- 1Department of General Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, China
- 2Central Laboratory, The Second Hospital of Hebei Medical University, Shijiazhuang, China
Background: Undifferentiated pleomorphic sarcoma (UPS) is a malignant tumor that originates in the mesenchymal tissue and is common in the extremities and retroperitoneum. Primary UPS of the duodenal papilla is rare and a distinct clinical entity.
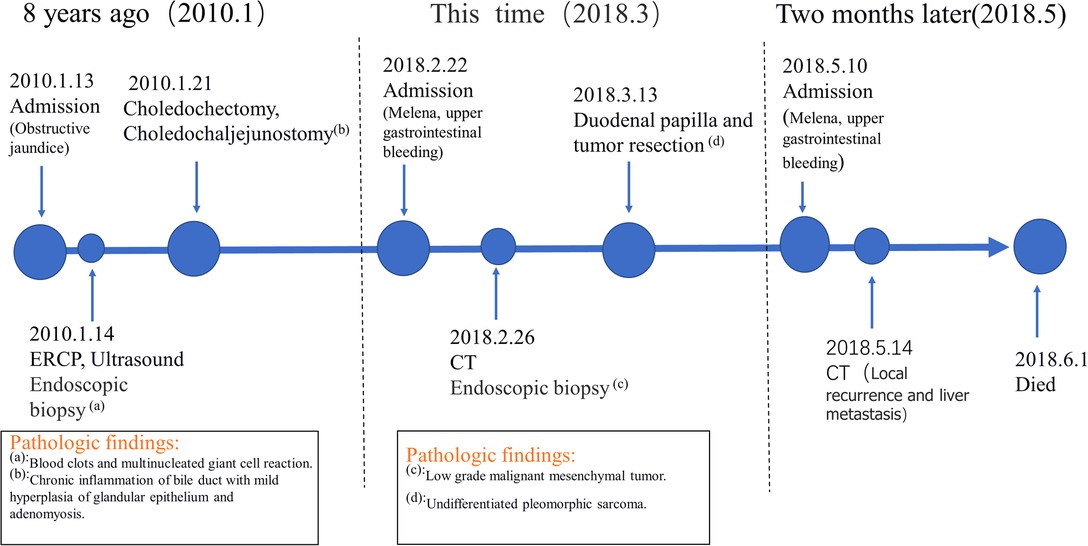
Case presentation: In this report, a 48-year-old Chinese man was admitted to our hospital with symptoms of melena. The patient underwent choledochectomy and choledochaljejunostomy for obstructive jaundice 8 years before admission. Endoscopic examination after admission confirmed a mass located at the duodenal papilla. Then, the duodenal papilla and tumor resection were performed, and the histopathology report confirmed the diagnosis of UPS. The patient refused further treatment and died 2 months later due to local recurrence and intrahepatic metastasis.
Conclusions: It is rare that the mass in the duodenal papilla is diagnosed as UPS. The unpredicted behavior of these tumors warrants a careful plan considering their indolent nature and possible recurrence and metastasis. The prognosis was poor despite the early complete resection.
Introduction
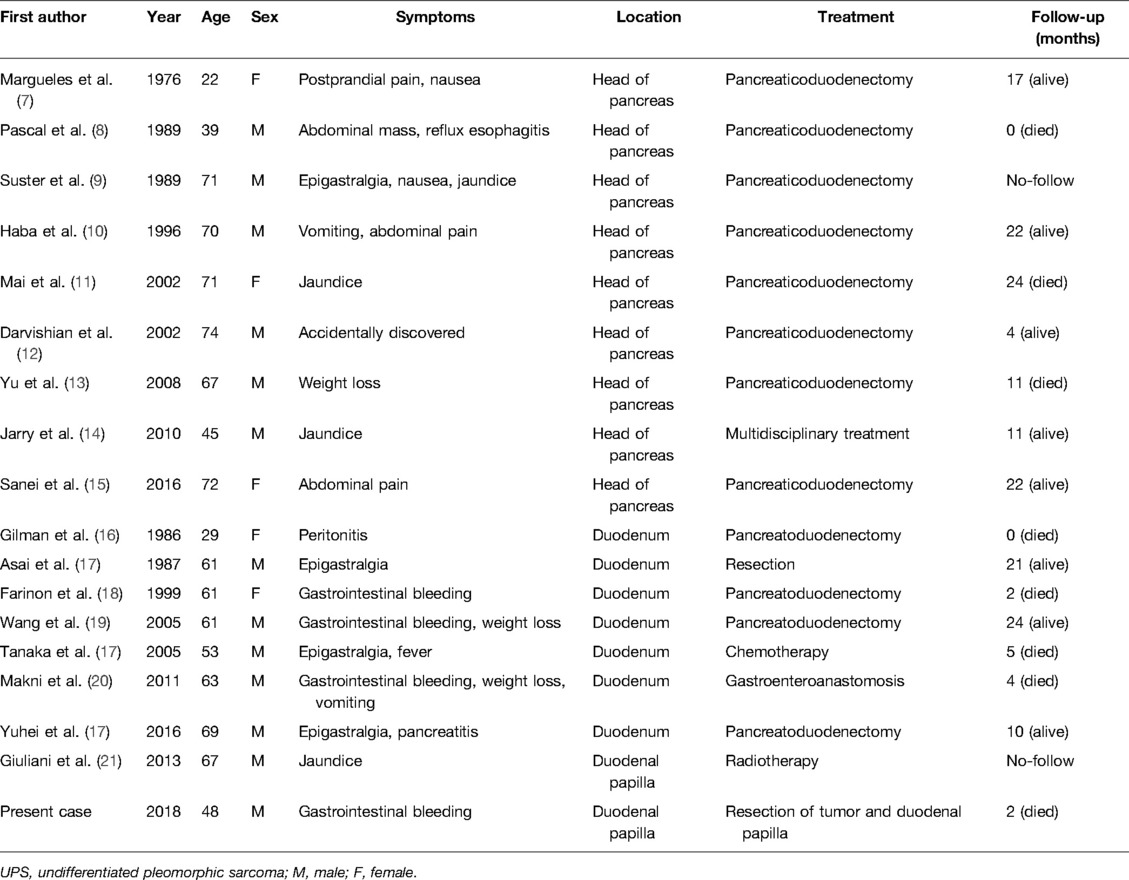
