- 1Department of Orthopedics, Lishui City People's Hospital, The Sixth Affiliated Hospital of Wenzhou Medical University, The First Affiliated Hospital of Lishui University, Lishui, China
- 2Department of Orthopedics, Qingtian People’s Hospital, Qingtian, China
- 3Department of Orthopedics, People’s Hospital of Jing Ning She Autonomous County, Jingning, China
- 4Department of Orthopedic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Purpose: With the gradual increase in the incidence of upper-tract urothelial carcinoma (UTUC), its metastatic disease has attracted much attention. The prognosis of UTUC patients with bone metastasis is worse than that of UTUC patients with other metastases. Therefore, the current study is performed to analyze the clinicopathologic features and survival predictors among UTUC patients with bone metastasis.
Patients and methods: We reviewed the Surveillance, Epidemiology, and End Results (SEER) database to select cases diagnosed with UTUC and bone metastasis at present from 2010 to 2016. Overall survival (OS) and cancer-specific survival (CSS) were first performed by applying univariate Cox regression analysis. Then we performed multivariate Cox analysis to determine independent predictors of survival. Forest plots were drawn by GraphPad 8.0.1 and used to visually display the results of multivariate analysis. Kaplan-Meier method was applied to intuitively show the prognosis difference of each independent risk factor.
Results: We finally identified 380 UTUC patients with bone metastasis for survival analysis, of which 230 males (60.5%) and 150 females (39.5%). The mean and median age at diagnosis were 71 and 72 years, respectively. Simultaneous lung metastasis (33.4%) and liver metastasis (31.1%) were more common in UTUC patients with bone metastasis. The 1-year OS and CSS rates of this research population were 23.8% and 26.6%, respectively. Multivariate Cox proportional hazards modeling controlling for surgery, chemotherapy, brain metastasis, liver metastasis, lung metastasis, and marital status, revealed that surgery, chemotherapy, no liver metastasis, no lung metastasis, and married status predicted for better OS and CSS.
Conclusion: Surgery and chemotherapy are optimal management of UTUC patients with bone metastasis. Active treatments on lung and liver metastases should be performed. The prognosis of patients with unmarried status or others should be further improved.
Introduction
Upper-tract urothelial carcinoma (UTUC) is a relatively uncommon disease of the urinary system, accounting for 5%–10% of all urothelial carcinomas (1). UTUC includes carcinoma of the renal pelvis and ureter (2, 3). Metastasis was observed in about 7% of UTUC patients and 3-year overall survival (OS) rates for metastatic UTUC were less than 10% (4). Compared to other metastatic sites, bone metastasis had less favorable prognosis (5). With the gradual increase in the incidence of UTUC (1) and its invasiveness at diagnosis (6), more and more studies have been conducted to analyze its prognosis (1, 7, 8). Giving the rarity of UTUC with bone metastasis, clinicopathologic features, epidemiological factors, and survival data are absent.
Mainstream treatments of UTUC include surgical resection and chemotherapy (9). Although there have been many studies on the treatment of UTUC, there have been few studies on metastatic UTUC, especially UTUC with bone metastasis. Whether surgical management and chemotherapy can prolong the survival of UTUC with bone metastasis remains unknown. Radiotherapy is usually used as one of the palliative treatments for patients with advanced tumors. Whether radiotherapy is also suitable for UTUC with bone metastasis remains to be further explored.
To obtain insight into UTUC with bone metastasis, we used data from the Surveillance, Epidemiology, and End Results (SEER) database to explore the clinicopathologic features and risk factors of survival. To our knowledge, this is the largest population study to date to analyze UTUC with bone metastasis, which will provide evidence for clinical practice.
Materials and methods
Patient selection
Clinical data from the SEER database on UTUC with bone metastasis were obtained by using the case-listing session on the SEER*Stat version 8.3.9 software. We selected the primary tumor sites of UTUC by using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) codes “C65.9-Renal pelvis” and “C66.9-Ureter.” Meanwhile, we set the SEER Combined Mets at DX-bone (2010+) to YES and finally identified the UTUC patients with bone metastasis. Patients with non-pathological diagnosis or death certificate diagnosis were excluded. In the current study, all UTUC patients had bone metastasis at initial diagnosis and were in M1 stage. After diagnosis, they received their treatments.
Information collected and also analyzed in from the SEER database includes race, gender, age at diagnosis, primary tumor site, pathological type, tumor size, treatment methods, visceral metastasis, marital status, vital status, survival time, and cause of death. Surgery or radiotherapy in the present study refers to treatment for primary tumor sites. According to previous studies (10, 11), OS and cancer-specific survival (CSS) were defined as the time from diagnosis till death due to any cause and due to the primary tumor, respectively.
Statistical analysis
All statistical and descriptive analyses were performed by using the SPSS 23.0 software. Univariate Cox regression analyses were performed by analyzing race, gender, age at diagnosis, primary tumor site, pathological type, tumor size, treatment methods, visceral metastasis, marital status. Important variables from univariate analysis were incorporated for multivariate Cox regression analysis. Meanwhile, hazard ratio (HR) and their 95% confidence interval (95% CIs) were presented in univariate and multivariate analysis. Forest plots were drawn by GraphPad 8.0.1 and used to visually display the results of multivariate analysis. Kaplan-Meier method was applied to intuitively show the prognosis difference of each independent risk factor. Two-sided p value less than 0.05 was considered of significance.
