- 1Department of Gastroenterology, The Third Central Hospital of Tianjin, Tianjin Key Laboratory of Extracorporeal Life Support for Critical Diseases, Artificial Cell Engineering Technology Research Center, Tianjin Institute of Hepatobiliary Disease, Tianjin, China
- 2Department of Gastroenterology, People’s Hospital Affiliated to Nankai University, Tianjin, China
Aim: To identify the association between endoscopic primary prophylaxis and the risk of rebleeding in patients with liver cirrhosis receiving endoscopic therapy.
Methods: This cohort study involved in 944 liver cirrhosis patients with esophagogastric variceal bleeding (EGVB) receiving endoscopic therapy. All participants were divided into two groups: rebleeding group (n = 425) and non-rebleeding group (n = 519) according to the occurrence of rebleeding in patients. Rebleeding indicated any bleeding after endoscopic therapy for the first bleeding of esophagogastric varices in liver cirrhosis patients. Univariate and multivariate logistic analyses were employed to identify the association between endoscopic primary prophylaxis and rebleeding in patients with liver cirrhosis after endoscopic therapy.
Results: In total, 425 patients rebleeded at the end of the follow-up. The risk of rebleeding in patients with endoscopic primary prophylaxis decreased by 0.773 times (OR = 0.227, 95%CI: 0.139–0.372, P < 0.001) after adjusting covariables. Subgroups were divided according to the Child-Pugh (CP) score, and the results revealed that the risk of rebleeding in patients with endoscopic primary prophylaxis decreased by 0.858 times in Grade A patients (OR = 0.142, 95%CI: 0.066–0.304, P < 0.001) and 0.804 times in Grade B patients (OR = 0.196, 95%CI: 0.085–0.451, P < 0.001) compared with patients without endoscopic primary prophylaxis, but showed no difference in Grade C patients.
Conclusion: Endoscopic primary prophylaxis was associated with a decreased risk of rebleeding in liver cirrhosis patients with EGVB after endoscopic therapy, which suggested that clinicians should pay more attention to endoscopic primary prophylaxis to prevent the occurrence of rebleeding in these patients.
Introduction
Liver cirrhosis is a major cause of death all over the world, and its mortality rate is still on the rise (1). Portal hypertension is a serious complication of liver cirrhosis, which may result in esophagogastric variceal bleeding (EGVB) in patients (2). Esophagogastric varices were found in about 50% of newly diagnosed liver cirrhosis patients (3). Every year, 12% of liver cirrhosis patients suffered from the occurrence of first variceal bleeding (4). Previous studies have also revealed that the mortality of acute variceal bleeding in patients with liver cirrhosis was about 15%–20% within 6 weeks and 40% within 1 year (5, 6). Worse still, 60% of liver cirrhosis patients surviving EGVB may have a higher risk of esophagogastric variceal rebleeding (EGVR) and the mortality rate is up to 33% (7). There is an urgent need to improve the management of liver cirrhosis patients with EGVB.
Currently, the first-line standard treatment for liver cirrhosis with variceal bleeding is the combination of vasoactive drug therapy and endoscopic methods of hemostasis (8, 9). Several treatment methods were applied for varices and variceal hemorrhage, including transjugular intrahepatic portosystemic shunt (TIPS), and liver transplantation (10). The lack of liver donors and high medical costs limit the application of liver transplantation and TIPS may cause the reduction of liver function in patients (11). Additionally, the risk of rebleeding and mortality due to EGVB remains high despite the improvements in these therapies (12). Recently, primary prophylaxis was recommended for liver cirrhosis patients with esophagogastric varices, which has been reported to prevent the first bleeding in liver cirrhosis patients (13, 14). Non-selective beta-blockers (NSBBs) are widely utilized for primary and secondary prophylaxis of esophageal variceal bleeding in liver cirrhosis, which can reduce the portal vein velocity (15). Antibiotic prophylaxis was applied in cirrhosis patients, especially Child-Pugh B and C (16). Endoscopic therapy to ligate the varices was also a choice for the prevention and treatment of bleeding in cirrhosis patients, but the association of endoscopic therapy as primary prophylaxis and rebleeding in patients with liver cirrhosis and EGVB was still unclear.
The purpose of this study was to identify whether endoscopic primary prophylaxis had any association with the risk of rebleeding in patients with liver cirrhosis, which might provide a reference for preventing the occurrence of rebleeding in patients with liver cirrhosis complicated with EGVB using endoscopic therapy.
