- 1Department of Gastrointestinal Surgery, Northern Jiangsu People’s Hospital, Clinical Medical College, Yangzhou University, Yangzhou, China
- 2Department of Hernia Surgery, Northern Jiangsu People’s Hospital, Clinical Medical College, Yangzhou University, Yangzhou, China
Background: Retroperitoneal liposarcoma (RPLS) is a variety of soft tissue sarcoma that originates from mesenchymal cells. A tumor measuring greater than 30 cm is called a “giant liposarcoma.” A part of the neoplasm tends to grow in size, recur locally, or metastasize distantly. In those with such a condition, long-term survival is uncommon. Therefore, it is necessary to present a uniform and optimized program to improve the prognosis.
Methods: By successfully treating a multiple-recurrent giant retroperitoneal dedifferentiated liposarcoma (RP DDLPS) in July 2010, we hope to devise more comprehensive strategies to improve diagnosis, therapy, and outcome.
Results: In July 2010, we thoroughly resected a giant multifocal RPLS with a concomitant part of the gastric wall. The histopathological examination revealed a high-grade (grade III) dedifferentiated liposarcoma. The patient was discharged uneventfully on the 15th postoperative day. She relapsed after 16 months and needed another complete excision. After 9 months, she died after the fourth recidive. The patient had experienced four recurrences and underwent operations with 15 years of follow-up.
Conclusions: The above demonstrates that we were able to successfully treat the multirecurrent giant RPLS, despite the patient’s poor medical condition, with meticulous management. Moreover, this indicates that long-term survival could be achieved for high-grade RP DDLPS.
Introduction
Retroperitoneal sarcomas (RPSs) are rare malignancies that develop from mesenchymal tissues. They account for approximately 15% of all sarcomas, with an estimated incidence of 3–4/1,000,000 of the population per year (1). Liposarcoma is the most common RPS and accounts for 41% of all RPS (2). RPLS may frequently occur at any age, with a peak incidence between 40 and 60 years of age, and the distribution is equal between genders (3, 4). As protuberances develop in the vast, expandable retroperitoneum, they often present with atypical symptoms and grow to enormous proportions before being detected. Lewis reported that masses greater than 10 cm account for approximately 60% of all cases (5), while diameters greater than 30 cm are termed “giant liposarcoma” but are rarely diagnosed. RPLS is one of several pathological variants.
Furthermore, they tend to recur locally and distantly, possibly infiltrating adjacent organs or tissues, with the former being the primary cause of death (6, 7). The mainstay of treatment is en bloc resection of tumors and contiguous structures. Patients often undergo multiple operations after relapses, but the resection rate tends to decline gradually, leaving few long-term survivors (5). This paper focuses on the diagnosis, treatments, and prognosis of managing a multiple-recurrent giant RP DDLPS. Meanwhile, we also reviewed the literature of 12 cases with a giant RPLS measuring over 30 cm by searching English language articles through the PubMed database. Until now, this case represents the longest duration of follow-up in this variety of cancers reported in the literature in English.
Case report
Case description I
In July 2010, a 50-year-old female with a poor medical condition was admitted to our medical center with abdominal distension for 2 months. Her past surgical history included a complete resection of RPLS in 1997 with four courses of postoperative chemotherapy, another thorough excision for the first recurrence in 1999 with no further treatments, and laparoscopic cholecystectomy in 2003, all in other hospitals. Her family and psychosocial history were unremarkable, and she had no known genetic diseases. The physical examination revealed abdominal distension. Meanwhile, a diffuse and tough mass was palpated in the right abdomen with local tenderness and was stable with ill-defined margins. As for laboratory results, hemograms indicated mild anemia (hemoglobin 101 g/L), hepatorenal functions revealed hypoproteinemia (albumin 27 g/L), and tumor antigens showed CA125 185.80 KU/L. The chest radiography demonstrated bilateral pleural effusion and no space-occupying lesions.
The features of abdominal and pelvic contrast-enhanced computed tomography (CT) included multifocal, giant tumors of roundish shape with adipose density that were suspicious for liposarcoma (Figure 1A); the nodular masses existed in the liver–stomach clearance; the right kidney and liver were both compressed to deformation, and the hepatic cysts were obvious; the left kidney was dislocated to the intraperitoneal cavity, and abundant ascites was observed.