Results
Demographic and clinical characteristics
Figure 1 showed the flow chart for selection of study population. In total, 380 cases who met the eligibility criteria were included in this study (Table 1), of which 230 males (60.5%) and 150 females (39.5%). More than four out of five (85.5%) of patients were white race. The mean and median age at diagnosis were 71 and 72 years, respectively. In terms of primary tumor site, 71.6% tumors were located in the renal pelvis, and 28.4% in the ureter. The majority of the patients (87.4%) were diagnosed as transitional cell papillomas and carcinomas. Other tumor types including (1) epithelial neoplasms, NOS, (2) squamous cell neoplasms, (3) adenomas and adenocarcinomas, (4) cystic, mucinous and serous neoplasms, (5) complex epithelial neoplasms, (6) complex mixed and stromal neoplasms, accounted for 12.6%. Tumor size <5 accounted for 24.5% and tumor size ≥5 accounted for 33.2%. Overall, only 29.7% of the patients received surgery, 37.4% received radiotherapy, and over half of patients (52.1%) had chemotherapy. There were 8(2.1%) patients with brain metastasis, 118 (31.1%) patients with liver metastasis, and 127 (133.4%) patients with lung metastasis. The 1-year OS and CSS rates of this research population were 23.8% and 26.6%, respectively. The median follow-up time for survivors was 2 years.
