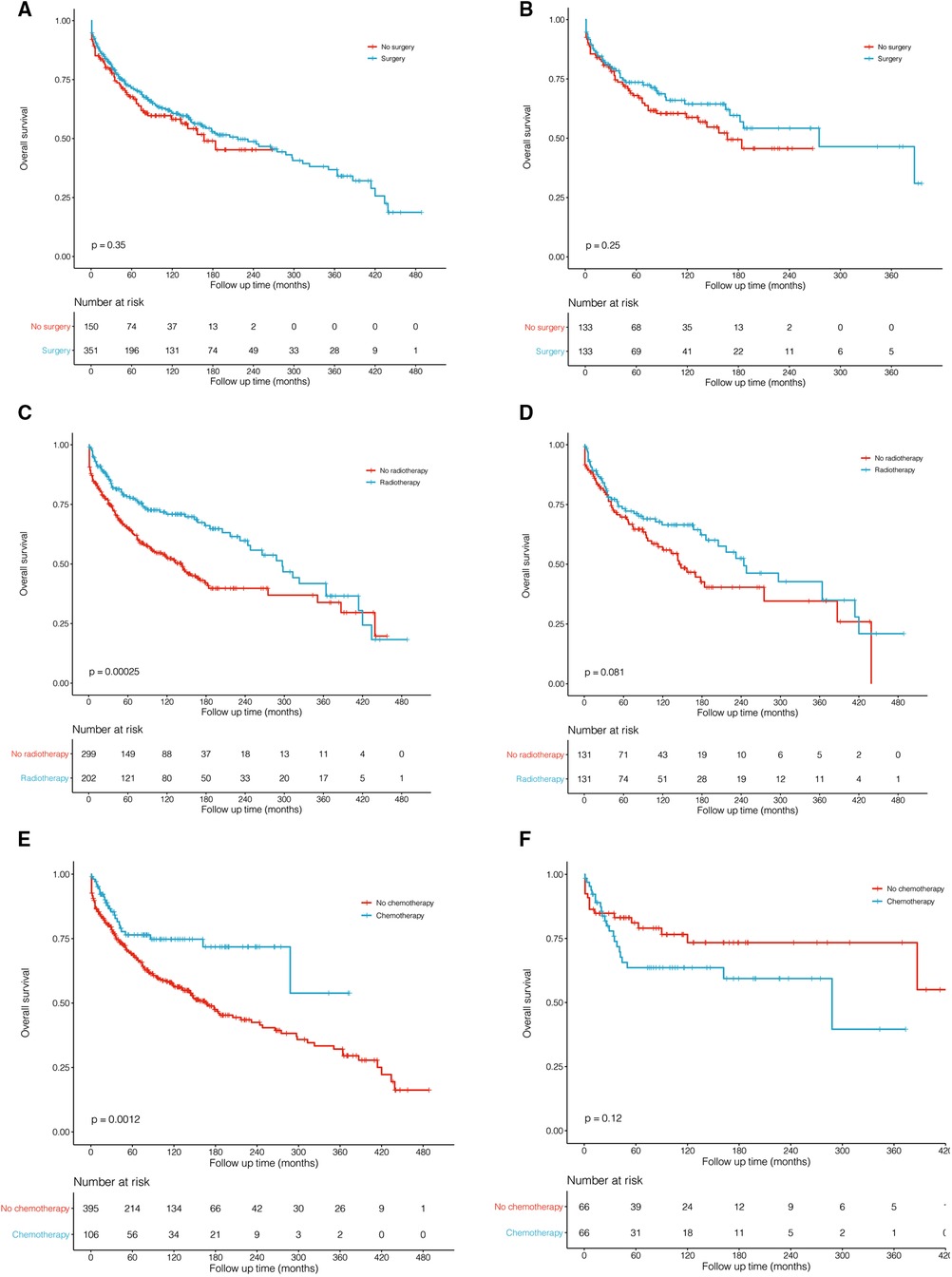
Figure 4. Kaplan–Meier survival curves and log-rank tests for treatments before (A,C,E) and after (B,D,F) propensity score matching (PSM). (A,B). Surgery vs. no surgery. (C,D) Radiotherapy vs. no radiotherapy. (E,F) Chemotherapy vs. no chemotherapy.