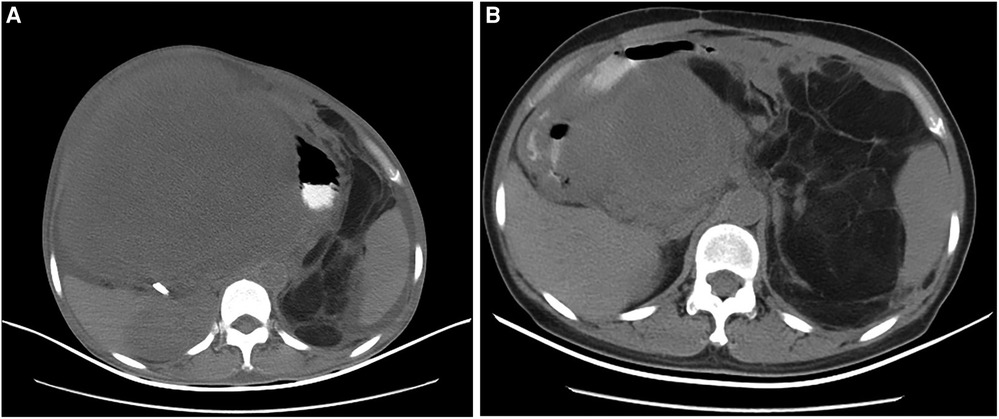
Figure 1. The imaging of CT scanning indicated for the second and third recurrence of the female patient in July 2010 and November 2011, respectively. (A) The giant tumors with adipose density occupied almost the entire abdominal cavity (July 2010); (B) The tumors originated from the hepatic hilar region and oppressed adjacent organs and tissues (November 2011).
After meticulous planning, we performed laparotomy through a right exploratory incision, indicating approximately 800 ml of light hemorrhagic ascites and extensive adherence of the omentum and intestinal loops. A giant tumor originated from the right middle and upper quadrant of the retroperitoneum, which crushed the liver upward to the right and posterior of the subdiaphragm, oppressed the flexura hepatica coli and abdominal wall rightward, pushed the mesocolon transversum downward to 10 cm below the umbilicus, and crushed the hepatogastric and gastrocolic ligaments anterior to the abdominal wall. The left margin of the tumor was adjacent to the hilus lienis. The tumor was shaped like a lobulated dumbbell, measuring 45 cm × 30 cm × 20 cm in size. Another yellow and white tumor in the mesocolon transversum measured 20 cm × 8 cm × 5 cm in diameter. Three small masses linked by the basis pontis of the greater gastric curvature extruded to the hilus lienis. In surgery, we separated gastrohepatic and hepatocolic ligaments, dissociated the capsule of the tumor, and separated the tumor from the adjacent tissues. Due to the enormous size, we had to separate a part of the mesocolon transversum and keep the tumor from metastasizing into it. We discovered that the tumor was hiding behind the transverse colon and the stomach, turned out it, and therefore, we detached the root of its capsule and severed and ligated the feeding veins before completely resecting the giant tumor. All tumors were well-encapsulated and completely removed with a concomitant part of the gastric wall (Figures 2A, B). The specimen weighed 6.65 kg, and the estimated blood loss was 2,800 mL.
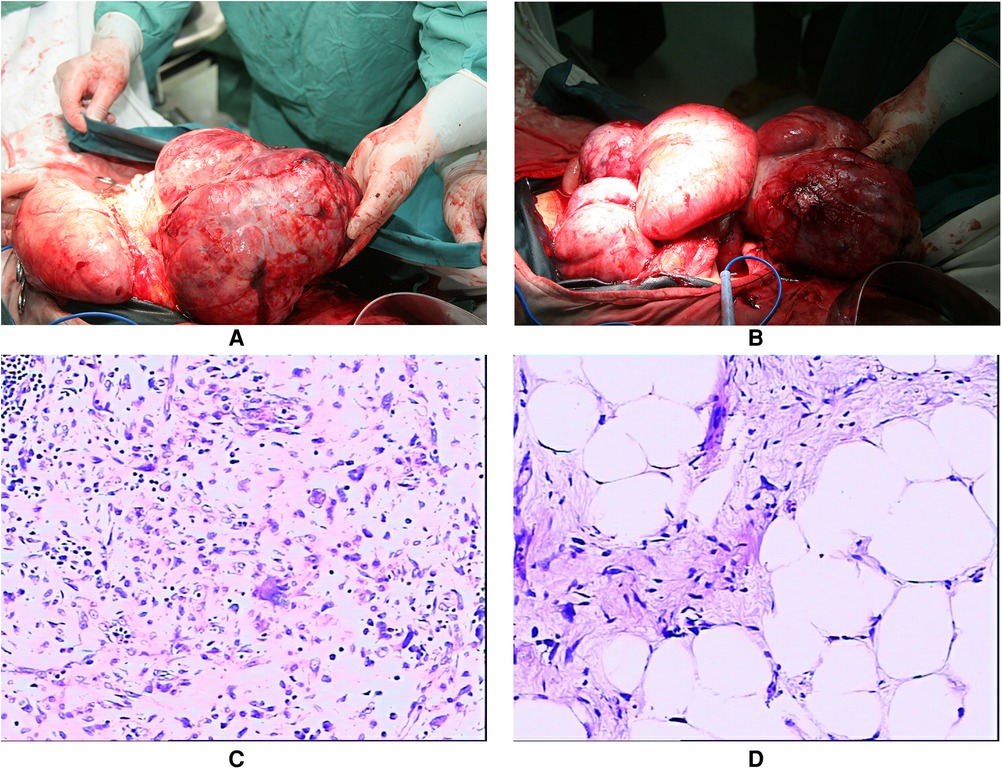