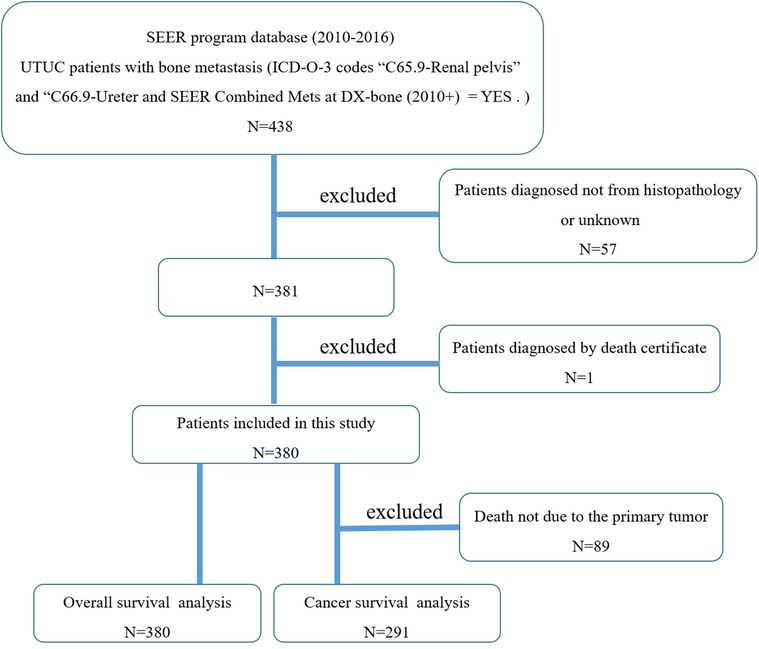
Figure 1. The flow chart for selection of study population. (SEER, Surveillance, Epidemiology, and End Results; ICD-O-3, international classification of diseases for oncology, 3rd edition; UTUC, upper-tract urothelial carcinoma).
Univariate cox regression analysis
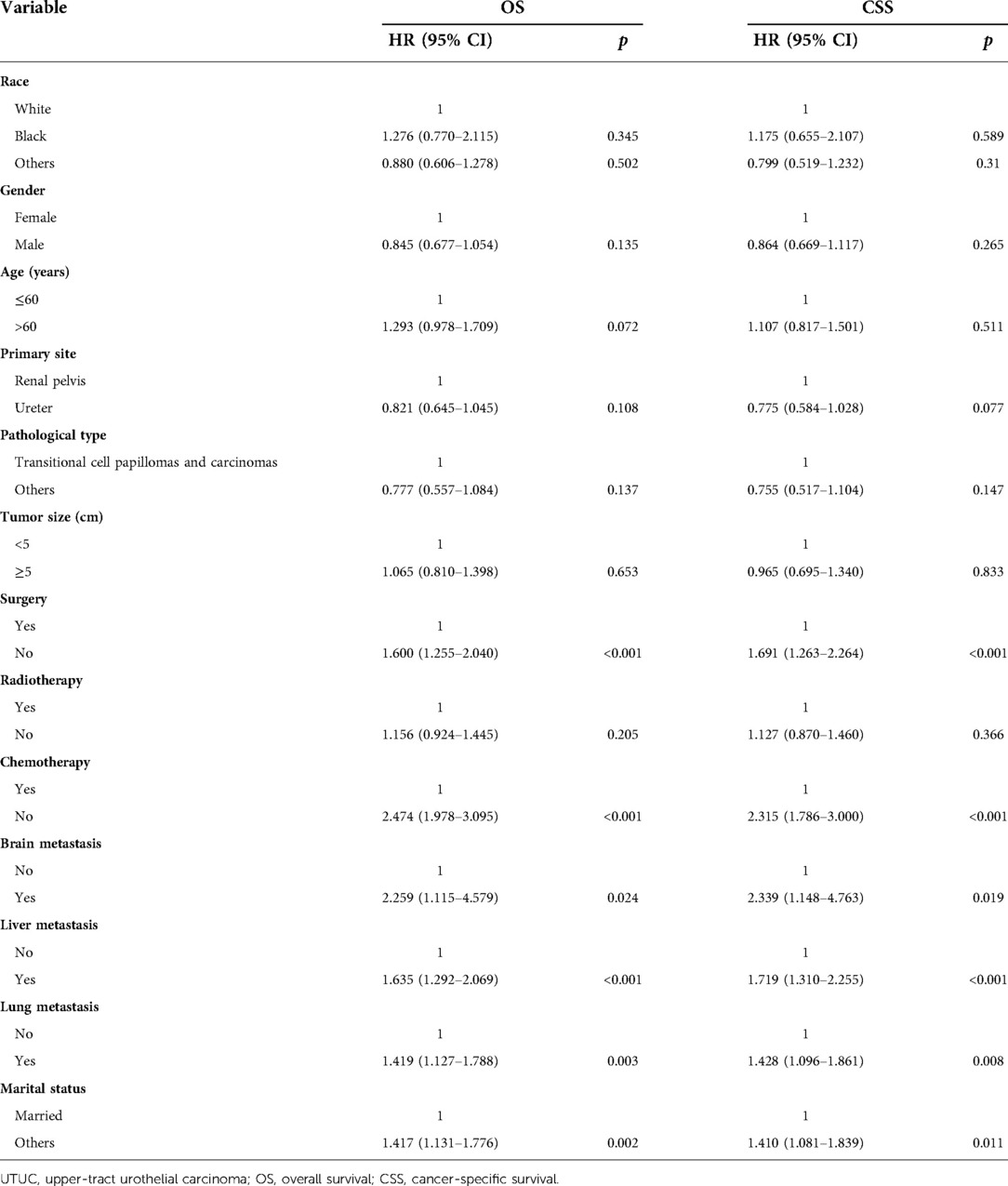
Statistical results of univariate analysis of UTUC with bone metastasis were presented in Table 2. No significance on OS and CSS were observed in terms of race, gender, age, primary site, pathological type, tumor size, and radiotherapy. Patients receiving surgery and chemotherapy experienced the better OS and CSS. Kaplan-Meier plots of surgery and chemotherapy were shown in Figure 2. Patients with brain or liver or lung metastasis were significantly correlated with worse OS and CSS. Figure 3 showed the Kaplan-Meier plots of brain, liver and lung metastasis. Moreover, married patients had a significant prolonged prognosis (Figure 4).
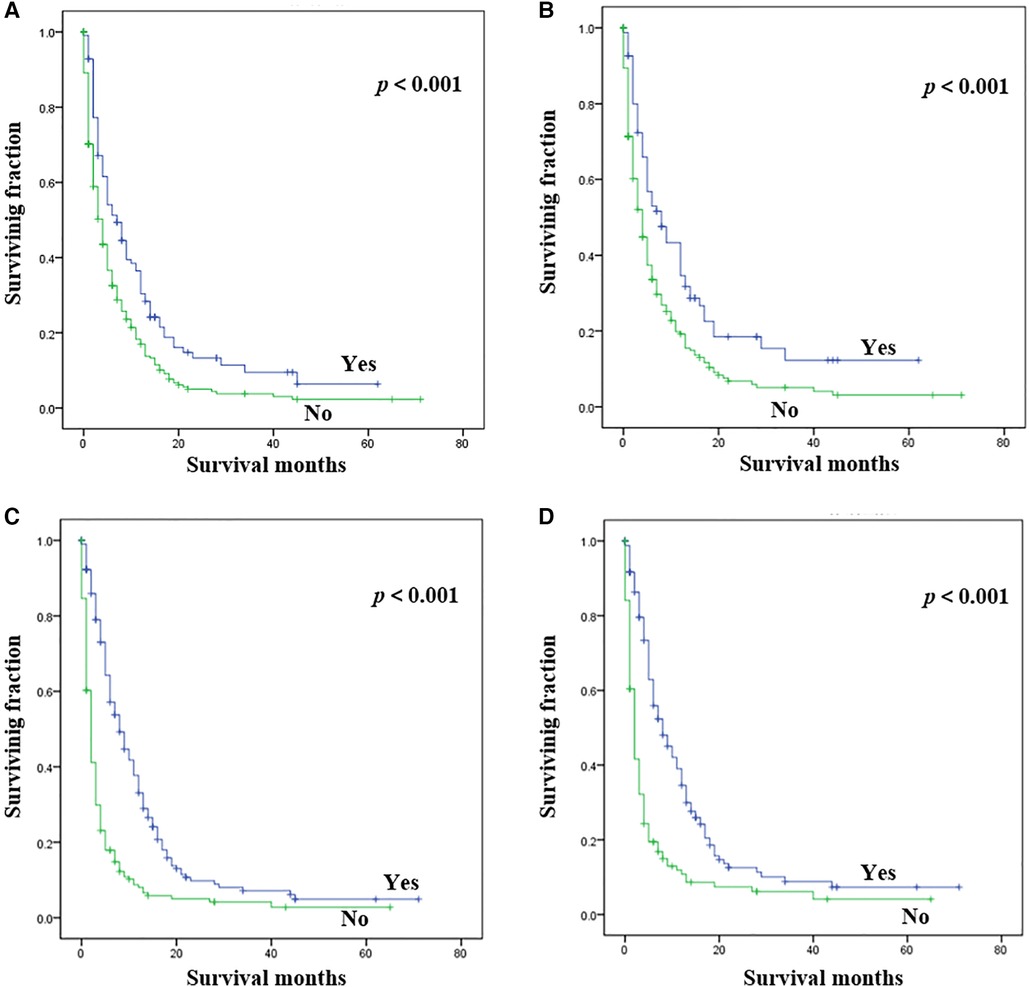
Figure 2. Kaplan-Meier method estimated OS and CSS in UTUC patients with bone metastasis stratified by treatment methods. (A) OS stratified by surgery; (B) CSS stratified by surgery; (C) OS stratified by chemotherapy; (D) CSS stratified by chemotherapy. (UTUC, upper-tract urothelial carcinoma; OS, overall survival; CSS: cancer-specific survival).