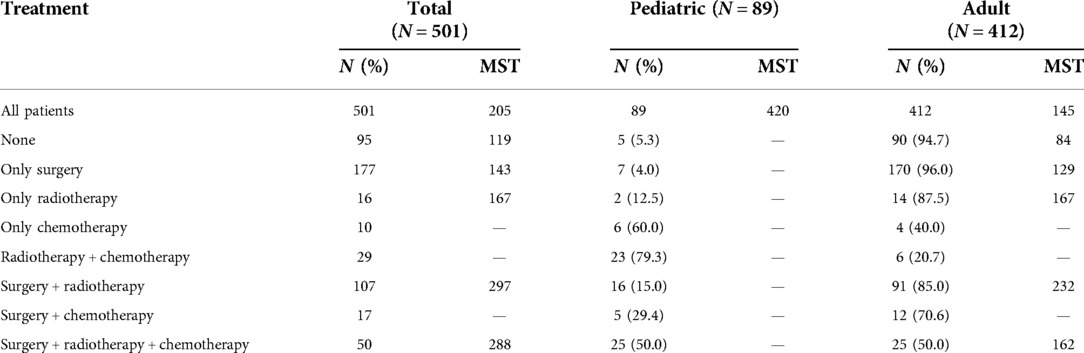
Table 3. Number and median survival time (MST) of patients with primary malignant pituitary tumors in different treatment groups.
Until the last update of follow-up in November 2018, there was a sum of 212 (42.3%, 212/501) PMPT patients who died, which involved 10 (11.2%, 10/89) pediatric cases and 202 (49.0%, 202/412) adults. Figure 5 shows death causes of pediatric and adult patients. Ten pediatric deaths included benign or unknown behavior neoplasm (in situ; N = 3), brain and other nervous systems (N = 3), chronic liver disease and cirrhosis (N = 1), testis disease (N = 1), and other unknown causes (N = 2). Among adults, five of the most common causes were other endocrine diseases (N = 40, 19.8%), benign or unknown behavior neoplasm (in situ; N = 25, 12.4%), heart diseases (N = 22, 12.9%), brain and other nervous systems (N = 17, 8.4%); miscellaneous malignant cancer (N = 15, 7.4%).
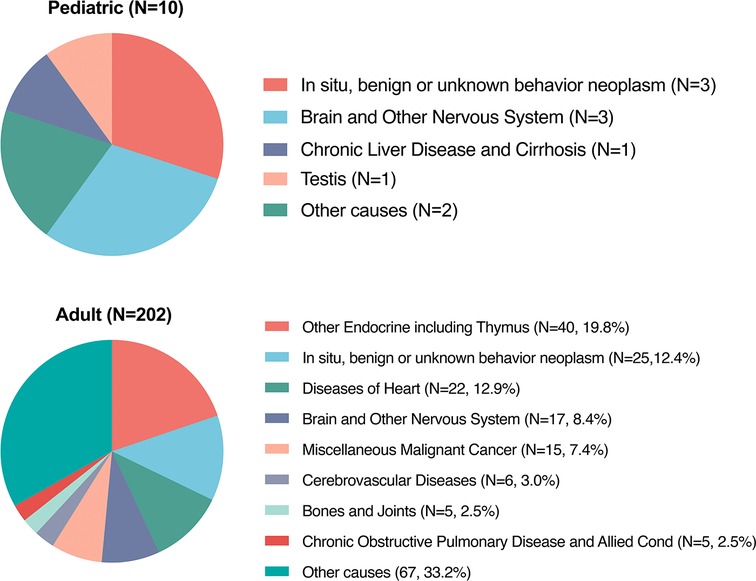
Figure 5. Causes of death among pediatric and adult patients with primary malignant pituitary tumor from the SEER 18 registries custom data.
A pituitary tumor is a kind of common intracranial tumor that has been poorly studied in previous pieces of literature. It was currently considered that pituitary tumors, especially adenoma, originated from the abnormal differentiation of anterior pituitary cells or craniofacial epithelial cells. At present, the treatment for pituitary adenomas includes drug therapy, radiotherapy, and surgery, among which surgery is the most effective and thorough method (6). Clinically, pituitary adenomas can be divided into functional and non-functional types. The active endocrine state is closely related to the functioning tumors, which can lead to a series of symptoms, such as acromegaly caused by high levels of growth hormone and insulin growth factor one, amenorrhea or sexual dysfunction caused by hyperprolactinemia, Cushing's disease caused by hypercorticosteremia, etc (7). The non-functional tumors do not cause hypersecretory symptoms and signs but have more obvious space-occupying effects, which can show several manifestations including headache, visual field defect, visual acuity, hypophysis, etc (8, 9). However, few studies focused on the rare malignant tumor at the pituitary gland, mainly including adenocarcinoma, invasive adenoma, germinoma, and chordoma. We aimed in this retrospective cohort study to provide an overview of the incidence, demographics, treatments, and long-term survival of PMPT patients. Moreover, causes of death were also first analyzed among these patients.