Figure 2. The gross appearance of the totally excised tumors presented in the operation (July 2010). (A) The giant tumor shaped like lobulated or dumbbell (measured 45×30×20 cm) with rich blood supply; (B) The multifocal tumors were well-encapsulated with macroscopic safe margins. The histopathological presentations of the tumors originated from the retroperitoneum and the tumors infiltrated the stomach (July 2010). (C) The retroperitoneal tumors were diagnosed as dedifferentiated liposarcoma; (D) Tumors of the stomach were diagnosed as sclerosing liposarcoma, dedifferentiation in focal areas.
The histopathological report indicated that in the retroperitoneal tumors, the oncocytes were heterogeneous and distributed like star networks with loose mesenchyme. Small fatty components were near the capsules in focal areas (diagnosed as dedifferentiated liposarcoma); in the tumors of the gastric wall, the nuclei were heterogeneous with clear cytoplasm. The lip blasts with abundant vessels were in focal areas (diagnosed as sclerosing liposarcoma, dedifferentiation in focal areas) (Figures 2C, D).
The patient was discharged on the 15th postoperative day with an uneventful course. The follow-up was done every 3 months for 16 months, and no signs of recurrence or metastasis were detected.
Case description II
The patient was readmitted to our hospital on November 2011. The abdominal and pelvic CT revealed multifocal, class-round tumors originating from the hepatic hilar region and oppressing the adjacent intestinal canal, caudate lobe, inferior vena cava (IVC), and aorta abdominalis (Figure 1B); another mass was located in the middle and lower abdomen; the tumors were all enhanced inhomogeneously; the left kidney was displaced anterior to the intraperitoneal cavity; and a class-round tumor and low density existed in the right ovary but was not enhanced, which was diagnosed as a recurrence of multifocal RPLS. The mass was considered a teratoma of the right ovary. She was submitted for another laparotomy. We resected a mass adjacent to the porta hepatis, a tumor and several small masses in the mesocolon transversum, and a tumor behind the mesosigmoid. The excised tumors measured 15 cm × 10 cm × 6 cm, 10 cm × 6 cm × 5 cm, and 8 cm × 5 cm × 5 cm, respectively. The pathological results confirmed dedifferentiated RPLS. She was discharged without incident but unfortunately died during the 4th recurrence in August 2012.
(The timeline is shown in Figure 3.)
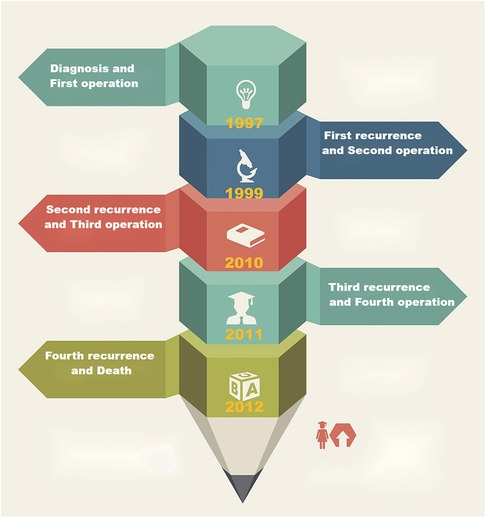
Figure 3. The timeline shows that the patient underwent operation four times and experienced recurrences, respectively, and 15 years of follow-up in all.