Methods
Study Design and Population
In this cohort study, the data of 944 liver cirrhosis patients with EGVB receiving endoscopic therapy after first bleeding were collected in the Third Central Hospital of Tianjin from Jan 2015 to June 2020. EGVB was caused by portal hypertension due to liver cirrhosis. The diagnosis of liver cirrhosis was based on the pathological examination or clinical diagnosis according to physical signs, ultrasound, computed tomography (CT), or biochemical indices (17). General gastroscopy was applied for the determination of esophageal varices. Esophageal varices were divided into three: mild (G1): esophageal varices were linear or slightly circuitous, without any red sign. Moderate (G2): esophageal varices were linear or slightly tortuous, with a red sign or serpentine protuberance but no red sign. Severe (G3): esophageal varices were serpentine and tortuous, with a red sign, or they were in the form of beads, nodules, or tumors (with or without any red sign) (18). Gastric varices were classified as gastroesophageal varices (GOV) and isolated gastric varices (IGV) (19). Variceal bleeding was defined according to Baveno VI criteria (8). The informed consents were collected from the participants, and our study was approved by the Ethics Committee of the Third Central Hospital of Tianjin (IRB2021-028-01).
Endoscopic Treatment
In our study, endoscopic treatment was applied in the primary prophylaxis and treatment after first bleeding in liver cirrhosis. Endoscopy was conducted by the use of OLYMPUS 260 or 290 (Japan). For patients with gastric varices, lauromacrogol and tissue adhesive injection were applied. For patients with esophageal varices, endoscopic variceal ligation (EVL) was performed with a multiband ligator (Wilson Cook, USA). For patients with gastroesophageal varices, lauromacrogol and tissue adhesive injection, combined with EVL, were used.
Data Collection
The baseline data of patients were collected: They are as follows: gender, age (year), the pathogenesis of liver cirrhosis [Hepatitis B Virus (HBV), hepatitis C virus (HCV), alcoholic, cryptogenic, autoimmune, and other reasons], time from admission to receiving endoscopic treatment (<6 h, ≥6 h and <12 h, ≥12 h and <24 h, and ≥24 h and <48 h), patients receiving painless endoscopy (yes or no), the frequency of endoscopic treatment, whether having a CT portosystemic shunt, prothrombin activity (PTA, %), international normalized ratio (INR), white blood cell (WBC, 10⁹/l), neutrophil (NEUT, 10⁹ g/l), lymphocyte (10⁹ g/l), hemoglobin (Hb, g/l), platelet (PLT, 10⁹ g/l), albumin (ALB, g/l), alanine transaminase (ALT, U/l), aspartate aminotransferase (AST, U/l), total bilirubin (TBIL, μmol/l), creatinine (CR, μmol/l), sodium (Na), alpha fetoprotein (AFP, μg/l), varices invalid or alleviated after treatment, whether having portal vein thrombosis, the width of the portal vein (mm), spleen thickness (mm), ascites grade, hepatic encephalopathy, shock, infection, and antibiotics using status, Child-Pugh (CP) score (Grade A: 5–6 points, Grade B: 7–9 points and Grade C: ≥10 points), and whether there is any occurrence of rebleeding.
Outcome Variable
Inpatient or outpatient follow-up was performed every 3 months after the treatment of the first bleeding, until the variceal obliteration. According to the Baveno VI document, a combination of either a 3 g/dl drop in hemoglobin, fresh hematemesis, or death within 5 days is defined as rebleeding.
Statistical Analysis
Statistical analyses were performed using SAS 9.4 software. All statistical analyses were subjected to a two-side test. The Shapiro test was conducted to evaluate the normality of measurement data. The measurement data with normal distribution were displayed as mean ± standard deviation (SD), and comparisons between groups were based on the independent sample t-test. The measurement data with non-normal distribution were depicted as M (Q1, Q3), and the differences between groups were evaluated using the Mann–Whitney U test. The enumeration data were described as n (%), and Chi-square (χ2) test or Fisher's exact probability method were used for making comparisons between groups. Variables with statistical differences between the rebleeding group and the non-rebleeding group were adjusted as covariates in multivariate logistic regression analysis to identify the association of endoscopic primary prophylaxis and rebleeding in patients with liver cirrhosis. The univariate model was the crude model, the multivariable modela adjusted for painless endoscopic therapy and endoscopic therapy frequency. In the multivariate model b, painless endoscopic therapy, endoscopic therapy frequency, lymphocyte, Hb, AST, varices status, spleen thickness, ascites grade, and shock were adjusted. A score of P < 0.05 was considered statistically significant.