Undifferentiated pleomorphic sarcoma (UPS) is the most common soft tissue sarcomas in the elderly (1, 2). It is a neoplasm considered to originate from primitive mesenchymal cells, arising from soft tissue or bone, usually in the extremities or retroperitoneum (3). The Vater papilla region is uncommon and primary duodenal papilla UPS is exceedingly rare, with only one case confirmed in the literature to date (Table 1). At present, the epidemiology, diagnosis, and treatment of this disease remain unclear (4–6). The following case documents a primary lesion of the duodenum papilla, and its biological behavior characterizing a worth discussing history and an extremely poor prognosis.
Case Presentation
A 48-year-old Chinese man was admitted with melena (hemoglobin = 64 g/L). Contrast-enhanced CT scans showed that the mass was located in the descending duodenum, a cross section of approximately 1.8 cm × 2.0 cm. The values of plain and three-phase contrast-enhanced CT scans were about 35/90/90/73HU (Figure 1). Duodenoscopy confirmed that the mass originated from the duodenal papilla and projected into the lumen of the duodenum without invading the intestinal wall (Figure 2A). Endoscopic biopsy of the tumor showed a proliferation of polygonal cells, which were a dense arrangement and accompanied by visible mitosis and pleomorphic giant cells. Tumor cells were positive for CD68, Ki67 (50%), and Vimentin (Figures 2B–E), while negative for CD117, CD20, CD3, CD34, chromogranin A, CK7, Desmin, DOG1, HMB-45, melon-A, and Synaptophysin (data not provided). Then, the diagnosis considered a high-grade sarcoma, and a complete resection of the tumor was recommended for further examination.