Literature review
In the literature in the English language published on PubMed from 1982 to December 2021, only 12 cases with giant RPLS greater than 30 cm in diameter have been reported (2, 8–18). Among the 13 patients (including ours), nine were male (69.2%) and four were female (30.8%), with a median age of 64 years (24–82). They mostly complained of vague symptoms such as abdominal discomfort and distension. CT imaging was the main examination for diagnosis, while only two patients (15.4%) received fine-needle aspiration cytology for the preoperative diagnosis. All the 13 patients underwent surgery, and seven of them (53.8%) were combined resections, including six nephrectomies (46.2%), one left colectomy (7.7%), one partial diaphragmatic resection (7.7%), one adrenalectomy (7.7%), and one part of gastric wall excision (7.7%). A total of 12 out of 13 cases (92.3%) achieved R0 resection. Furthermore, all 13 patients were discharged uneventfully without any complications. With regard to the histopathological types, six were dedifferentiated (46.2%), five were well-differentiated (38.5%), one was pleomorphic (7.7%), and one was well-differentiated/myxoid (7.7%). Meanwhile, in accordance with the grading system of the French Federation Cancer Centers (FNCLCC), six cases were grade I (46.2%), two cases were grade II (15.3%), and the remaining five cases were grade III (38.5%). In the group of 13 cases, one patient accepted neoadjuvant chemotherapy (7.7%) with no benefit, and one received postoperative radiotherapy (7.7%) but with no further evaluation. The months of follow-up were in the range of 8–181, during which two patients had recurrences at 16 and 21 months. Besides, two longer-surviving patients had local recurrences at 60, 108, and 120 and at 24, 156, 172, and 181 months after initial surgeries, respectively (Table 1).
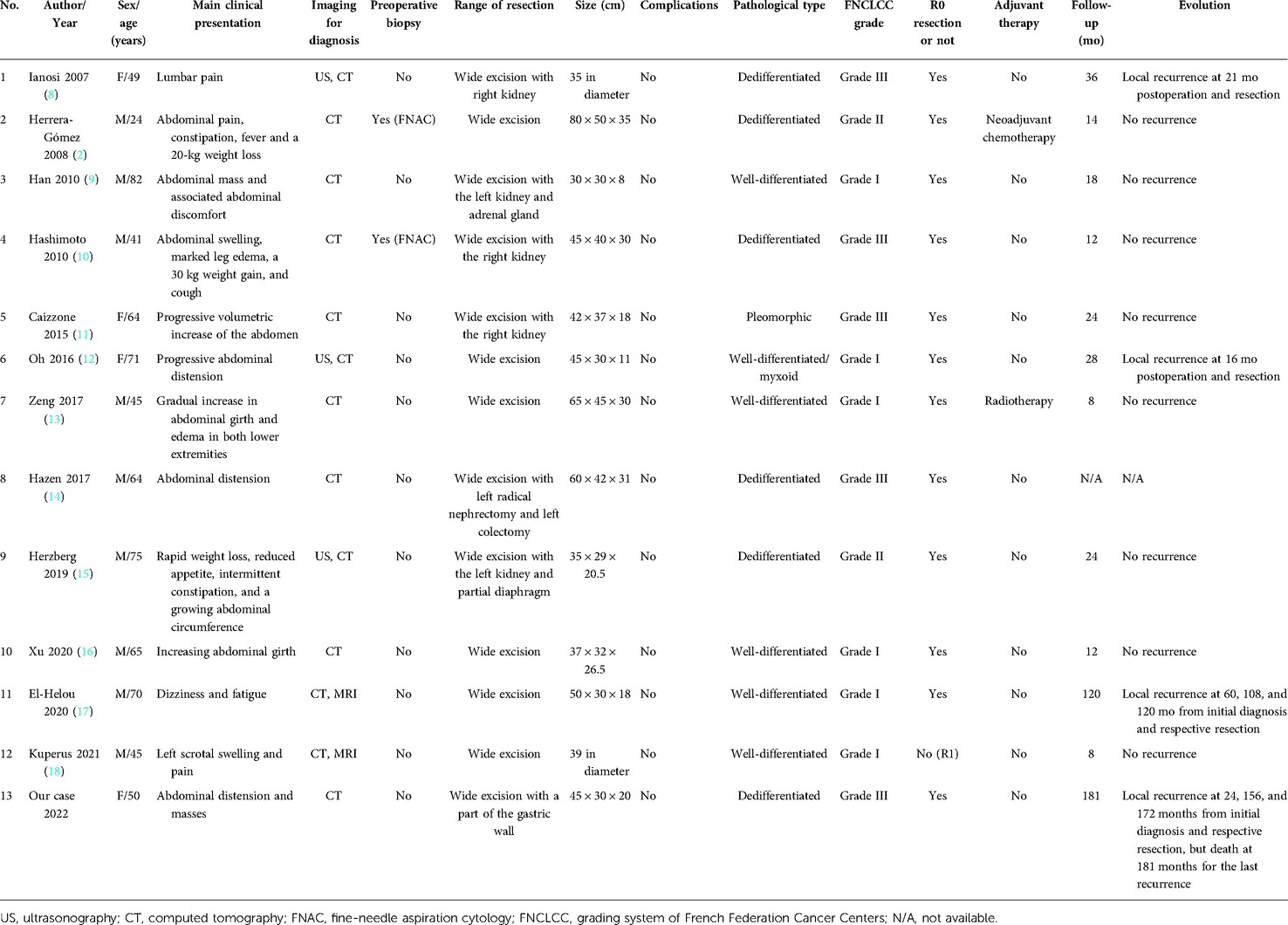
Table 1. Clinical characteristics, managements, histological features, follow-up, and outcomes of 13 patients with a giant RPLS measuring greater than 30 cm.