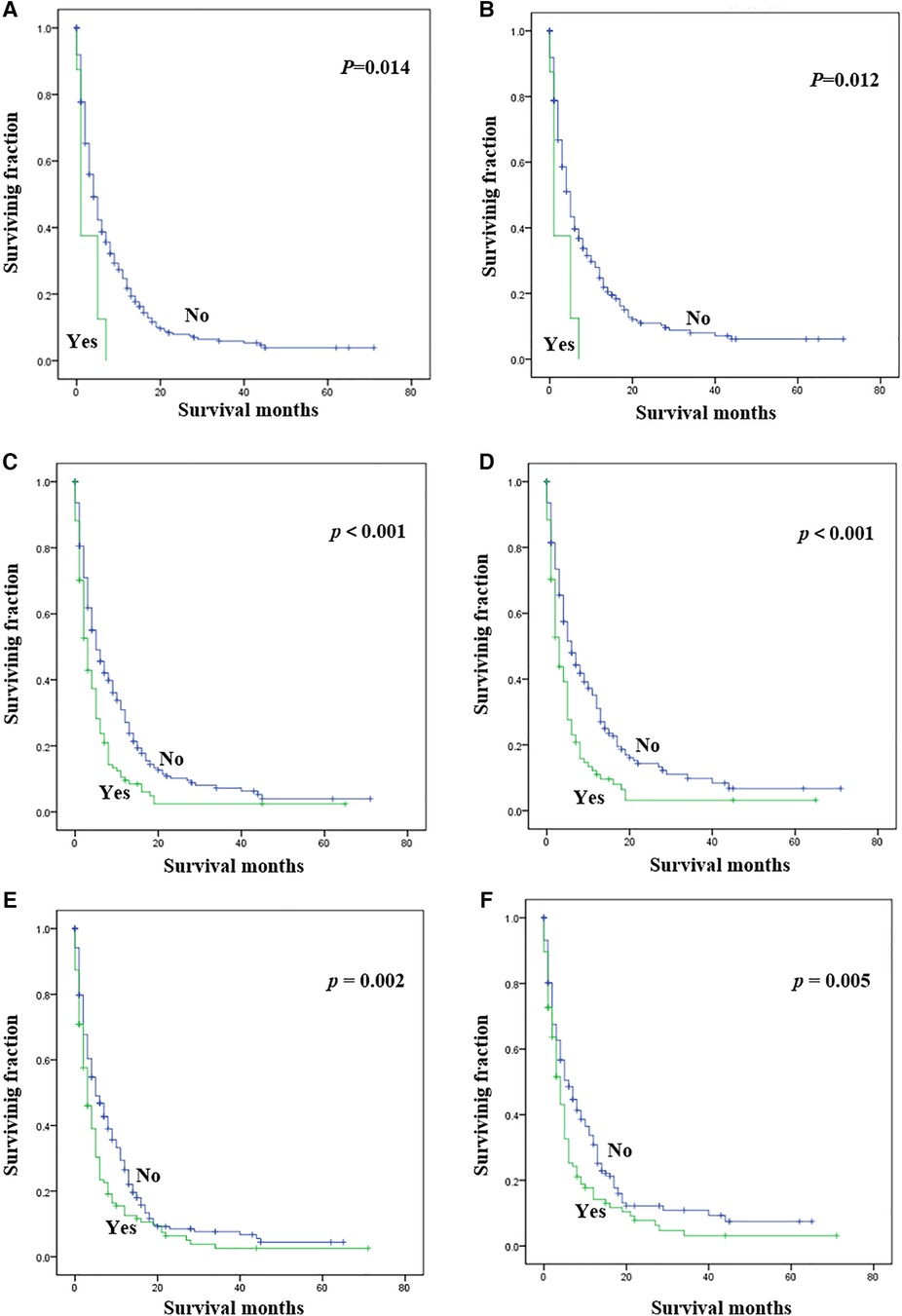
Figure 3. Kaplan-Meier method estimated OS and CSS in UTUC patients with bone metastasis stratified by visceral metastasis. (A) OS stratified by brain metastasis; (B) CSS stratified by brain metastasis; (C) OS stratified by liver metastasis; (D) CSS stratified by liver metastasis; (E) OS stratified by lung metastasis; (F) CSS stratified by lung metastasis. (UTUC, upper-tract urothelial carcinoma; OS, overall survival; CSS, cancer-specific survival).