The incidence of pituitary tumors varied in different reports (1, 10, 11). The average prevalence rate of pituitary adenomas in autopsy data was estimated to be about 10.7% (11). PMPT occurred more rarely. According to the SEER data, the AAR of PCL between 1975 and 2017 was 0.233 per 1,000,000 population. The rate increasingly changed over age among both pediatric and adult populations. These results were consistent with the previous report of the Central Brain Tumor Registry of the United States (CBTRUS) between 2004 and 2009 (12), which demonstrated that malignant pituitary tumors occurred most commonly among 65–74 years old and were most rare among those 15–24 years old (Figure 2). However, the incidence rate of malignant pituitary tumors seemed to become lower over time from 1975 to 2017 without any join point, the APC was −1.04% per year (Figure 1). This study first described the changing trend of incidence in patients with a malignant pituitary tumor in the United States, but we could not estimate the rates of pituitary adenoma for comparisons due to the limitations of the SEER database.
Our analysis showed that age (adult vs. pediatric, HR = 3.18, 95%CI: 1.49–6.82, P = 0.003; Table 2) was also an independent prognostic factor for pituitary tumors. The older age (HR = 1.046, 95%CI: 1.038–1.053, P < 0.001, Table 1) and male gender (female vs. male, HR = 0.56, 95%CI: 0.41–0.74, P < 0.001; Table 2) were significantly associated with poorer prognosis. Additionally, among all patients, histological types seemed not to affect overall survival outcomes. However, marital status was surprisingly identified as a significant prognostic factor. Compared with married patients, the single status cases had a better prognosis (HR = 0.62, 95%CI: 0.41–0.93, P = 0.022), but the widowed (HR = 2.47, 95%CI: 1.62–3.75, P < 0.001) and the divorced/separated (HR = 1.72, 95%CI: 1.05–2.84, P = 0.033) indicated a higher risk of poor prognosis, which was not reported before. The results may be attributed to the confounding effect of different ages, for most patients with single status (never married) were at a younger age. The previous research reported an increase in mortality among widowed and never-married cancer patients (13), partly consistent with our study. These results may present the critical effects of marital status in the management and survival outcomes for cancer patients.
Patients with pituitary tumors showed great survival outcomes. In our study, a total of 501 patients were identified with an MST of 205 months (Table 3). The pediatric (MST = 420 months) demonstrated significant overall survival in adults (MST = 145 months; P < 0.001; Figure 3). Pituitary tumors are commonly and easily treated by surgery or other medical treatments in most cases. Radiation therapy is usually considered when initial treatment fails or disease recurs. It was previously reported that pituitary surgery was the most effective treatment for most endocrinal pituitary adenomas, non-functioning pituitary adenomas causing mass effect, and pituitary cancer (14). In this cohort that we studied, the most commonly used therapy for them was surgery plus radiotherapy (MST: 297 months; Table 3) and only surgery (MST: 143 months). However, surgery may not be an effective treatment for our cohort (Figure 4; before matching: P = 0.35, after matching: P = 0.25), which only included pituitary tumors with malignant behavior. Furthermore, radiotherapy and chemotherapy may also not significantly affect overall survival after matching among malignant pituitary tumors (Figure 4). Considering the limited number of patients, future studies are required to focus on systematical therapy for malignant pituitary tumors.
The present study also first reported the death causes among malignant primary tumors (Figure 5). Among the 212 deaths, most were adults (202/212, 95.3%). Among adults, the most common causes were from other endocrine organs (N = 40, 19.8%), followed by unknown neoplasm (N = 25, 12.4%), heart diseases (N = 22, 12.9%), and brain and other nervous systems (N = 17, 8.4%), which indicated that few patients died of pituitary tumors.
Several limitations of this study should be considered. First, the retrospective cohort was retrieved from the SEER database, and some natural data bias cannot be avoided totally. Second, the database did not include some important characteristics such as imaging features, detailed information on chemotherapy and radiotherapy, etc. Third, the SEER database only included tumors with malignant behavior, while most pituitary adenomas were actually benign. Thus, we could not compare characteristics and survival between malignant and benign pituitary tumors.
In conclusion, the AAR for PMPT from 1975 to 2017 was 0.233 (95%CI: 0.205–0.264) per million population. The Join-point analysis indicated an insignificantly decrease in incidence rates over years. Older age, tumor extent, and marital status were independent prognostic factors for PMPT. The PSM analyses demonstrated no significant survival benefits from surgery, radiotherapy, and chemotherapy among PMPT patients. More future studies should be carried on to focus on therapy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital. The patients/participants provided their written informed consent to participate in this study.