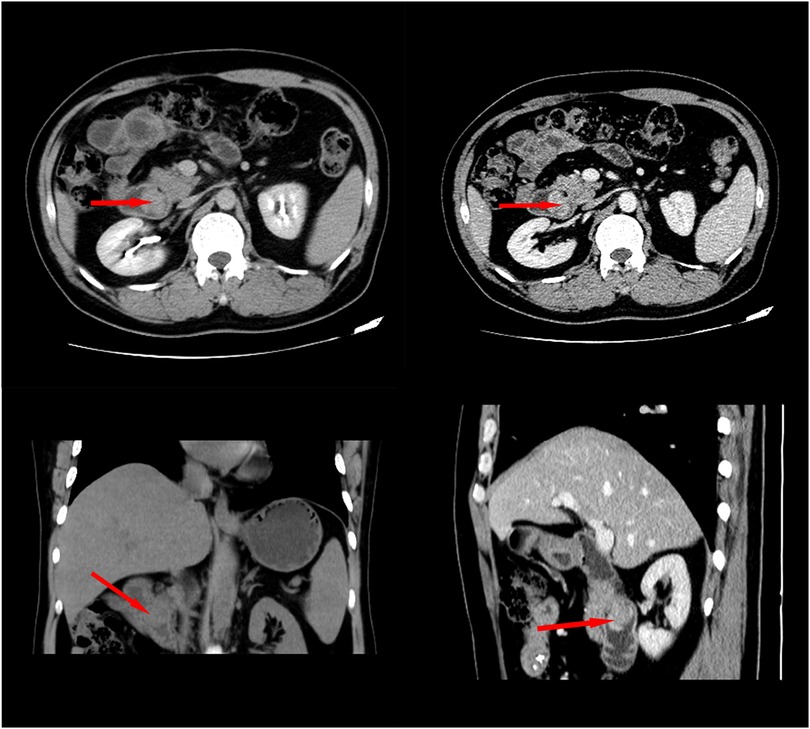
Figure 1. Contrast-enhanced CT showed that the tumor was located in the duodenal cavity (approximately 1.8 cm × 2.2 cm) without penetrating the duodenal wall.
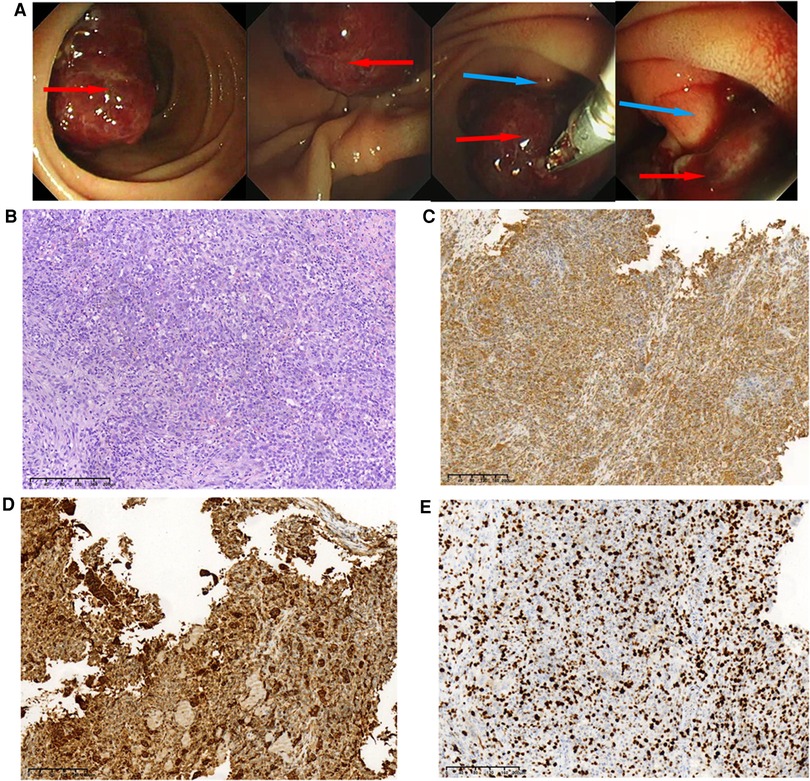
Figure 2. Duodenoscopic images of the tumor and pathological results of endoscopic biopsy during hospitalization. (A) Duodenoscopic images of the tumor; the red arrow indicates tumor tissue, and the blue arrow indicates duodenal papilla. (B)Histopathological examination of the biopsy (H&E staining) of the tumor, original magnification, ×100. These cells were positive for Vimentin, ×100 (C), CD68, ×100 (D), and Ki-67 was about 50% upon immunostaining, ×100 (E).
What is noteworthy is that the patient was admitted to our hospital 8 years ago with obstructive jaundice (bilirubin = 286 μmol/L, especially direct). Ultrasound and endoscopic retrograde cholangiopancreatography (ERCP) were performed at that time, both of which indicated mass in the distal common bile duct (Figures 3A, B). A biopsy specimen obtained from the mass revealed blood clots and multinucleated giant cell reaction (Figure 3C). Subsequently, the patient underwent cholecystectomy, choledochectomy, and Roux-en-Y hepaticojejunostomy. No mass was found in the bile duct during the operation, but stenosis was observed in the pancreatic segment of the common bile duct with a thickness of 0.3 cm. The distal bile duct was resected approximately 0.6 cm below the stenosis. Postoperative pathology showed chronic inflammation of the bile duct with mild hyperplasia of glandular epithelium and adenomyosis. The patient was asymptomatic for 8 years.