Discussion
We report a patient from China with multiple-recurrent and multi-operative giant RP DDLPS for a 15-year follow-up. She was first diagnosed in 1997, experienced four recurrences, and underwent subsequent surgeries four times. This case was rare due to the more challenging treatment involved and was, by far, the longest follow-up. The recurrence of giant RP DDLPS many times caused greater operative difficulties and a poorer prognosis. The 8-year overall survival (OS) rate is only 30% of the neoplasms with high grade III(GIII). Meanwhile, the high-grade II(GII) tumor usually causes death by recurrence or metastasis (7). When managed in our hospital in July 2010, the patient suffered from a poor medical condition during the perioperative period. The multifocal giant tumors invaded the stomach with 2800 mL of estimated blood loss, high-grade and advanced pathological presentations, and complicated surgery. However, we accomplished R0 resection and a smooth postoperative process, which would be instructive for improving the management standards of such complicated cases. Because of the fear of the disease and the pain of each operation, the patient felt miserable on account of all these recurrences. However, she felt no particular discomfort and recovered smoothly. The levels of adherence and tolerability of the patient were favorable. Moreover, there were no adverse or unanticipated events.
Soft tissue sarcomas account for less than 1% of all neoplasms (19). While RPLS is more uncommon and hardly diagnosed early, it progresses to present some manifestations such as stomachache, ventosity, palpable abdominal mass, compressed surrounding viscera, and gastrointestinal hemorrhage. Computed tomography (CT) or magnetic resonance imaging (MRI) is the primary diagnosis (20, 21). CT may frequently establish a preliminary diagnosis by indicating the location, size, consistency, and relationships with adjacent structures and can provide some proposals for surgery. For possible seeding in the needle tract and low accuracy in diagnosing RP DDLPS, we do not generally recommend core needle biopsy guided by CT (22, 23). However, it can be applied to preoperative radiotherapy and radiochemotherapy or target genomic therapy for unresectable tumors (24).
According to the 2013 World Health Organization (WHO) classification of soft tissue and bone tumors, RPLS is classified into four main subtypes: well-differentiated, myxoid/round cell, dedifferentiated, and pleomorphic (25). The 5-year survival rates of well-differentiated and myxoid/round cell variations are 90% and 60%–90%, respectively, with relatively better prognoses; the rates of dedifferentiated and pleomorphic varieties are 75% and 30%–50%, respectively (24). The pleomorphic subtype is most likely to metastasize distantly. A total of 83% of RP DDLPS patients experience locoregional recurrences, and 10%–15% of them develop distant metastasis postoperatively. Furthermore, 30% of recurrent tumors metastasize within the first 3 years (26). According to FNCLCC, RPLS is classified as grade I, II, and III (27). The well-differentiated and myxoid/round cell subtypes are low grade,, although the pleomorphic and dedifferentiated subtypes have higher grades with poorer outcomes (28). The histological subtype, grade, and complete surgical resection (R0) are the main prognostic factors (29). Moreover, the multifocal growth and rapid growth rate after recurrence (an average of more than 0.9 cm/month) are the influencing factors (30, 31).
