- 1Department of Radiology, Hunan Key Laboratory of Oral Health Research & Xiangya Stomatological Hospital & Xiangya School of Stomatology, Central South University, Changsha, China
- 2Department of Periodontology, Hunan Key Laboratory of Oral Health Research & Xiangya Stomatological Hospital & Xiangya School of Stomatology, Central South University, Changsha, China
Background: Drug-induced gingival overgrowth is common but neglected in patients with systemic disease medications until it seriously affects the quality of life.
Methods: Initial periodontal treatment, combined with water laser surgery, was performed sequentially in two cases.
Results: The therapeutic effect was good, and there was no recurrence along with good oral hygiene.
Conclusion: Water laser equipment surgery, as well as initial periodontal treatment, required that surgeons are trained specifically. A tool was devised for various oral diseases, and it was safer, more efficient and more comfortable than others.
Introduction
Predisposing drugs for gingival enlargement mainly fall into three types: antiepileptic drugs (such as Phenytoin), immunosuppressive agents (such as Cyclosporin), and calcium channel antagonists (such as Nifedipine), while its severity can be modified by the degree of primary gingival inflammation and oral hygiene conditions (1). Gingival enlargement may lead to chewing and pronunciation difficulties regardless of oral facial aesthetics, and the major managements are initial periodontal treatment and surgical periodontal therapy. In the past few years, increasing studies have revealed the superiority of laser therapy over conventional surgical treatment, which manifested as less infection, better hemostatic effect, clearer surgical field, shorter operative time, less anesthetic dosage, and less postoperative discomfort with remarkable therapeutic effect (2). The water laser technique, other than having the above advantages, overcomes the deficiency of heat production caused by conventional laser techniques owing to its unique therapeutic mechanism, allowing its wide applicability in the treatment of diseases of oral soft and hard tissues. Here, we report the cases of two patients who underwent water laser-based gingivectomy for drug-induced gingival overgrowth (DIGO) following initial periodontal treatment. The patients suffered from slight bleeding and discomfort during the operation, with no postoperative pain or bleeding and showed rapid recovery. The gingiva of the patients gradually recovered to normal after 1 year of follow-up, and there were no signs of recurrence. Written informed consent was obtained from the patients for the publication of any potentially identifiable images or data included in this article.
Case Description 1
General Information
The first patient who was a male and 42 years old complained of 1 year of gingival overgrowth with bleeding from brushing at his first visit. Three years prior to this diagnosis, the patient underwent a kidney transplant in another hospital and took Tacrolimus and Felodipine after this. He had a history of kidney disease and hypertension but no allergies to medications or food.
Examinations
Intraoral Examination (Figure 1)
The patient’s oral hygiene was in a poor condition, which manifested as a dental calculus (++), with a large amount of materia alba, swelling and tumor-like overgrowth of the gingiva, obtuse morphology, a nodular gingival enlargement of the anterior teeth covering more than 2/3 of the crown with a wide base, a barely noticeable pedicle, little mobility and a tough and substantial texture, PD: 4–6 mm, BI: 3–4.
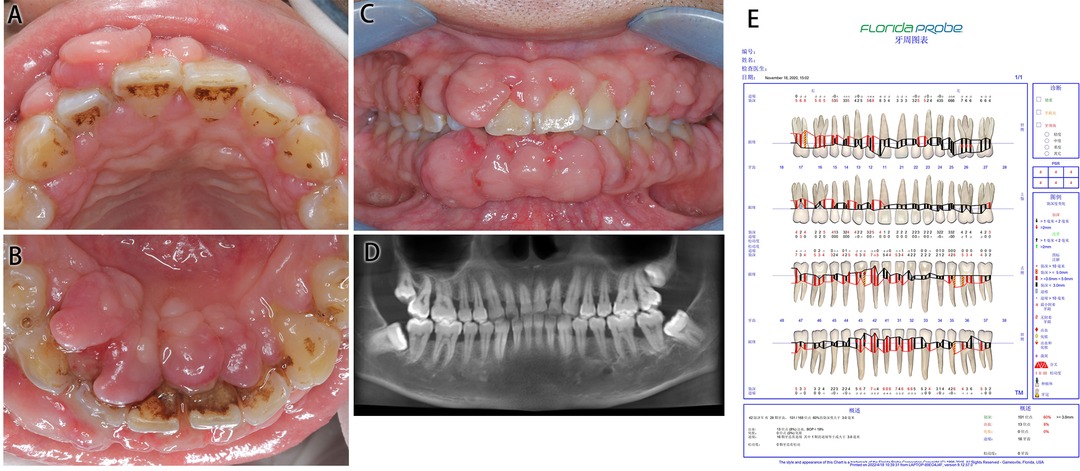
Figure 1. Medical information on Case 1 at first diagnosis (before treatment). (A) Maxillary view. (B) Mandibular view. (C) Frontal view images. (D) Radiological images. (E) Periodontal examination tables.