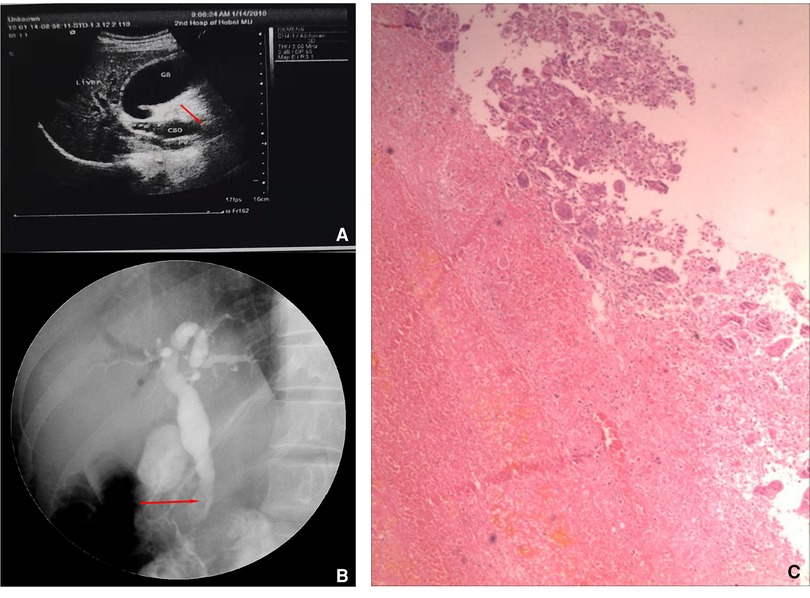
Figure 3. Ultrasound, endoscopy, and biopsy results 8 years ago. Preoperative ultrasound (A) and endoscopic retrograde cholangiopancreatography (ERCP) images showed a mass (red arrow) in the distal bile duct (B), and the histological manifestation of biopsy specimen (C).
Based on medical history and the biopsy results during the second admission, the final surgical method was determined as duodenal papilla and tumor resection, rather than pancreaticoduodenectomy. The histopathological findings revealed an UPS. Microscopically, multiple multinucleated osteoclast-like giant cells were evenly distributed on the mononucleated stromal cells. Giant cells contained numerous nuclei with eosinophilic cytoplasm. The pleomorphic stromal cells were spindle-shaped and atypical round, which were similar to the high-grade sarcoma. Nucleoli could be seen in several stromal cells. A small number of stromal cells showed a high degree of mitosis, which is a sign of malignancy. Immunohistochemical stains for CD68 and Vimentin were positive in the neoplastic cells, the proliferative marker Ki67 showing approximately 80% nuclear staining (Figure 4), and CD117, CD34, HMB45, CD45, CD56, S-100, smooth muscle actin (SMA), or desmin were negative (data not provided). The patient declined further chemotherapy and was discharged.
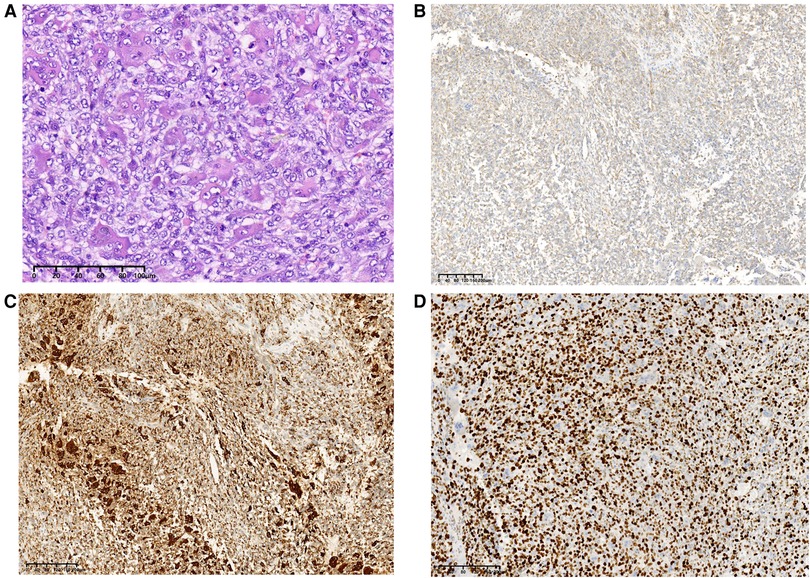
Figure 4. Final pathological results after surgery. Histopathological examination of the tumor (H&E staining), original magnification, ×200 (A). These cells were positive for Vimentin, ×100 (B), CD68, ×100 (C), and Ki-67 was about 80% upon immunostaining, ×100 (D).