Results
The Baseline Characteristics of All Participants
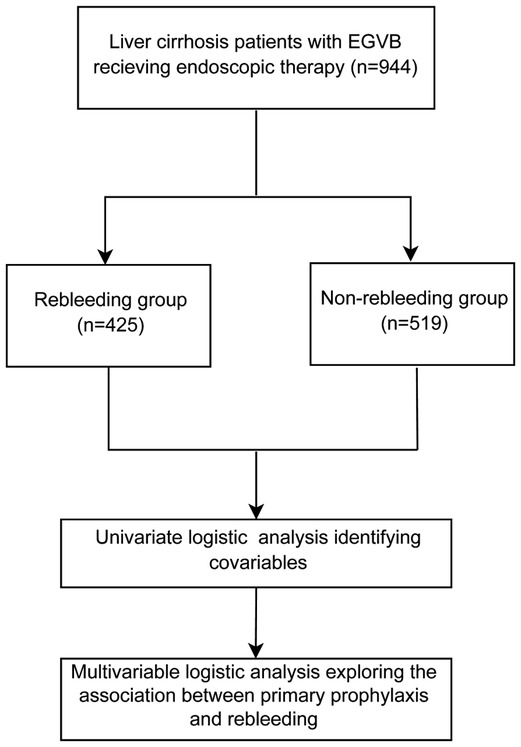
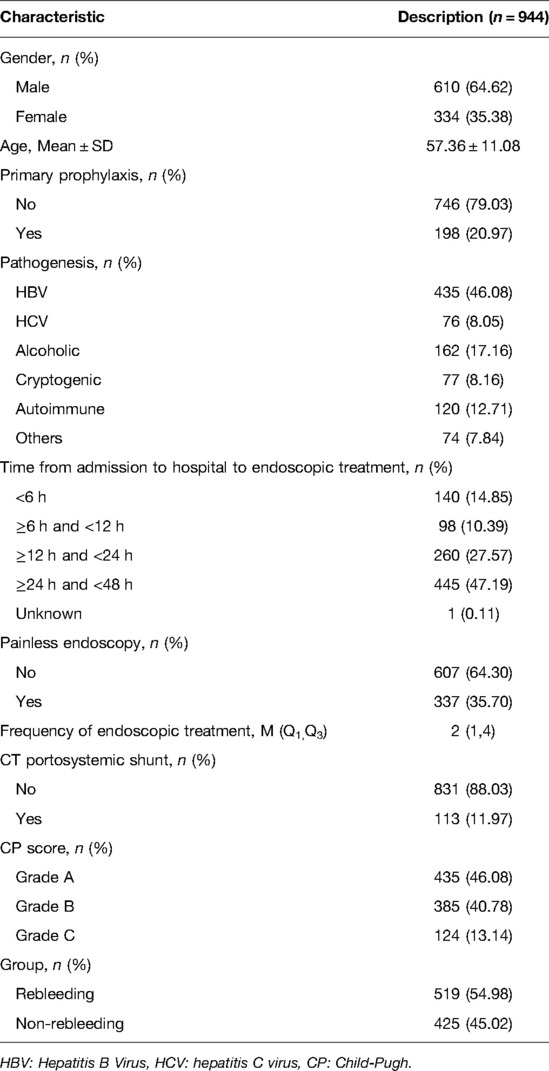
This study involved 944 patients with liver cirrhosis (Figure 1). All participants were divided into two groups, the rebleeding group (n = 425) and the non-rebleeding group (n = 519), according to the occurrence of rebleeding in patients receiving endoscopic therapy. The average age of all participants was 57.36 ± 11.08 years. Among them, 610 (64.62%) were males and 334 (35.38%) were females. A total of 198 (20.97%) patients had endoscopic primary prophylaxis. The pathogenesis of 435 (46.08%) patients was HBV, 76 (8.05%) were HCV, 162 (17.16%) were alcoholic, 77 (8.16%) were cryptogenic, 120 were autoimmune (12.71%), and 74 were others (7.84%). A total of 337 (35.70%) patients received painless endoscopy, and the median frequencies of endoscopic treatment in all patients were 2 (1, 4) times. According to the CP scores, 435 (46.08%) patients were in Grade A, 385 (40.78%) participants were in Grade B, and 124 (13.14%) patients were in Grade C. There were 425 (45.02%) patients in the rebleeding group and 519 (54.98%) patients in the non-rebleeding group (Table 1).