Extraoral Examination
The patient had facial symmetry, no obvious deformity or defects, normal mouth opening and shape, and no enlargements of bilateral cervical or submandibular lymph nodes.
Auxiliary Examination (Figure 1)
Cone beam computed tomography (CBCT) showed a slightly horizontal alveolar bone absorption of full teeth and a slightly vertical alveolar bone absorption of the bilateral posterior teeth. The periodontal examination tables show the presence of deep pockets around the periodontium.
Diagnosis
DIGO and chronic periodontitis were diagnosed.
Treatment Plan
Oral hygiene education; initial periodontal treatment; surgical periodontal therapy; periodontal maintenance therapy.
Treatment Procedure
Initial Periodontal Treatment
Oral hygiene education was imparted for this patient. The patient was informed of the correct control methods for dental plaque and was advised to develop good oral hygiene habits. Complete supragingival and subgingival scaling and root planning were recommended successively (cleansing with hydrogen peroxide after treatment followed by local iodophenol application). Regular re-examination was required.
Surgical Periodontal Therapy
A total of 4–6 weeks after the periodontal therapy, the gingival overgrowth of the upper and lower anterior teeth reduced, while aesthetics, mastication, and oral hygiene maintenance were still affected. Er,Cr:YSGG laser was used for the removal of the enlarged gingiva at labial (palate) 13–23 and 33–43 based on the external oblique incision. The gingival morphology was trimmed and hemostasis was performed. Postoperative anti-inflammatory treatment and chlorhexidine gargle rinse were managed for 5 days.
Periodontal Maintenance Therapy (Figure 3)
One year after the operation, the patient’s oral hygiene was in good condition upon re-examination, with less plaque and pigmentation. The mouth gingiva was pinkish in color, with a tough texture and an improved gingival morphology, and there was no recurrence. The enlarged gingiva of the posterior teeth was not managed by periodontal surgery, but it greatly reduced after treatment.
Case Description 2
General Information
The second patient who was a female and 67 years old gradually developed gingival swelling with bleeding 6 years ago and visited our hospital for a recent difficulty in food intake. The patient suffered from diabetes, coronary heart disease and hypertension for more than 10 years. She had well-controlled blood glucose and pressure by daily oral administration of Metformin Hydrochloride tablets and Amlodipine Besylate tablets.
Examination
Intraoral Examination (Figure 2)
This patient had poor oral hygiene, a large amount of plaque and materia alba and dental calculus (+++). The whole gingiva was bright red in color and manifested as spherical gingival enlargements covering more than 2/3 of the crown surface, with a tough texture and a tendency to bleed on touching. The upper and lower anterior teeth were spread, and teeth 31 and 41 were dislocated with 3° loosening.