Two months later, the patient was admitted to our hospital again with complaints of melena. CT scan showed a recurrent duodenal mass and multiple metastatic foci in the liver (Figure 5), and the patient died of metastasis 2 weeks later. The episode of care is organized as a timeline in Figure 6.
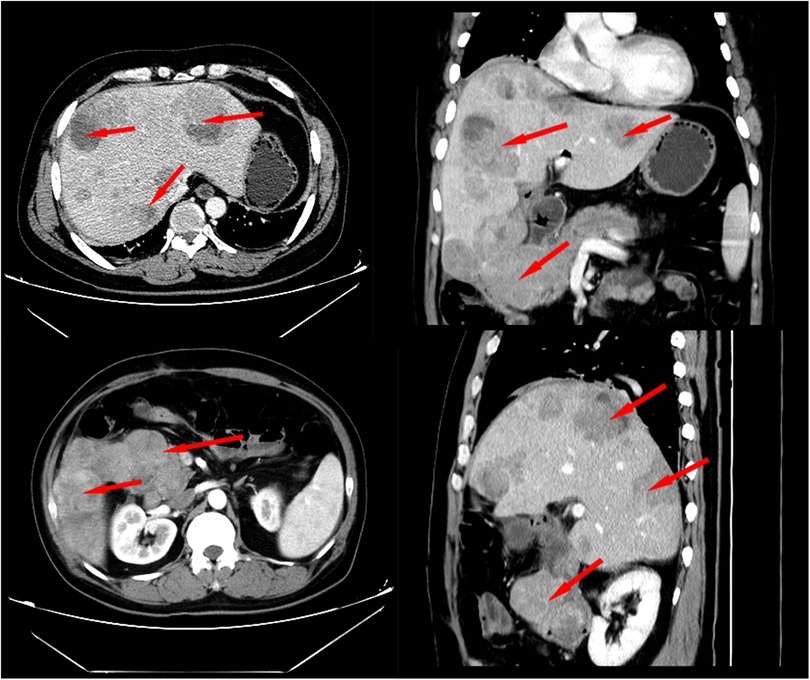
Figure 5. Contrast-enhanced CT scan 2 months after surgery revealed local recurrence and intrahepatic metastasis.
Discussion
UPS, or previously known malignant fibrous histiocytoma (MFH), is considered the most common type of soft tissue sarcoma. It often occurs in the limbs, trunk, and retroperitoneal tissues (3, 22), but it has been rarely observed in the digestive organ (15). The nomenclature and categorization of this neoplasm have changed several times over the years. In 2002, the World Health Organization (WHO) believed that MFH should be synonymous with UPS and divided into three subtypes, polymorphic MFH/UPS, giant cell MFH/with giant cell UPS, and inflammatory MFH/ with prominent inflammation UPS (23). In 2013, the WHO removed MFH and replaced it with UPS, which was classified into the newly established undifferentiated soft tissue sarcoma (USTS), a group of polymorphic heterogenous stromal tumors with no clear differentiation direction (24, 25).
UPS located at the same site often have similar clinical features. UPS in the head of the pancreas is characterized by epigastric pain, nausea, and vomiting. Some patients may present with weight loss and abdominal mass. Jaundice is also possible, depending on whether the tumor is compressing or invading the bile ducts. Primary UPS of the duodenum is often invasive and can penetrate the intestinal wall, leading to perforation or ulceration bleeding (20). Therefore, gastrointestinal bleeding and melena are the main symptoms of primary UPS in the duodenum. The symptoms caused by UPS arising in the Vater papilla region are similar to those caused by other tumors in this location and include melena, recurrent acute pancreatitis, jaundice, and abdominal pain. Similar to previous cases, melena was the main symptom accompanied by upper abdominal discomfort in this case. The difference is that this patient had undergone a Roux-en-Y hepaticojejunostomy, so jaundice was not present. The histopathology confirmed the diagnosis of UPS.