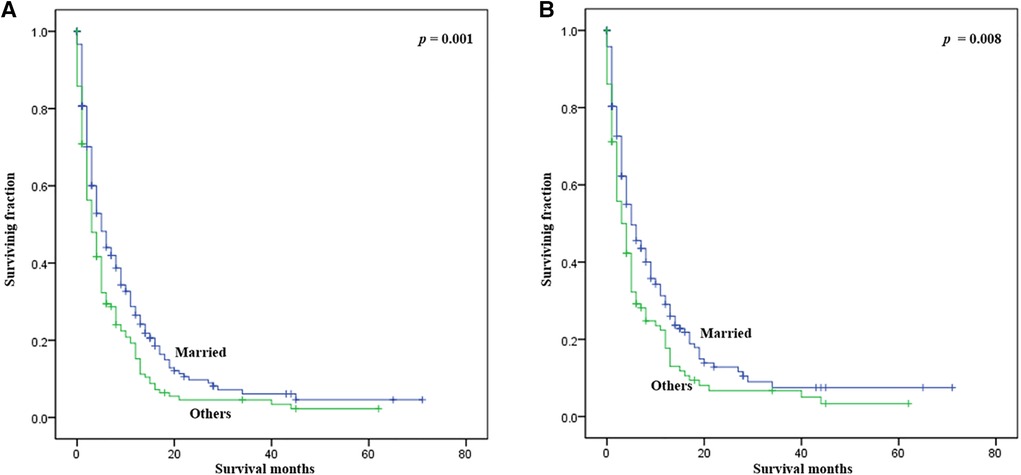
Figure 4. Kaplan-Meier method estimated OS and CSS in UTUC patients with bone metastasis stratified by marital status. (A) OS stratified by marital status; (B) CSS stratified by marital status. (UTUC, upper-tract urothelial carcinoma; OS, overall survival; CSS: cancer-specific survival).
Multivariate cox regression analysis
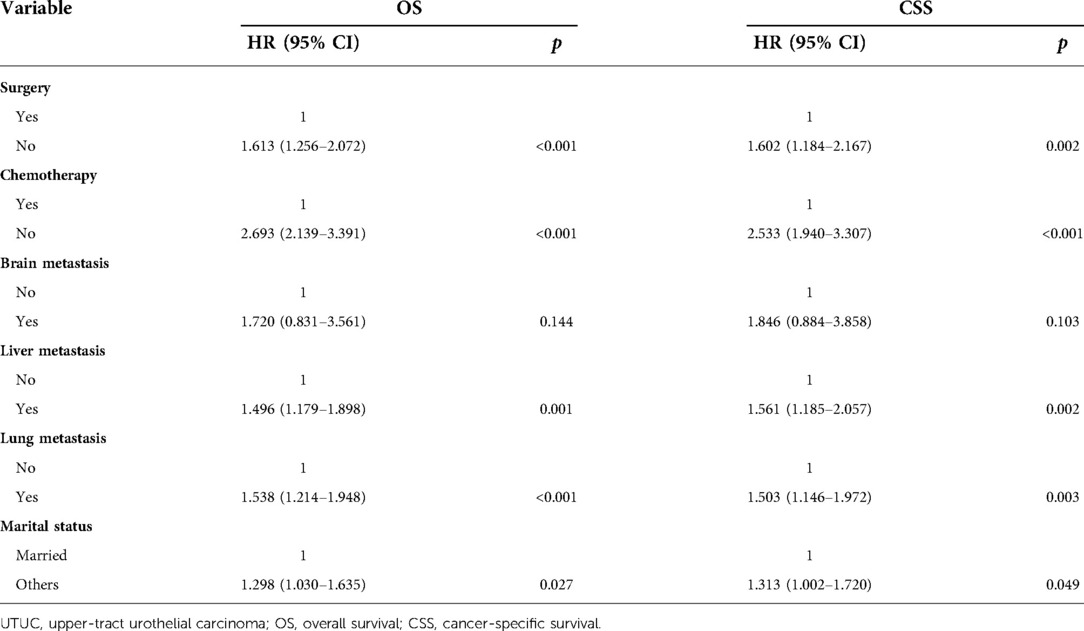
Table 3 and Figure 5 presented statistical results of multivariate analysis of UTUC with bone metastasis. Age and primary tumor site were identified as independent risk factors of OS and CSS. Surgery and chemotherapy were the beneficial factors for OS and CSS. Liver and lung metastasis were significantly correlated with worse rates of OS and CSS. Brain metastasis and marital status did not confer a disadvantage to the survival for this population.
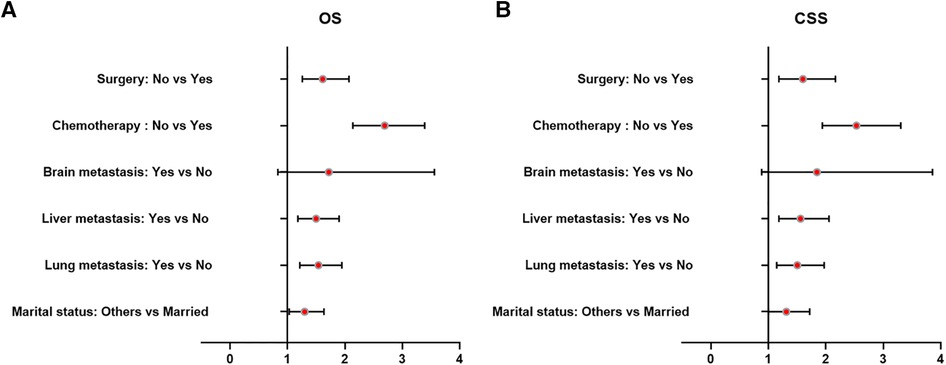
Figure 5. Forest plots of the predictors of OS (A) and CSS (B) in UTUC patients with bone metastasis. (UTUC, upper-tract urothelial carcinoma; OS, overall survival; CSS, cancer-specific survival).