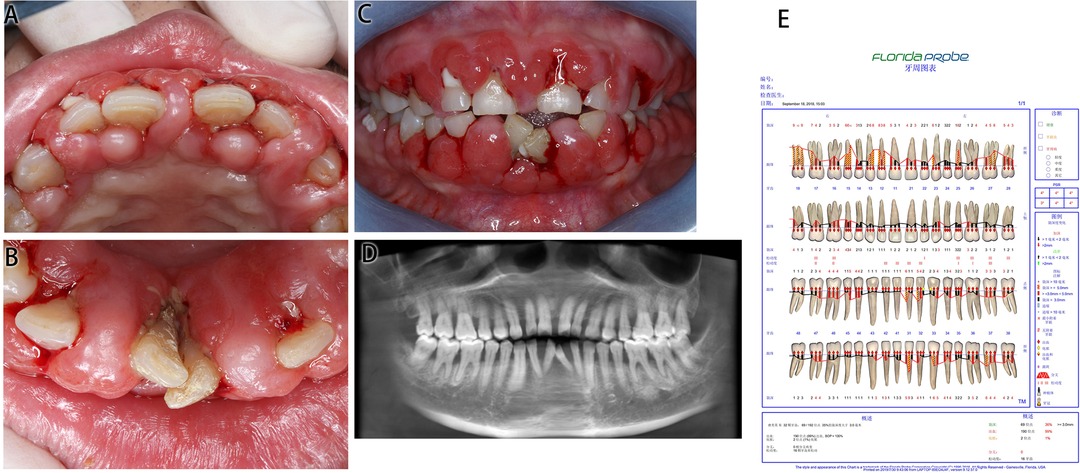
Figure 2. Medical information on Case 2 at first diagnosis (before treatment). (A) Maxillary view. (B) Mandibular view. (C) Frontal view images. (D) Radiological images. (E) Periodontal examination tables.
Auxiliary Examination (Figure 2)
CBCT presented an alveolar bone absorption of teeth 31 and 41 to the apical part, and an absorption of teeth 32 and 42 to the 1/3 apical part. Full mouth examination was referred to the periodontal specialist examination table.
Diagnosis
DIGO was diagnosed.
Treatment Plan
Oral hygiene education; initial periodontal treatment; surgical periodontal therapy; supportive periodontal therapy.
Treatment Procedure
Initial Periodontal Treatment
Oral hygiene education was imparted. Complete supragingival and subgingival scaling and root planning were recommended to be performed consecutively. Inflammation was basically controlled in 4–6 weeks and regular re-examination was required.
Surgical Periodontal Therapy
Gingival swelling greatly subsided after initial treatment. The laser technique was used to generate a gingival physical appearance favorable for follow-up self-cleaning, by performing gingivectomy on 16–26 and 36–46 based on external oblique incision. The gingival morphology was trimmed and hemostasis was performed. The incision was protected by a periodontal pack. The patient was informed of postoperative precautions and asked to orally take antibiotics followed by chlorhexidine gargle rinse.
Supportive Periodontal Therapy (Figure 3)
In 1 week after the operation, the gingival swelling subsided significantly, and basically, a normal scallop-shaped gingiva was revealed.
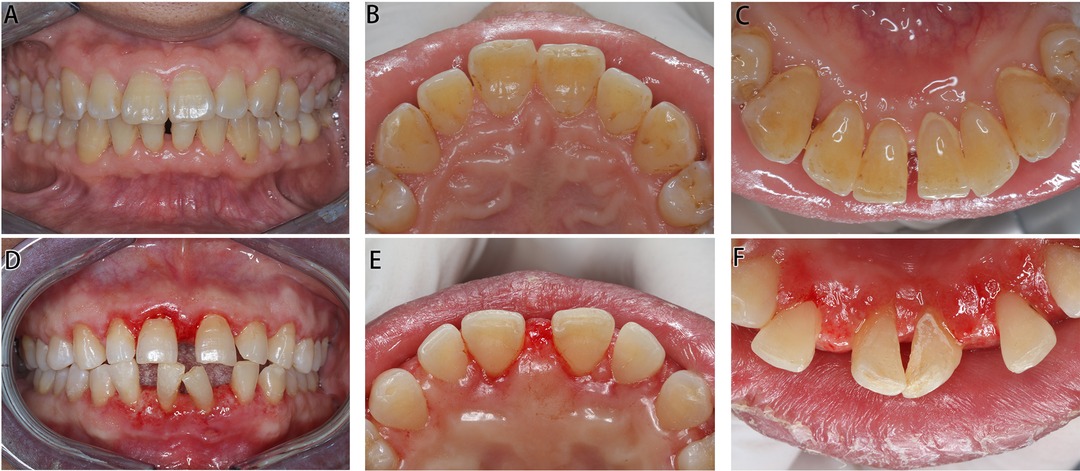
Figure 3. Intraoral images of Cases 1 and 2 post operation. (A–C) Images of Case 1 in 1 year post operation. (D–E) Images of Case 2 in 1 week post operation.