Patients with UPS in the duodenum often die months or years after diagnosis (18, 20). It is not clear whether the tumor has undergone a long growth process or grown rapidly from the beginning. The endoscopic biopsy result of 8 years ago showed blood clots and multinucleated giant cell reaction in this case, which could not be confirmed as UPS, giant cell tumor, or foreign body giant cell reflection now. A similar case has been reported in the central nervous system where a mass was found in the same area on a plain CT scan 5 years before the diagnosis of primary UPS in the brain (26). In addition, traumatic factors such as surgery are also one of the possible causes of UPS (27). The patient had undergone cholecystectomy and choledochectomy 8 years ago, and traumatic factors may have contributed to the occurrence of UPS in this rare site.
The diagnosis of UPS is important in its treatment process. UPS is defined as a group of sarcomas in which any attempt to disclose their line of differentiation has failed (28). Their immunophenotype is plastic and they may focally express CD34, SMA, and CD68. Immunohistochemistry can be used as a means to rule out melanoma, sarcomatoid carcinoma, and malignant lymphomas, which have respective tumor markers (29), but its role is still auxiliary. The diagnosis continues to presuppose thorough sampling and evaluation of hematoxylin–eosin-stained sections (29). Another differential diagnosis of this case is giant cell tumor of soft tissue because of the existence of multinucleated giant cells (30, 31). However, considering the patient's high Ki67 index (80%) and rapidly progressing history, our final pathological diagnosis was UPS rather than a giant cell tumor of soft tissue (30, 32).
The surgical procedure, in this case, is different from the previous cases. Treatment with UPS emphasizes early complete resection of the tumor to obtain an adequate margin and reduce tumor recurrence. There is no consensus on what is an adequate margin distance for minimizing the risk of local recurrence. When UPS occurs in the extremities, the resection margin should be at least 1–3 cm away from the gross border (33, 34). However, if UPS is located in the retroperitoneum, complete compartmental resection will produce significantly better results (34, 35). Due to the low risk of lymphatic metastasis, routine lymph node dissection is not recommended during surgery (36). In this case, the tumor protruded into the intestinal lumen without invading the intestinal wall, and the intraoperative frozen section results showed a negative resection margin. Therefore, only the tumor and duodenal papilla were removed in this operation, instead of pancreaticoduodenectomy.
Conclusion
Primary UPS of the duodenal papilla is a highly malignant sarcoma with a poor prognosis. There are no specific clinical manifestations, which may only be jaundice with upper abdominal discomfort. When the tumor is enlarged and necrotic, upper gastrointestinal bleeding such as melena may occur. This tumor represents a challenge in management due to the lack of data in the literature. Early radical surgery is the key to better efficacy. The prognosis was poor despite the early complete resection. Early endoscopy in elderly patients with upper abdominal discomfort may yield early detection of this rare tumor, which needs to be verified in more cases.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JW was responsible for the conception, design, and writing of the draft. BL, JH, and TL did the proof check. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Medical Science Research Project of Hebei Province (No. 20211760) and the National Natural Science Foundation of China (No. 81402510).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. (1978) 41:2250–66. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w