Discussion
UTUC with bone metastasis is a relatively rare urothelial carcinoma, and little attention has been given to it (12). However, its incidence has been increasing in the past 30 years (13). Thus, it is necessary to investigate the independent survival predictors affecting UTUC with bone metastasis. To our knowledge, this is the largest population-based study to describe the demographic and clinical characteristics, and analyze the prognosis for UTUC patients with bone metastasis. The 1-year OS and CSS rates of 380 UTUC patients with bone metastasis were 23.8% and 26.6%, respectively, indicating a poor prognosis. Furthermore, our findings showed that surgery, chemotherapy, liver metastasis, lung metastasis, and marital status were significant independent predictors of survival, which provides a good assistance for clinicians and patients in treatment decisions.
Meaningful difference was not observed in terms of race, which was consistent with previous researches on UTUC (14, 15). However, some researchers reported that race was an independent prognostic factor of UTUC (16, 17). Although the male to female ratio in this study cohort was close to the overall UTUC patients (18), it was not a risk factor affecting the prognosis, which was in line with results of Mori et al. (19). However, Huang et al. (20) found that gender was a significant prognostic factor for all UTUC patients and females had significantly improved survival. Li et al. (17) identified that female patients had worse survival. The mean age of the study population was similar to that of UTUC patients overall. Previous studies identified older age as a poor survival predictor for UTUC (14, 17, 21). However, our study found that age was not correlated with survival among UTUC with bone metastasis. Primary tumor site was not a survival predictor of UTUC with bone metastasis, which was consistent with overall UTUC (22, 23). Additionally, Alessandro Veccia et al. (24) showed that tumor location in UTUC seems to be associated with outcomes, especially in the case of advanced disease. Although some studies suggest that tumor size was related to the prognosis of UTUC (17, 21), our present study revealed no association between tumor size and survival among UTUC patients with bone metastasis. Additionally, pathological type was not correlated with survival.
Univariate and multivariate analysis showed that marital status significantly impacted survival in UTUC with bone metastasis. Many studies have shown that marital status is an important factor affecting the prognosis of cancer patients, and married patients generally have a better prognosis due to the economic and emotional support (25–27). Married patients were more likely to gain curative treatment, high-quality care, and support of their spouse (28, 29). Thus, it is imperative to provide more support to those divorced, single, separated, and widowed patients. Overall, UTUC patients with bone metastasis exhibited different demographic and clinical characteristics compared with overall UTUC patients.
Simultaneous lung metastasis (33.4%) and liver metastasis (31.1%) were more common in UTUC patients with bone metastasis. Synchronous metastases significantly decreased the survival of patients with bone metastasis (10). Similarly, our study revealed that liver and lung metastases were independent prognostic factors of both OS and CSS. Brain metastasis is generally considered to be a poor prognostic factor in patients with bone metastasis (30). However, our multivariate analysis did not identify brain metastasis as an independent prognostic factor. It is possible that the number of patients with brain metastasis in this cohort was relatively small (8, 2.1%).
A recent meta-analysis revealed that perioperative chemotherapy for UTUC was beneficial for prolonging survival (31). Additionally, there is growing evidence that neoadjuvant chemotherapy has beneficial effects on pathologic downstaging of patients with UTUC (32). Surgical excision and chemotherapy were also suitable for UTUC patients with bone metastasis, which was consistent with previous mainstream treatments for patients with primary tumors (17, 33, 34). In advanced UTUC, radical nephroureterectomy (RNU) still remains the standard of care (35). Alberto Martini et al. (36) reported that neoadjuvant chemotherapy may be an option in patients with UTUC and bone metastasis. Although radiotherapy had no influence on survival of UTUC patients with bone metastasis, it may offer local control and reduce pain (37). Huang et al. (38) found that radiotherapy provided no significant benefit in survival of UTUC patients. Li et al. (17) reported that radiotherapy actually reduced the UTUC patient's prognosis. Thus, surgery and chemotherapy are recognized as optimal treatments to improve the survival of UTUC with bone metastasis.
The SEER database is a very powerful cancer research tool, which provides advantages for the study of patients with rare tumors. Of course, this study has some shortcomings. First, this study was an observational study design. Second, type of surgical treatment, radiotherapy and chemotherapy procedure, and immunotherapy were not defined in this cancer database. Third, the SEER database does not include information of local recurrence or distant metastasis during follow-up, which may affect the survival. Additionally, the SEER database does not contain information on the performance status of the patients and given this, it is usually not well characterized the decision to perform a treatment rather than others. Future randomized trials should be performed to solve the above problems.