Discussion
A transplant operation often requires the use of immunosuppressants such as Cyclosporine and Tacrolimus to reduce the rejection of recipients (3). Renal hypertension is prone to become a complication requiring a regular use of antihypertensive drugs, which is significantly associated with DIGO (4–6). Such disease is reported to occur at a probability range of 8%–70% (7), modified by a patient's age, drug dosage, duration of action, combination therapy, and other factors (8). Drugs of different types function behind diverse mechanisms. Phenytoin mainly causes gingival fibrosis, Cyclosporine is mainly associated with inflammatory responses with less effect on gingival fibrosis, while Nifedipine induces both fibrosis and inflammation, resulting in gingival overgrowth (9). Additionally, plaque microorganisms play an important role in the occurrence and development of DIGO (10, 11). Therefore, initial periodontal treatment is a necessity that can greatly control gingival enlargement-related inflammation by removing plaque and calculus, decreasing stimulation on periodontal tissue and reducing periodontal inflammation.
Predominantly, DIGO is managed by flap surgery, gingivectomy, and laser resection (12). Conventional gingivectomy involves a 45° oblique incision with a scalpel under anesthesia, which generates greater pain post operation (13) compared with flap surgery but preserves more aesthetic gingival morphology (14). However, the risks of anesthesia pain and accident, postoperative swelling and pain and infection cannot be negated. Besides, the surgical field of surgeons might be stained with the effects of massive bleeding during operation, and postoperative complications are prone to develop. Laser-guided gingival resection is emerging as an alternative with many advantages such as less infection, better hemostatic effect, clearer surgical field, shorter operative time, less anesthetic dosage, and less postoperative discomfort with remarkable therapeutic effect (2). Such a technique, on the one hand, makes up for the disadvantages in conventional gingivectomy, contributing to easier hemostasis, clearer surgical field and favorable therapeutic effect and prognosis. On the other hand, postoperative morphological recovery of gingiva is more aesthetic in nature owing to the procedure of oblique incision. Mavrogiannis et al. (13) compared the efficacy of traditional gingivectomy with laser resection on DIGO and postoperative recurrence, and they indicated that laser resection was superior in terms of hemostasis and the reduction of recurrence rates. Another compelling evidence by Campos et al. (15) also revealed that, in two recurrent cases of DIGO by laser treatment, only slight bleeding and discomfort occurred during operation with no postoperative pain, bleeding or recurrence signs in 1 year of follow-up. This indicated that laser treatment might be associated with increased therapeutic efficacy and favorable outcome of DIGO.
Currently, the main laser types available for gingivectomy are semiconductor diode laser, CO2 laser, Nd:YAG laser, Er:YAG laser, and Er,Cr:YSGG laser (16–19). Among these types, except the first one (semiconductor diode laser), the others are all capable of performing soft-tissue cutting and function to achieve hemostasis and sterilization (17, 20).
Er,Cr:YSGG laser therapy involves the release of a laser light of 2,780 mm in wavelength to activate water molecules and convert them into particles with high-speed kinetics, which enables the cutting function, and then causes the energy-released water to re-condense into normal water droplets. This allows the laser to perform its cutting function while protecting normal tissue and removing heat and debris from damaged tissue, which is in contrast to conventional surgery and the working of other laser types. The Er,Cr:YSGG laser during treatment can produce a kind of morphine-like electrical biological stimulation at the surgical site, which blocks nerve conduction and achieves analgesia (21). This can contribute to a reduction of pain during treatment, which is particularly suitable for those who are sensitive to pain, such as children and the elderly, and those incapable of tolerating pain. The patients in this report had poor pain tolerance due to DIGO induced by immunosuppressants and antihypertensive drugs after a kidney transplant. Er,Cr:YSGG laser resection was then managed for gingival enlargements, resulting in obvious discomfort during and after operation. The patients actively cooperated with the doctors and recovered well post operation. Soares et al. (22) applied the Er,Cr:YSGG laser to treat the case of gingival enlargement in a child, which showed remarkable results. Besides, favorable gingival healing was observed upon oral examination in 1 week and 3 months of follow-up visits. This is also in agreement with the views mentioned earlier. Current studies have identified that the Er,Cr:YSGG laser, apart from being used for the resection of an enlarged gingiva, is also used for soft tissue mass resection of gingiva and for the treatment of oral mucosal diseases and hard tissue diseases with favorable therapeutic effects.