XS, LH, CZ, and AZ contributed to the conception and design of the study; XS and XW contributed to the provision of study materials or patients; LH contributed to the collection and assembly of data; XS and XW contributed to the data analysis and interpretation; All authors contributed to the manuscript writing; All authors contributed to the final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Post-Doctor Research Project, West China Hospital, Sichuan University (No. 2020HXBH101), “from zero to one” Innovation Research Project of Sichuan University (No. 2022SCUH0025), Chengdu Science and Technology Program (No. 2022-YF05-01443-SN), China Postdoctoral Science Foundation (No. 2021M692310), and National Natural Science Foundation of China (No. 82204490).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The prevalence of pituitary adenomas: a systematic review. Cancer. (2004) 101(3):613–9. doi: 10.1002/cncr.20412
2. Freda PU, Beckers AM, Katznelson L, Molitch ME, Montori VM, Post KD, Vance ML. Pituitary incidentaloma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2011) 96(4):894–904. doi: 10.1210/jc.2010-1048
3. Sivakumar W, Chamoun R, Nguyen V, Couldwell WT. Incidental pituitary adenomas. Neurosurg Focus. (2011) 31(6):E18. doi: 10.3171/2011.9.Focus11217
4. Villwock JA, Villwock M, Deshaies E, Goyal P. Significant increases of pituitary tumors and resections from 1993 to 2011. Int Forum Allergy Rhinol. (2014) 4(9):767–70. doi: 10.1002/alr.21356
5. Fernández-Balsells MM, Murad MH, Barwise A, Gallegos-Orozco JF, Paul A, Lane MA, Lampropulos JF, Natividad I, Perestelo-Pérez L, Ponce de León-Lovatón PG, Erwin PJ, Carey J, Montori VM. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab. (2011) 96(4):905–12. doi: 10.1210/jc.2010-1054
6. Karamouzis I, Caputo M, Mele C, Nuzzo A, Zavattaro M, Car P, Panzarasa G, Prodam F, Marzullo P, Aimaretti G. Transsphenoidal surgery for pituitary adenomas: early results from a single center. Hormones. (2018) 17(4):551–6. doi: 10.1007/s42000-018-0082-9
7. Trouillas J, Jaffrain-Rea ML, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers (Basel). (2020) 12(2):514. doi: 10.3390/cancers12020514
8. Losa M, Donofrio CA, Barzaghi R, Mortini P. Presentation and surgical results of incidentally discovered nonfunctioning pituitary adenomas: evidence for a better outcome independently of other patients’ characteristics. Eur J Endocrinol. (2013) 169(6):735–42. doi: 10.1530/eje-13-0515
9. Robenshtok E, Benbassat CA, Hirsch D, Tzvetov G, Cohen ZR, Iraqi HM, Gorshtein A, Toledano Y, Shimon I. Clinical course and outcome of nonfunctioning pituitary adenomas in the elderly compared with younger age groups. Endocr Pract. (2014) 20(2):159–64. doi: 10.4158/ep13182.Or
10. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncology. (2012) 14:1. doi: 10.1093/neuonc/nos218
11. Molitch ME. Pituitary tumours: pituitary incidentalomas. Best Pract Res Clin Endocrinol Metab. (2009) 23(5):667–75. doi: 10.1016/j.beem.2009.05.001
12. Gittleman H, Ostrom QT, Farah PD, Ondracek A, Chen Y, Wolinsky Y, Kruchko C, Singer J, Kshettry VR, Laws ER, Sloan AE, Selman WR, Barnholtz-Sloan JS. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009. J Neurosurg. (2014) 121(3):527–35. doi: 10.3171/2014.5.Jns131819
13. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. (2011) 11:804. doi: 10.1186/1471-2458-11-804
14. Casanueva FF, Barkan AL, Buchfelder M, Klibanski A, Laws ER, Loeffler JS, Melmed S, Mortini P, Wass J, Giustina A, Pituitary Society, Expert Group on Pituitary Tumors. Criteria for the definition of pituitary tumor centers of excellence (PTCOE): a pituitary society statement. Pituitary. (2017) 20(5):489–98. doi: 10.1007/s11102-017-0838-2
Keywords: primary malignant pituitary tumor, incidence, death, treatment, survival
Citation: Sun X, Huo L, Wang X, Zhang C and Zhao A (2022) Comprehensive clinical analysis of patients with primary malignant tumor of pituitary gland: A population-based study. Front. Surg. 9:933168. doi: 10.3389/fsurg.2022.933168
Received: 30 April 2022; Accepted: 21 September 2022;
Published: 18 October 2022.
Edited by:
Shanqing Li, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Zhisong Liu, Tianjin University of Finance and Economics, China© 2022 Sun, Huo, Wang, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ailin Zhao aXJlbmV6MjBAb3V0bG9vay5jb20= Chunlan Zhang bWVyZXpjbEAxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.