2. Liang Z, Han J, Tuo H, Xue D, Yu H, Peng Y. Undifferentiated pleomorphic sarcoma of the pancreas: a rare case report and literature review. World J Surg Oncol. (2022) 20:55. doi: 10.1186/s12957-022-02525-1
3. Winchester D, Lehman J, Tello T, Chimato N, Hocker T, Kim S, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. (2018) 79:853–9. doi: 10.1016/j.jaad.2018.05.022
4. Teng H, Xinghai Y, Wei H, Huang Q, Xiao J, Zhang C. Malignant fibrous histiocytoma of the spine: a series of 13 clinical case reports and review of 17 published cases. Spine (Phila Pa 1976). (2011) 36:E1453–62. doi: 10.1097/BRS.0b013e318203e292
5. Takahashi H, Fujiwara T, Noro A, Orii S, Sato K, Sato S, et al. A case of malignant fibrous histiocytoma of mesentery of the ileum. Nihon Shokakibyo Gakkai Zasshi. (2000) 97:186–90. doi: 10.3919/ringe1963.54.2130
6. Pathak S, Soni TP, Gupta AK, Sharma LM. Retrovesical malignant fibrous histiocytoma: a rare tumor. BMJ Case Rep. (2017) 2017. doi: 10.1136/bcr-2017-222612
7. Margules RM, Allen RE, Dunphy JE. Pancreatic tumor of mesenchymal origin presenting as obstructive jaundice. Am J Surg. (1976) 131:357–9. doi: 10.1016/0002-9610(76)90131-8
8. Pascal RR, Sullivan L, Hauser L, Ferzli G. Primary malignant fibrous histiocytoma of the pancreas. Hum Pathol. (1989) 20:1215–7. doi: 10.1016/s0046-8177(89)80015-2
9. Suster S, Phillips M, Robinson MJ. Malignant fibrous histiocytoma (giant cell type) of the pancreas. A distinctive variant of osteoclast-type giant cell tumor of the pancreas. Cancer. (1989) 64:2303–8. doi: 10.1002/1097-0142(19891201)64:11<2303::aid-cncr2820641120>3.0.co;2-s
10. Haba R, Kobayashi S, Hirakawa E, Miki H, Okino T, Kurokawa T, et al. Malignant fibrous histiocytoma of the pancreas. Pathol Int. (1996) 46:515–9. doi: 10.1111/j.1440-1827.1996.tb03647.x
11. Mai G, Baer HU, Mittler M, Uhl W, Büchler MW, Rodriguez RU, et al. Malignant fibrous histiocytoma of the pancreas. Pancreas. (2002) 25:320–4. doi: 10.1097/00006676-200210000-00018
12. Darvishian F, Sullivan J, Teichberg S, Basham K. Carcinosarcoma of the pancreas: a case report and review of the literature. Arch Pathol Lab Med. (2002) 126:1114–7. doi: 10.5858/2002-126-1114-cotp
13. Yu RS, Wang JW, Chen Y, Ding WH, Xu XF, Chen LR. A case of primary malignant fibrous histiocytoma of the pancreas: CT and MRI findings. World J Gastroenterol. (2008) 14:2942–5. doi: 10.3748/wjg.14.2942
14. Jarry J, Belleannee G, Laurent C, Coindre JM, Evrard S. Primary malignant fibrous histiocytoma of the pancreas: benefit of the multidisciplinary approach. Eur J Gastroenterol Hepatol. (2010) 22:765–8. doi: 10.1097/MEG.0b013e32832e0a05
15. Sanei B, Kefayat A, Samadi M, Goli P, Sanei MH, Ghahremani F, et al. Undifferentiated pleomorphic sarcoma of pancreas: a case report and review of the literature for the last updates. Case Rep Med. (2018) 2018:1510759. doi: 10.1155/2018/1510759
16. Gilman JK, Sievers DB, Thornsvard CT. Malignant fibrous histiocytoma manifesting as a cavitary lung metastasis. South Med J. (1986) 79:376–8. doi: 10.1097/00007611-198603000-00031
17. Endo Y, Kobayashi T, Irie S, Mori K, Minamimura K, Hirata T, et al. Primary duodenal undifferentiated pleomorphic sarcoma that recurred after resection. Jpn J Gastroenterol Surg. (2016);49:989–96. doi: 10.5833/jjgs.2015.0190
18. Farinon AM, Bock E, Federico F, Rulli F, D'Antini P. Primary malignant fibrous histiocytoma of the duodenum. Dig Surg. (1999) 16:425–30. doi: 10.1159/000018760
19. Wang ZS, Xiong CL, Zhan N, Xiong GS, Li H, Hu H. Primary malignant fibrous histiocytoma of the small bowel: a report of an additional case in duodenum. Int J Gastrointest Cancer. (2005) 36:105–12. doi: 10.1385/ijgc:36:2:105
20. Makni A, Chebbi F, Azzouz H, Magherbi H, Jouini M, Kacem M, et al. A case of primary malignant fibrous histiocytoma of the duodenum. Int J Surg Case Rep. (2011) 2:103–5. doi: 10.1016/j.ijscr.2011.01.011