Patient Perspective
Due to its advantageous characteristics of efficient cutting function, less heat generation, less pain, active hemostasis, and coagulation, the Er,Cr:YSGG laser has been widely seen in oral and maxillofacial surgery, bone and soft tissue repair, and other oral surgeries (23). In addition, because of the safe, painless and comfortable treatment process, the fear of patients with regard to postoperative pain can be greatly alleviated, making it particularly suitable for children, the elderly and those incapable of tolerating pain. This also supports its promising application prospect. Although Er,Cr:YSGG laser equipment, as well as treatment cost, is huge, and surgeons are required to be trained specifically in its use, it is a tool that is safer, more efficient and more comfortable than others for the treatment of various oral diseases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board and human subject ethics board of Xiangya Stomatological Hospital of Central South University. Written informed consent was obtained from the patients for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Clinical Medical Technology Innovation Guide Project [No. 010092628040] and China Hunan Provincial Science & Technology Department.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yan L, Meng C, Xu Q. Etiology and treatment of drug-induced gingival hyperplasia. J Int J Stomatol. (2020) 47(06):693–8. doi: 10.7518/gjkq.2020097
2. Li X, Luan QX, Li P, Sha YQ, Wang SY, Cao CF. Analysis of risk indicators of drug-induced gingival hyperplasia induced by Nifedipine. J Chin J Stomatol. (2007) 42(11):677–80. doi: 10.3760/j.issn:1002-0098.2007.11.011
3. Kataoka M, Kido J, Shinohara Y, Nagata T. Drug-induced gingival overgrowth – a review. Biol Pharm Bull. (2005) 28(10):1817–21. doi: 10.1248/bpb.28.1817
4. Rapone B, Ferrara E, Santacroce L, Cesarano F, Arazzi M, Di Liberato L, et al. Periodontal microbiological status influences the occurrence of cyclosporine-A and tacrolimus-induced gingival overgrowth. Antibiotics (Basel). (2019) 8(3):124. doi: 10.3390/antibiotics8030124
5. Gong YM, Xu M, Gu ZY. Investigation of the prevalence of drug-induced gingival overgrowth in renal transplant recipients medicated with cyclosporine A or tacrolimus. J Stomatol. (2008) 17(2):121–4. doi: 10.3969/j.issn.1006-7248.2008.02.003
6. de Oliveira Costa F, Diniz Ferreira S, de Miranda Cota LO, da Costa JE, Aguiar MA. Prevalence, severity, and risk variables associated with gingival overgrowth in renal transplant subjects treated under tacrolimus or cyclosporin regimens. J Periodontol. (2006) 77(6):969–75. doi: 10.1902/jop.2006.050327
7. Bartoli F, Castronovo G, Stabile A. Risk factors conditioning the incidence and severity of cyclosporine A-induced gingival overgrowth and methods of prevention. Minerva Stomatol. (2004) 53(4):165–70.15107773
8. Weir MR, Burgess ED, Cooper JE, Fenves AZ, Goldsmith D, Dianne M, et al. Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. (2015) 26(6):1248–60. doi: 10.1681/ASN.2014080834
9. Trackman PC, Kantarci A. Molecular and clinical aspects of drug-induced gingival overgrowth. J Dent Res. (2015) 94(4):540–6. doi: 10.1177/0022034515571265
10. Liu H, Pan Y. Relationship between periodontal disease-causing microorganisms and drug-induced gingival hypertrophy. J Stomatol Res. (2015) 31(04):370–372 + 376. doi: 10.13701/j.cnki.kqyxyj.2015.04.015