Comparisons of the Data in Patients Between the Rebleeding Group and the Non-Rebleeding Group
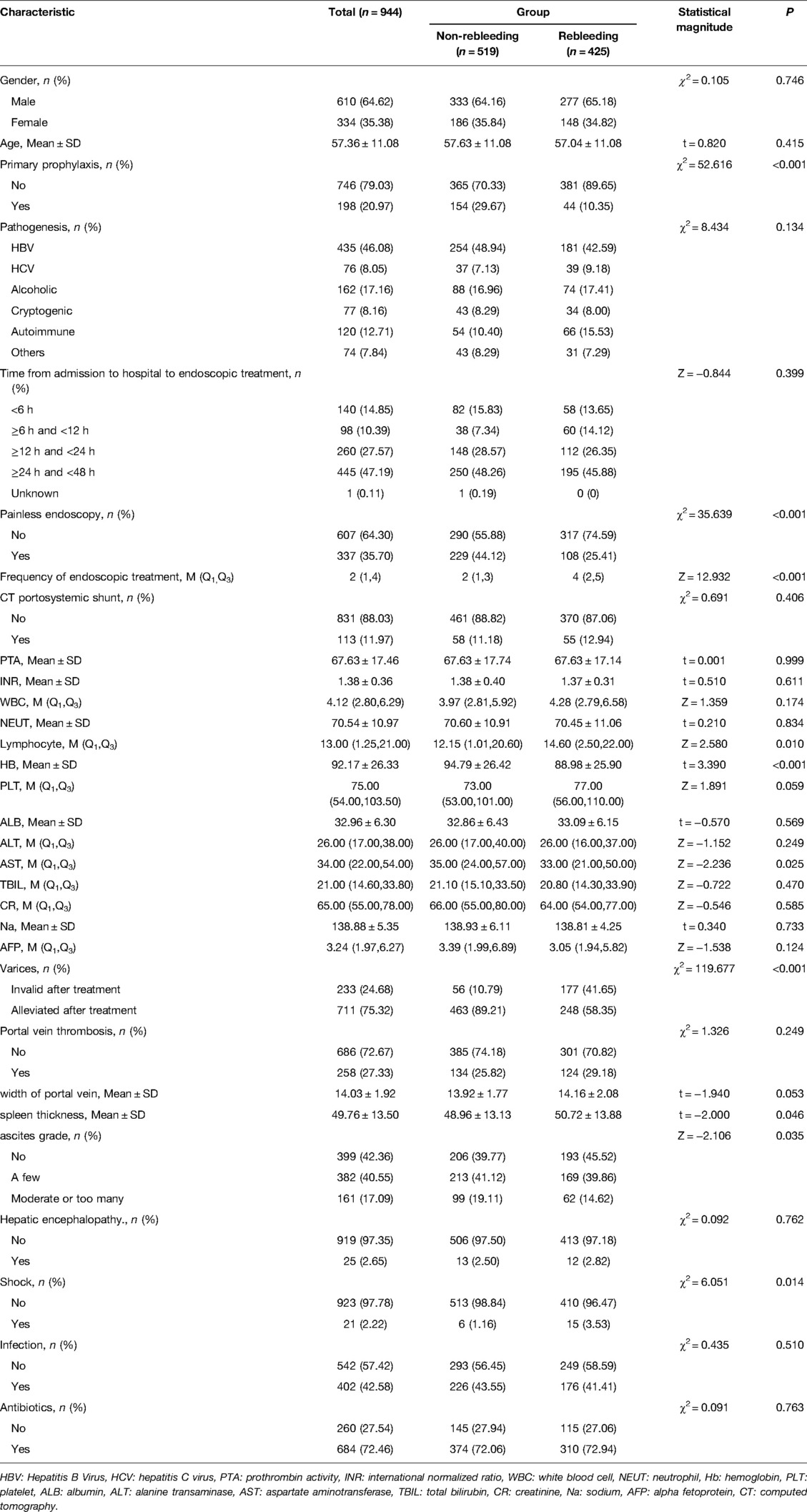
As observed in Table 2, the proportions of patients receiving endoscopic primary prophylaxis (10.35% vs 29.67%, χ2 = 52.616, P < 0.001) and painless endoscopy (25.41% vs 44.12%, χ2 = 35.639, P < 0.001) in the rebleeding group were lower than those in the non-rebleeding group. The proportions of patients with varices becoming invalid after endoscopic therapy (41.65% vs 10.79%, χ2 = 119.677, P < 0.001) and shock (3.53 vs 1.16, χ2 = 6.051, P = 0.014) were significantly higher in the rebleeding group than those in the non-rebleeding group. The frequencies of endoscopic therapy (4 times vs 2 times, Z = 12.932, P < 0.001), the average lymphocyte level (14.60 10⁹ g/l vs 12.15 10⁹ g/l, Z = 2.580, P = 0.010), the mean spleen thickness (50.72 mm vs 48.96 mm, t = −2.000, P = 0.046), and ascites grade (Z = −2.106, P = 0.035) were higher in the rebleeding group than in the non-rebleeding group. The mean level of Hb (88.98 g/l vs 94.79 g/l, P < 0.001) and average level of AST (33.00 U/l vs 35.00 U/l, P = 0.025) were lower in the rebleeding group than in the non-rebleeding group (Table 2).
Association Between Endoscopic Primary Prophylaxis and Rebleeding
According to the results in univariate and multivariate analyses, the data delineated that the risk of rebleeding after endoscopic therapy in patients with endoscopic primary prophylaxis was decreased by 0.733 times compared with patients without endoscopic primary prophylaxis (OR = 0.267, 95%CI: 0.185–0.385, P < 0.001). After adjusting for variables including painless endoscopic therapy and endoscopic therapy frequency, patients with endoscopic primary prophylaxis were associated with a 0.226-fold decrease of risk of rebleeding compared with patients without endoscopic primary prophylaxis (OR = 0.226, 95%CI:0.150–0.339, P < 0.001). After adjusting for variables including painless endoscopic therapy, endoscopic therapy frequency, lymphocyte, Hb, AST, varices status, spleen thickness, ascites grade and shock, the risk of rebleeding in patients with endoscopic primary prophylaxis was decreased by 0.773 times (OR = 0.227, 95%CI: 0.139–0.372, P < 0.001) (Table 3).