21. Giuliani J. Primary malignant fibrous histiocytoma of Vater’s Papilla: first reported case. J Gastrointest Cancer. (2013) 44:366–7. doi: 10.1007/s12029-012-9439-5
22. Xu F, Zhao F, Feng X, Li C, Han D, Zheng S, et al. Nomogram for predicting cancer-specific survival in undifferentiated pleomorphic sarcoma: a surveillance, epidemiology, and end results-based study. Cancer Control. (2021) 28:10732748211036775. doi: 10.1177/10732748211036775
23. Matushansky I, Charytonowicz E, Mills J, Siddiqi S, Hricik T, Cordon-Cardo C. MFH Classification: differentiating undifferentiated pleomorphic sarcoma in the 21st Century. Expert Rev Anticancer Ther. (2009) 9:1135–44. doi: 10.1586/era.09.76
24. Kikuta K, Morioka H, Kawai A, Kondo T. Global protein-expression profiling for reclassification of malignant fibrous histiocytoma. Biochim Biophys Acta. (2015) 1854:696–701. doi: 10.1016/j.bbapap.2014.08.012
25. Jo VY, Fletcher CD. WHO Classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. (2014) 46:95–104. doi: 10.1097/pat.0000000000000050
26. Baehring JM, Alemohammed S, Croul SE. Malignant fibrous histiocytoma presenting as an intraventricular mass five years after incidental detection of a mass lesion. J Neurooncol. (2001) 52:157–60. doi: 10.1023/a:1010685020995
27. Froehner M, Gaertner HJ, Hakenberg OW, Wirth MP. Malignant fibrous histiocytoma of the ileum at a site of previous surgery: report of a case. Surg Today. (2001) 31:242–5. doi: 10.1007/s005950170177
28. Thway K, Fisher C. Undifferentiated and dedifferentiated soft tissue neoplasms: immunohistochemical surrogates for differential diagnosis. Semin Diagn Pathol. (2021) 38:170–86. doi: 10.1053/j.semdp.2021.09.005
29. Goldblum JR. An approach to pleomorphic sarcomas: can we subclassify, and does it matter? Mod Pathol. (2014) 27(Suppl 1):S39–S46. doi: 10.1038/modpathol.2013.174
30. Folpe AL, Morris RJ, Weiss SW. Soft tissue giant cell tumor of low malignant potential: a proposal for the reclassification of malignant giant cell tumor of soft parts. Mod Pathol. (1999) 12:894–902.
31. Carvalho SD, Pissaloux D, Crombé A, Coindre JM, Le Loarer F. Pleomorphic sarcomas: the state of the art. Surg Pathol Clin. (2019) 12:63–105. doi: 10.1016/j.path.2018.10.004
32. Xu Z, Qu W, Yu Y. Primary giant cell malignant fibrous histiocytoma of the lung: a rare case report and literature review. Transl Cancer Res. (2020) 9:7350–8. doi: 10.21037/tcr-20-2297
33. Jibbe A, Worley B, Miller CH, Alam M. Surgical excision margins for fibrohistiocytic tumors, including atypical fibroxanthoma and undifferentiated pleomorphic sarcoma: a probability model based on a systematic review. J Am Acad Dermatol. (2021). doi: 10.1016/j.jaad.2021.09.036. [Epub ahead of print]
34. Fujiwara T, Stevenson J, Parry M, Tsuda Y, Tsoi K, Jeys L. What is an adequate margin for infiltrative soft-tissue sarcomas? Eur J Surg Oncol. (2020) 46:277–81. doi: 10.1016/j.ejso.2019.10.005
35. Li B, Luo CH, Zheng W. Risk factors for recurrence and survival in patients with primary retroperitoneal tumors. J Buon. (2013) 18:782–7.
Keywords: undifferentiated pleomorphic sarcoma, duodenal papilla, duodenoscopy, duodenal papillectomy, case report
Citation: Wang J, Liu B, Hou J and Li T (2022) Undifferentiated Pleomorphic Sarcoma of the Duodenal Papilla: A Rare Case and Worth Discussing History. Front. Surg. 9:926003. doi: 10.3389/fsurg.2022.926003
Received: 22 April 2022; Accepted: 3 June 2022;
Published: 6 July 2022.
Edited by:
Qi Liu, Fudan University, ChinaReviewed by:
Masachika Ikegami, Tokyo Metropolitan Komagome Hospital, JapanMariam Zaghloul, Kafrelsheikh University, Egypt
Copyright © 2022 Wang, Liu, Hou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Li bGl0YW83NDEwMkAxMjYuY29t
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Jianlong Wang1
Jianlong Wang1 Tao Li
Tao Li