Conclusion
UTUC patients with bone metastasis had a poor prognosis, with 1-year OS and CSS rates 23.8% and 26.6%, respectively. Surgery and chemotherapy were beneficial for prolonging the survival of UTUC with bone metastasis. Liver and lung metastases were associated with worse prognosis. Additionally, patients with married status experienced better survival. Future randomized trials are needed to confirm these prognostic factors to better guide the management of such patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZW, and WL conceived and designed the study. MZ, JZ, and XC collected the data. MZ, and JZ performed the statistical analysis. MZ wrote the manuscript. JZ, XC, ZW, and WL revised it. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Zhejiang Provincial Natural Science Foundation (LQ22H160040), National Natural Science Foundation of China (82103499), and China Postdoctoral Science Foundation (2021M692792).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Soria F, Shariat SF, Lerner SP, Fritsche HM, Rink M, Kassouf W, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. (2017) 35(3):379–87. doi: 10.1007/s00345-016-1928-x
2. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. (2018) 73(1):111–22. doi: 10.1016/j.eururo.2017.07.036
3. Favaretto RL, Shariat SF, Chade DC, Godoy G, Adamy A, Kaag M, et al. The effect of tumor location on prognosis in patients treated with radical nephroureterectomy at memorial sloan-kettering cancer center. Eur Urol. (2010) 58(4):574–80. doi: 10.1016/j.eururo.2010.07.003
4. Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. (2011) 107(7):1059–64. doi: 10.1111/j.1464-410X.2010.09675.x
5. Cheaib JG, Claus LE, Patel HD, Kates MR, Matoso A, Hahn NM, et al. Site of metastatic recurrence impacts prognosis in patients with high-grade upper tract urothelial carcinoma. Urol Oncol. (2021) 39(1):74.e9–.e16. doi: 10.1016/j.urolonc.2020.09.029
6. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer. (2009) 115(6):1224–33. doi: 10.1002/cncr.24135
7. Davaro F, May A, McFerrin C, Raza SJ, Siddiqui S, Hamilton Z. Chemotherapy increases survival and downstaging of upper tract urothelial cancer. Can J Urol. (2019) 26(5):9938–44.31629443
8. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol. (2021) 79(1):62–79. doi: 10.1016/j.eururo.2020.05.042
9. Leow JJ, Liu Z, Tan TW, Lee YM, Yeo EK, Chong YL. Optimal management of upper tract urothelial carcinoma: current perspectives. Onco Targets Ther. (2020) 13:1–15. doi: 10.2147/OTT.S225301
10. Wang Z, Cheng Y, Chen S, Shao H, Chen X, Wang Z, et al. Novel prognostic nomograms for female patients with breast cancer and bone metastasis at presentation. Ann Transl Med. (2020) 8(5):197. doi: 10.21037/atm.2020.01.37
11. Wang Z, Chen G, Chen X, Huang X, Liu M, Pan W, et al. Predictors of the survival of patients with chondrosarcoma of bone and metastatic disease at diagnosis. J Cancer. (2019) 10(11):2457–63. doi: 10.7150/jca.30388
12. Zhang X, Wang P, Qi K, Qiao Q, Jiang Y. The role of surgery on primary site in metastatic upper urinary tract urothelial carcinoma and a nomogram for predicting the survival of patients with metastatic upper urinary tract urothelial carcinoma. Cancer Med. (2021) 10(22):8079–90. doi: 10.1002/cam4.4327
13. Leow JJ, Chong KT, Chang SL, Bellmunt J. Upper tract urothelial carcinoma: a different disease entity in terms of management. ESMO Open. (2016) 1(6):e000126. doi: 10.1136/esmoopen-2016-000126
14. Cohen A, Kuchta K, Park S. Neoadjuvant and adjuvant chemotherapy use in upper tract urothelial carcinoma. Urol Oncol. (2017) 35(6):322–7. doi: 10.1016/j.urolonc.2016.11.018
15. Wright JL, Hotaling J, Porter MP. Predictors of upper tract urothelial cell carcinoma after primary bladder cancer: a population based analysis. J Urol. (2009) 181(3):1035–9doi: 10.1016/j.juro.2008.10.168
16. Godfrey MS, Badalato GM, Hruby GW, Razmjoo M, McKiernan JM. Prognostic indicators for upper tract urothelial carcinoma after radical nephroureterectomy: the impact of lymphovascular invasion. BJU Int. (2012) 110(6):798–803. doi: 10.1111/j.1464-410X.2011.10893.x
17. Li C, Yang J, Xu F, Han D, Zheng S, Kaaya RE, et al. A prognostic nomogram for the cancer-specific survival of patients with upper-tract urothelial carcinoma based on the surveillance, epidemiology, and end results database. BMC Cancer. (2020) 20(1):534. doi: 10.1186/s12885-020-07019-5
18. Shariat SF, Favaretto RL, Gupta A, Fritsche HM, Matsumoto K, Kassouf W, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol. (2011) 29(4):481–6. doi: 10.1007/s00345-010-0594-7
19. Mori K, Mostafaei H, Enikeev DV, Lysenko I, Quhal F, Kimura S, et al. Differential effect of sex on outcomes after radical surgery for upper tract and bladder urothelial carcinoma: a systematic review and meta-analysis. J Urol. (2020) 204(1):58–62. doi: 10.1097/JU.0000000000000788