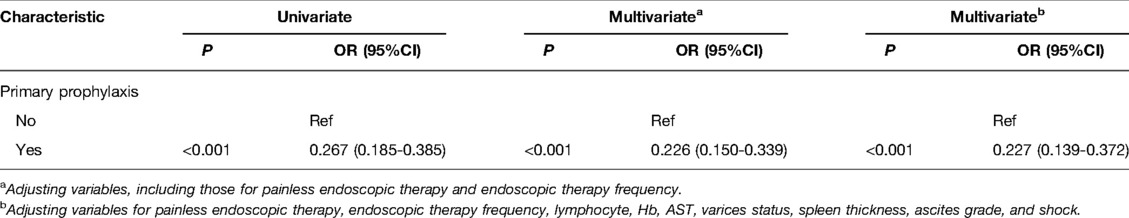
Table 3. Univariate and multivariate analyses of associations between primary prophylaxis and rebleeding.
Subgroup Analysis of the Association Between Endoscopic Primary Prophylaxis and Rebleeding in Patients With a Different CP Score
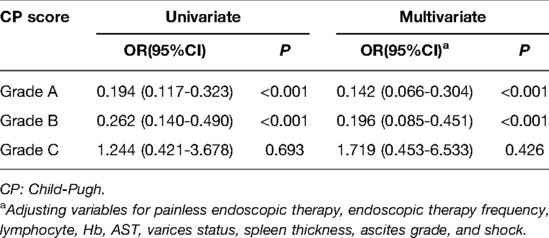
All subjects were divided into Grade A, Grade B, and Grade C groups according to their CP score. As depicted in Table 4, in the Grade A group, the risk of rebleeding decreased by 0.806 times in patients with endoscopic primary prophylaxis compared with patients without endoscopic primary prophylaxis (OR = 0.194, 95%CI: 0.117–0.323, P < 0.001). After adjusting for variables including painless endoscopic therapy, endoscopic therapy frequency, lymphocyte, Hb, AST, varices status, spleen thickness, ascites grade and shock, the risk of rebleeding after endoscopic therapy in patients with endoscopic primary prophylaxis decreased by 0.858 times in comparison with those without endoscopic primary prophylaxis (OR = 0.142, 95%CI: 0.066–0.304, P < 0.001). As for patients in the Grade B group, endoscopic primary prophylaxis decreased the risk of rebleeding by 0.738 times compared with those without endoscopic primary prophylaxis (OR = 0.262, 95%CI: 0.140–0.490, P < 0.001). After adjusting for variables such as painless, endoscopic therapy frequency, lymphocyte, Hb, AST, varicose veins, splenic thickness, ascites grade, and shock, endoscopic primary prophylaxis decreased the risk of rebleeding after endoscopic therapy by 0.804 times compared with those without endoscopic primary prophylaxis (OR = 0.196, 95%CI: 0.085–0.451, P < 0.001). In the Grade C group, endoscopic primary prophylaxis showed no statistical difference on rebleeding risk in patients (P > 0.05).
Discussion
In this study, the data of 944 liver cirrhosis patients with EGVB receiving endoscopic therapy were collected to examine whether endoscopic primary prophylaxis had an impact on the rebleeding risk in liver cirrhosis patients with EGVB after endoscopic therapy. The data revealed that endoscopic primary prophylaxis decreased the risk of rebleeding in liver cirrhosis patients with EGVB after endoscopic therapy. Additionally, for patients with a CP score in Grade A and Grade B, the risk of rebleeding reduced by endoscopic primary prophylaxis, but in Grade C, there was no difference in the risk of rebleeding in patients irrespective of whether they received endoscopic primary prophylaxis. The findings of our study might provide a reference for the use of endoscopic primary prophylaxis in the clinic.
Variceal bleeding is a serious complication of liver cirrhosis with a high risk of mortality (20). Due to the compression of regenerated nodular tissue on venous vessels in liver fibrosis patients, the vessels were distorted, and the pressure on hepatic sinus and the terminal portal vein increased, thereby inducing EGVB (21). In our study, the pathogeneses of patients with liver cirrhosis were mainly HBV, HCV, alcoholic, cryptogenic, and autoimmune hepatitis. Liver cirrhosis patients suffered from liver cell degeneration and necrosis, the collapse of the hepatic lobular fibrous scaffold, and irregular regeneration of liver cells. At the same time, inflammation and other pathogenic factors activated hepatic astrocytes and promoted the transformation of hepatic cells and bile duct cells into interstitial cells. Collagen synthesis in the liver increased and degradation reduced. Then, a large amount of collagen was deposited in the Disse space, which narrowed the hepatic sinuses, leading to a reduction of hepatic sinusoids and a compression of hepatic sinusoids and hepatic venous systems. Therefore, the portal vein blood flow into the hepatic sinuses and the outflow tract of the hepatic vein after the sinus were blocked, which increased the vascular resistance and elevated the risk of portal hypertension. The gastric coronary vein of the portal vein system was anastomosed with the azygos vein of the esophageal vein of the vena cava system at the lower esophagus and stomach fundus, forming esophagogastric varices.