11. Oliveira Costa F, Diniz Ferreira S, Pereira Lages EJ, Estáquio Costa J, Dutra Oliveira AMS, Miranda Cota LO. Demographic, pharmacologic, and periodontal variables for gingival overgrowth in subjects medicated with cyclosporin in the absence of calcium channel blockers. J Periodontol. (2007) 78(2):254–61. doi: 10.1902/jop.2007.050445
12. Ritchhart C, Joy A. Reversal of drug-induced gingival overgrowth by UV-mediated apoptosis of gingival fibroblasts – an in vitro study. Ann Anat. (2018) 217:7–11. doi: 10.1016/j.aanat.2018.01.001
13. Mavrogiannis M, Ellis JS, Seymour RA, Thomason JM. The efficacy of three different surgical techniques in the management of drug-induced gingival overgrowth. J Clin Periodontol. (2006) 33(9):677–82. doi: 10.1111/j.1600-051X.2006.00968.x
14. Muralikrishna T, Kalakonda B, Gunupati S, Koppolu P. Laser-assisted periodontal management of drug-induced gingival overgrowth under general anesthesia: a viable option. Case Rep Dent. (2013) 2013:387453. doi: 10.1155/2013/387453
15. Campos L, Gallottini M, Pallos D, Simões A, Martins F. High-power diode laser on management of drug-induced gingival overgrowth: report of two cases and long-term follow-up. J Cosmet Laser Ther. (2018) 20(4):215–19. doi: 10.1080/14764172.2017.1400165
16. To TN, Rabie AB, Wong RW, McGrath CP. The adjunct effectiveness of diode laser gingivectomy in maintaining periodontal health during orthodontic treatment. Angle Orthod. (2013) 83(1):43–7. doi: 10.2319/012612-66.1
17. Kazakova RT, Tomov GT, Kissov CK, Vlahova AP, Zlatev SC, Bachurska SY. Histological gingival assessment after conventional and laser gingivectomy. Folia Med (Plovdiv). (2018) 60(4):610–16. doi: 10.2478/folmed-2018-0028
18. Tao X, Yao JW, Wang HL, Huang C. Comparison of gingival troughing by laser and retraction cord. Int J Periodontics Restor Dent. (2018) 38(4):527–32. doi: 10.11607/prd.3551
19. Hegde R, Padhye A, Sumanth S, Sanjay JA, Thukral N. Comparison of surgical stripping; erbium-doped:yttrium, aluminum, and garnet laser; and carbon dioxide laser techniques for gingival depigmentation: a clinical and histologic study. J Periodontol. (2013) 84(6):738–48. doi: 10.1902/jop.2012.120094
20. Benjamin SD. Lasers in the dental office: treatment considerations for hard and soft tissue contouring. Pract Proced Aesthet Dent. (2003) 15(2):156.12772633
21. Liu H. Er,Cr: clinical application of YSGG laser in oral soft tissue surgery. J Stomatol. (2014) 34(03):200–3. doi: 10.13591/j.cnki.kqyx.2014.03.013
22. Soares FM, Tarver EJ, Bimstein E, Shaddox LM, Bhattacharyya I. Gingival overgrowth in a child with arthrogryposis treated with a Er,Cr:YSGG laser: a case report. Pediatr Dent. (2009) 31(1):8–13. doi: 10.1016/S0168-583X(98)00264-X
Keywords: laser therapy, drug-induced gingival overgrowth (DIGO), surgery, aesthetics, oral health
Citation: Liu YM, Peng Q, Liu BJ, Wang ZB and Cao Q (2022) Er,Cr:YSGG Laser Therapy for Drug-Induced Gingival Overgrowth: A Report of Two Case Series. Front. Surg. 9:922649. doi: 10.3389/fsurg.2022.922649
Received: 18 April 2022; Accepted: 2 May 2022;
Published: 24 May 2022.
Edited by:
Weiguo Li, Harbin Institute of Technology, ChinaReviewed by:
Zhengqiu Li, Hunan Children's Hospital, ChinaYunrun Liu, Hong Kong Baptist University, Hong Kong SAR, China
Copyright © 2022 Liu, Peng, Liu, Wang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Cao OTMyMDAxOEBjc3UuZWR1LmNu
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Yumei Liu1
Yumei Liu1 Binjie Liu
Binjie Liu Qiong Cao
Qiong Cao