20. Huang CC, Su YL, Luo HL, Chen YT, Sio TT, Hsu HC, et al. Gender is a significant prognostic factor for upper tract urothelial carcinoma: a large hospital-based cancer registry study in an endemic area. Front Oncol. (2019) 9:157. doi: 10.3389/fonc.2019.00157
21. Emamekhoo H, Dhillon P, Gopalakrishnan D, Elson P, Stephenson A, Magi-Galluzzi C, et al. Prognostic factors and risk stratification in invasive upper tract urothelial carcinoma. Clin Genitourin Cancer. (2018) 16(4):e751–e60. doi: 10.1016/j.clgc.2018.01.014
22. Joshi SS, Quast LL, Chang SS, Patel SG. Effects of tumor size and location on survival in upper tract urothelial carcinoma after nephroureterectomy. Indian J Urol. (2018) 34(1):68–73. doi: 10.4103/iju.IJU_216_17
23. Zhang GL, Zhou W. A model for the prediction of survival in patients with upper tract urothelial carcinoma after surgery. Dose-Response. (2019) 17(4):1559325819882872. doi: 10.1177/1559325819882872
24. Veccia A, Antonelli A, Martini A, Falagario U, Carrieri G, Grob MB, et al. Ureteral location is associated with survival outcomes in upper tract urothelial carcinoma: a population-based analysis. Int J Urol. (2020) 27(11):966–72. doi: 10.1111/iju.14336
25. Yuan R, Zhang C, Li Q, Ji M, He N. The impact of marital status on stage at diagnosis and survival of female patients with breast and gynecologic cancers: a meta-analysis. Gynecol Oncol. (2021) 162(3):778–87. doi: 10.1016/j.ygyno.2021.06.008
26. Zhou C, Zhang Y, Hu X, Fang M, Xiao S. The effect of marital and insurance status on the survival of elderly patients with stage M1b colon cancer: a SEER-based study. BMC Cancer. (2021) 21(1):891. doi: 10.1186/s12885-021-08627-5
27. Ding Z, Yu D, Li H, Ding Y. Effects of marital status on overall and cancer-specific survival in laryngeal cancer patients: a population-based study. Sci Rep. (2021) 11(1):723. doi: 10.1038/s41598-020-80698-z
28. Denberg TD, Beaty BL, Kim FJ, Steiner JF. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer. (2005) 103(9):1819–25. doi: 10.1002/cncr.20982
29. Zhang SL, Sun HT, Li ZM, Zhang ZY, Wang WR, Wang X, et al. A real-world 1:1 propensity-matched study revealed unmarried status was independently associated with worse survival for patients with renal clear cell carcinoma. J Cancer. (2019) 10(16):3767–77. doi: 10.7150/jca.31744
30. Younis MH, Fuentes-Rivera L, Summers S, Pretell-Mazzini J. Survival in patients with carcinomas presenting with bone metastasis at diagnosis: a SEER population-based cohort study. Arch Orthop Trauma Surg. (2021) 141(3):367–73. doi: 10.1007/s00402-020-03417-3
31. Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol. (2021) 79(5):635–54. doi: 10.1016/j.eururo.2020.07.003
32. Kim DK, Cho KS. Neoadjuvant chemotherapy for upper tract urothelial carcinoma. Transl Cancer Res. (2020) 9(10):6576–82. doi: 10.21037/tcr.2020.03.08
33. Gregg RW, Vera-Badillo FE, Booth CM, Mahmud A, Brundage M, Leveridge MJ, et al. Perioperative chemotherapy for urothelial carcinoma of the upper urinary tract: a systematic review and meta-analysis. Crit Rev Oncol Hematol. (2018) 128:58–64. doi: 10.1016/j.critrevonc.2018.05.005
34. Cutress ML, Stewart GD, Zakikhani P, Phipps S, Thomas BG, Tolley DA. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int. (2012) 110(5):614–28. doi: 10.1111/j.1464-410X.2012.11068.x
35. Veccia A, Antonelli A, Checcucci E, Falagario U, Carrieri G, Guruli G, et al. Segmental ureterectomy for upper tract urothelial carcinoma: a systematic review and meta-analysis of comparative studies. Clin Genitourin Cancer. (2020) 18(1):e10–20. doi: 10.1016/j.clgc.2019.10.015
36. Martini A, Falagario UG, Waingankar N, Daza J, Treacy PJ, Necchi A, et al. Neoadjuvant versus adjuvant chemotherapy for upper tract urothelial carcinoma. Urol Oncol. (2020) 38(8):684.e9–.e15. doi: 10.1016/j.urolonc.2020.03.008
37. Sprave T, Verma V, Förster R, Schlampp I, Hees K, Bruckner T, et al. Bone density and pain response following intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for vertebral metastases - secondary results of a randomized trial. Radiat Oncol. (2018) 13(1):212. doi: 10.1186/s13014-018-1161-4
Keywords: upper-tract urothelial carcinoma (UTUC), renal pelvis, ureter, bone metastasis, survival, risk factor
Citation: Zhou M, Zhang J, Chen X, Wang Z and Liang W (2022) Clinical features and prognostic indicators in upper-tract urothelial carcinoma with bone metastasis. Front. Surg. 9:928294. doi: 10.3389/fsurg.2022.928294
Received: 25 April 2022; Accepted: 25 July 2022;
Published: 30 August 2022.
Edited by:
Xun Sun, Tianjin Hospital, ChinaReviewed by:
Yucheng Wang, Taizhou Municipal Hospital, ChinaHeyong Yin, Capital Medical University, China
© 2022 Zhou, Zhang, Chen, Wang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhan Wang d2FuZ3poYW5oekB6anUuZWR1LmNu Wei Liang ZG9jbHc4ODg4OEAxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Mingping Zhou1,†
Mingping Zhou1,† Zhan Wang
Zhan Wang Wei Liang
Wei Liang