Previous researchers have made efforts to make an in-depth study of the pathogenesis of liver cirrhosis with portal hypertension, and new technologies have led to notable advances in treating EGVB. However, the mortality rate due to EGVB is still unfortunately high despite the application of current standards of treatment (22). Given the poor prognosis of EGVB patients, the primary prophylaxis of it in patients with esophagogastric varices has been widely proposed in recent years. EVL is recommended as a prevention method of primary prophylaxis for the first bleeding episode from esophagogastric varices (23). Endoscopic treatment of high risk lesions reduces the risk of bleeding and the need for surgery (24). EVL is an effective and safe treatment for the prevention of upper gastrointestinal bleeding in patients with portal hypertension (25). Another multicenter randomized-controlled trial revealed that as one of the primary prophylaxis, EVL is an effective method for preventing variceal hemorrhage in liver cirrhosis patients (26). In this study, we found that endoscopic primary prophylaxis using EVL was associated with a decreased risk of rebleeding in liver cirrhosis patients with EGVB after endoscopic therapy.
The CP classification was applied to assess the hepatic function according to the risk stratifications of ascites, encephalopathy, serum ALB, TBIL, and prothrombin time (27). The classification was graded based on the total score: Grade A: 5–6 points, Grade B: 7–9 points, Grade C: 10 points, or above. A previous study found that the CP score was an independent risk factor for immediate bleeding after colonoscopic polypectomy in liver cirrhosis patients, and the bleeding risk was higher in Grade C patients than in Grade A patients (28). Another large-scale cohort study also depicted that the 1-year and 3-year survival rates were 90.2% and 75.3% for Grade A, 73.5% and 53.9% for Grade B, and 41.9% and 28.9% for Grade C, indicating that patients in Grade C were associated with a poorer prognosis (29). In this study, subgroup analysis was performed to screen out patients who were found suitable for primary prophylaxis. The results demonstrated that endoscopic primary prophylaxis could decrease the risk of rebleeding in Grade A and Grade B patients, suggesting that endoscopic primary prophylaxis might be suitable for patients in these groups. This may be due to the fact that patients in Grade A and Grade B had better liver function and a longer survival time with appropriate treatment (30). Bleeding is a serious threat to the lives of patients, and primary prophylaxis decreased the risk of bleeding and caused little side effects in the recovery process in those patients (23). Additionally, the effect of endoscopic primary prophylaxis in decreasing the risk of rebleeding was better in the Grade A group than in the Grade B group. Endoscopic primary prophylaxis had no significant effects for Grade C patients because these patients had poor coagulation function, liver function, and nutritional status; the endoscopic treatment may increase the risk of bleeding, and it is difficult for them to recover after surgery (31). Therefore, clinicians might apply endoscopic primary prophylaxis based on the Child Pugh of patients.
The present study identified that endoscopic primary prophylaxis was associated with a reduced risk of rebleeding after endoscopic therapy in patients with cirrhosis complicated with EGVB. Endoscopic primary prophylaxis was performed before the first bleeding in liver cirrhosis patients with esophagogastric varices. Patients receiving endoscopic primary prophylaxis via endoscopic treatment had reduced varices, and the rebleeding risk decreased. Also, the volume of rebleeding reduced and the therapeutic effect was better (13). The risk of rebleeding after endoscopy is mainly related to the level of portal hypertension, the severity of varices, and the status of coagulation function in patients (32). Based on these results, we recommend endoscopic primary prophylaxis for patients with cirrhosis complicated with esophagogastric varices, rather than waiting for emergency treatment after the first episode of variceal bleeding.
This study was a case-control study including a large sample size to evaluate the association between endoscopic primary prophylaxis and rebleeding after endoscopic therapy in liver cirrhosis patients with EGVB. The variables were collected after admission to hospital, which decreased the recall bias. The first limitation of this study was that this was a single-center study. Was that the data of all participants from the time of endoscopic treatment for first bleeding to rebleeding, which might have association with the risk of rebleeding. In the future, the findings of our study should be validated by multicenter studies with a large sample size.
Conclusions
Our study collected the data of 944 liver cirrhosis patients with EGVB receiving endoscopic therapy to find whether endoscopic primary prophylaxis was associated with the risk of rebleeding after endoscopic therapy in these patients. The results showed that endoscopic primary prophylaxis was associated with a decreased risk of rebleeding in liver cirrhosis patients with EGVB after endoscopic therapy. These findings will serve to remind clinicians to pay more attention to endoscopic primary prophylaxis in liver cirrhosis patients with esophagogastric varices and provide proper and timely treatment in those patients. For patients in Grade C with poor liver function, endoscopic primary prophylaxis should be done with caution.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Third Central Hospital of Tianjin (IRB2021-028-01). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YG and TH designed the study. YG wrote the manuscript. HY, XZ, FL, FT, and HL collected, analyzed, and interpreted the data. TH critically reviewed, edited, and approved the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2015) 385(9963):117–71. doi: 10.1016/s0140-6736(14)61682-2
2. Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. Β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2019) 393(10181):1597–608. doi: 10.1016/s0140-6736(18)31875-0
3. Garcia-Pagan JC, Francoz C, Montagnese S, Senzolo M, Mookerjee RP. Management of the major complications of cirrhosis: beyond guidelines. J Hepatol. (2021) 75(Suppl 1):S135–s146. doi: 10.1016/j.jhep.2021.01.027
4. Ma JL, He LL, Li P, Jiang Y, Hu JL, Zhou YL, et al. Clinical features and outcomes of repeated endoscopic therapy for esophagogastric variceal hemorrhage in cirrhotic patients: ten-year real-world analysis. Gastroenterol Res Pract. (2020) 2020:5747563. doi: 10.1155/2020/5747563
5. Lv Y, Qi X, He C, Wang Z, Yin Z, Niu J, et al. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut. (2018) 67(12):2156–68. doi: 10.1136/gutjnl-2017-314634
6. Sharma M, Singh S, Desai V, Shah VH, Kamath PS, Murad MH, et al. Comparison of therapies for primary prevention of esophageal variceal bleeding: a systematic review and network meta-analysis. Hepatology. (2019) 69(4):1657–75. doi: 10.1002/hep.30220
7. Bari K, Garcia-Tsao G. Treatment of portal hypertension. World J Gastroenterol. (2012) 18(11):1166–75. doi: 10.3748/wjg.v18.i11.1166
8. de Franchis R. Expanding consensus in portal hypertension: report of the baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. (2015) 63(3):743–52. doi: 10.1016/j.jhep.2015.05.022
9. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American association for the study of liver diseases. Hepatology. (2017) 65(1):310–35. doi: 10.1002/hep.28906
10. Zheng S, Sun P, Liu X, Li G, Gong W, Liu J. Efficacy and safety of laparoscopic splenectomy and esophagogastric devascularization for portal hypertension: a single-center experience. Medicine (Baltimore). (2018) 97(50):e13703. doi: 10.1097/md.0000000000013703
11. Bosch J. Small diameter shunts should lead to safe expansion of the use of TIPS. J Hepatol. (2021) 74(1):230–4. doi: 10.1016/j.jhep.2020.09.018
12. García-Pagán JC, Villanueva C, Albillos A, Bañares R, Morillas R, Abraldes JG, et al. Nadolol plus isosorbide mononitrate alone or associated with band ligation in the prevention of recurrent bleeding: a multicentre randomised controlled trial. Gut. (2009) 58(8):1144–50. doi: 10.1136/gut.2008.171207
13. Robertson M, Hayes P. Primary prophylaxis of variceal bleeding. Hepatol Int. (2018) 12(1):1–5. doi: 10.1007/s12072-018-9846-1
14. Tantai XX, Liu N, Yang LB, Wei ZC, Xiao CL, Song YH, et al. Prognostic value of risk scoring systems for cirrhotic patients with variceal bleeding. World J Gastroenterol. (2019) 25(45):6668–80. doi: 10.3748/wjg.v25.i45.6668
15. Xu X, Guo X, De Stefano V, Silva-Junior G, Goyal H, Bai Z, et al. Nonselective beta-blockers and development of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Hepatol Int. (2019) 13(4):468–81. doi: 10.1007/s12072-019-09951-6
16. Fernández J, Tandon P, Mensa J, Garcia-Tsao G. Antibiotic prophylaxis in cirrhosis: good and bad. Hepatology. (2016) 63(6):2019–31. doi: 10.1002/hep.28330
17. Ding XC, Ma WL, Li MK, Liu SW, Liu XY, Hai L, et al. A meta-analysis of the value of vWF in the diagnosis of liver cirrhosis with portal hypertension. J Clin Transl Hepatol. (2019) 7(1):3–8. doi: 10.14218/jcth.2018.00036
18. The Chinese Society of Spleen and Portal Hypertension Surgery, Chinese Society of Surgery. Expert consensus on diagnosis and treatment of esophagogastric variceal bleeding in cirrhotic portal hypertension (2019 edition). Zhonghua Wai Ke Za Zhi. (2019) 57(12):885–92. doi: 10.3760/cma.j.issn.0529-5815.2019.12.002
19. Seo YS. Prevention and management of gastroesophageal varices. Clin Mol Hepatol. (2018) 24(1):20–42. doi: 10.3350/cmh.2017.0064
20. Mallet M, Rudler M, Thabut D. Variceal bleeding in cirrhotic patients. Gastroenterol Rep (Oxf). (2017) 5(3):185–92. doi: 10.1093/gastro/gox024
21. Rahimi RS, Rockey DC. Complications and outcomes in chronic liver disease. Curr Opin Gastroenterol. (2011) 27(3):204–9. doi: 10.1097/MOG.0b013e3283460c7d
22. Salman AA, Shaaban HE, Atallah M, Yousef M, Ahmed RA, Ashoush O, et al. Long-term outcome after endoscopic ligation of acute esophageal variceal bleeding in patients with liver cirrhosis. Acta Gastroenterol Belg. (2020) 83(3):373–80.
23. Lo GH. Endoscopic treatments for portal hypertension. Hepatol Int. (2018) 12(Suppl 1):91–101. doi: 10.1007/s12072-017-9828-8
24. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. (2012) 107(3):345–60; quiz 361. doi: 10.1038/ajg.2011.480
25. Quintero J, Juampérez J, Mercadal-Hally M, King ML, Ortega J, Molino JA, et al. Endoscopic variceal ligation as primary prophylaxis for upper GI bleeding in children. Gastrointest Endosc. (2020) 92(2):269–75. doi: 10.1016/j.gie.2020.02.035
26. Shah HA, Azam Z, Rauf J, Abid S, Hamid S, Jafri W, et al. Carvedilol vs. esophageal variceal band ligation in the primary prophylaxis of variceal hemorrhage: a multicentre randomized controlled trial. J Hepatol. (2014) 60(4):757–64. doi: 10.1016/j.jhep.2013.11.019
27. Elmeliegy M, Yang DZ, Salama E, Parivar K, Wang DD. Discordance between child-pugh and national cancer institute classifications for hepatic dysfunction: implications on dosing recommendations for oncology compounds. J Clin Pharmacol. (2021) 61(1):105–15. doi: 10.1002/jcph.1702
28. Lee S, Park SJ, Cheon JH, Kim TI, Kim WH, Kang DR, et al. Child-Pugh score is an independent risk factor for immediate bleeding after colonoscopic polypectomy in liver cirrhosis. Yonsei Med J. (2014) 55(5):1281–8. doi: 10.3349/ymj.2014.55.5.1281
29. Yatsuhashi H, Sano H, Hirano T, Shibasaki Y. Real-world hospital mortality of liver cirrhosis inpatients in Japan: a large-scale cohort study using a medical claims database: prognosis of liver cirrhosis. Hepatol Res. (2021) 51(6):682–93. doi: 10.1111/hepr.13635
30. Tandon P, Abraldes JG, Keough A, Bastiampillai R, Jayakumar S, Carbonneau M, et al. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on child-pugh class, and effects of antibiotics. Clin Gastroenterol Hepatol. (2015) 13(6):1189–96.e1182. doi: 10.1016/j.cgh.2014.11.019
31. Miaglia C, Guillaud O, Rivory J, Lépilliez V, Chambon-Augoyard C, Hervieu V, et al. Safe and effective digestive endoscopic resection in patients with cirrhosis: a single-center experience. Endoscopy. (2020) 52(4):276–84. doi: 10.1055/a-1089-9459
32. Torres P, Best MC, Freeman LM, Roberts SC, Cooper D, Sutton NJ, et al. Secondary prevention of variceal bleeding in adults with previous oesophageal variceal bleeding due to decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. (2021) 3:Cd013122. doi: 10.1002/14651858.CD013122.pub2
Keywords: primary prophylaxis, rebleeding, endoscopic therapy, liver cirrhosis, esophagogastric variceal bleeding
Citation: Gao Y, Yuan H, Han T, Zhang X, Li F, Tang F and Liu H (2022) Associations Between Endoscopic Primary Prophylaxis and Rebleeding in Liver Cirrhosis Patients with Esophagogastric Variceal Bleeding. Front. Surg. 9:925915. doi: 10.3389/fsurg.2022.925915
Received: 22 April 2022; Accepted: 21 June 2022;
Published: 12 July 2022.
Edited by:
Andrea Laurenzi, IRCCS Azienda Ospedaliero-Universitaria di Bologna, ItalyReviewed by:
Erlei Zhang, Huazhong University of Science and Technology, ChinaRiccardo Memeo, Ospedale Generale Regionale F. Miulli, Italy
Copyright © 2022 Gao, Yuan, Han, Zhang, Li, Tang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Han VGFvaGRjdEAxNjMuY29t
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Yanying Gao1
Yanying Gao1 Tao Han
Tao Han Fei Tang
Fei Tang