Radical surgery, including invading tissues and organs, is the mainstay of treatment that can effectively cure RPLS, reduce the risk of a recurrence, and improve disease-free survival (DFS) and overall survival (OS) rates (32). Lewis reported a cohort of 500 patients with retroperitoneal soft-tissue sarcoma in a single institution. The median survival was 103 months for patients with thorough surgery vs. 18 months for those with incomplete resection (5). Zeng presented that they organized a multidisciplinary team to draw up a meticulous plan by establishing abdominal CT aortography and applying intraureteral catheterization to achieve complete resection (13). The application of adjuvant radiochemotherapy is still controversial (24). Haas reported that neoadjuvant radiotherapy might improve the local recurrence rate but with no benefit in OS (33). Postoperative radiotherapy, even if applied restrictedly, can cause some damage to the normal surrounding tissues and lead to related complications (34). The effectiveness of chemotherapy has not been clearly demonstrated, and this treatment has not been generally carried out so far; only in some isolated reports does it find a mention (35, 36).
Nevertheless, radiochemotherapy is still applied as palliative treatment to patients with unresectable or distant metastasis, which may improve the quality of life to some extent and prolong the period of survival(24). Research on target genomic therapy has become a hotspot, aiming to improve surgical outcomes in recent years in order to overcome the various limitations of radiochemotherapy. For instance, the dedifferentiated variety, aimed at the genes harboring the amplified sequences on chromosome 12(12q13–15), the antagonists or inhibitors of MDM2, CDK4, HMGA2, et al., and the ligand for PPAR-γ have been developed. However, to reduce the impact of the adverse effects and resistance of MDM2 inhibitors, we should pay more attention to translating research with YEATS4 knockdown and the genes outside the amplicon. Therefore, inhibiting JUN, DDR2, FGFR3, NTRK1, lowering or absenting ZIC-1, and recovering the normal expressions of RB1 and CEBPA are all research approaches(37).
However, our research did not involve any systematic and comprehensive study of the number of case limits, the patient's complicated illness, and managerial criteria. Nevertheless, it would provide some scientific evidence and support for the standardized management of such cases in our tertiary medical center or at WHO. In the future, we hope to establish multicenter databases, formulate standardized operative procedures, study target genomic therapy, and, if possible, normalize diagnostic and therapeutic programs.
Conclusion
An RPLS measuring greater than 30 cm in diameter is extremely rare and is considered a “giant liposarcoma.” The neoplasms tend to multiple-recur locally. Approximately 10%–15% of patients with RP DDLPS may develop distant metastasis, with rare chances of long-term survival. The primary diagnostic tools are CT and MRI scans. Complete surgical resection with infiltrated structures is the cornerstone of cancer and its locoregional relapses. The prognosis of RPLS is associated with pathological subtype, grade, and radical surgery (R0); hence, genomic treatment has been receiving a lot of attention recently. Given the recurrent tendency and genomic characteristics of RPLS, we may hope to establish genetic screening of primary and recurrent patients to prevent recidive by target therapy and consequently improve the prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary files, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Northern Jiangsu People's Hospital, Clinical Medical College, Yangzhou University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FF and HY analyzed the data. YT was the surgical assistant. HX reviewed the literature, designed the study, and drafted and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mettlin C, Priore R, Rao U. Results of the national soft tissue sarcoma registry. J Surg Oncol. (1982) 19:224–7. doi: 10.1002/jso.2930190410
2. Herrera-Gómez A, Ortega-Gutiérrez C, Betancourt AM, Luna-Ortiz K. Giant retroperitoneal liposarcoma. World J Surg Oncol. (2008) 6:115. doi: 10.1186/1477-7819-6-115
3. Mack TM. Sarcomas and other malignancies of soft tissue, retroperitoneum, peritoneum, pleura, heart, mediastinum, and spleen. Cancer. (1995) 75:211–44. doi: 10.1002/1097-0142(19950101)75:1+%3C211::AID-CNCR2820751309%3E3.0.CO;2-X
4. Mendenhall WM, Zlotecki RA, Hochwald SN, Hemming AW, Grobmyer SR, Cance WG. Retroperitoneal soft tissue sarcoma. Cancer. (2005) 104:669–75. doi: 10.1002/cncr.21264
5. Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. (1998) 228:355–65. doi: 10.1097/00000658-199809000-00008
6. Bagaria SP, Gabriel E, Mann GN. Multiply recurrent retroperitoneal liposarcoma. J Surg Oncol. (2018) 117:62–8. doi: 10.1002/jso.24929
7. Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg. (2016) 263:1002–9. doi: 10.1097/SLA.0000000000001447
8. Ianoşi G, Neagoe D, Buteică E, Ianoşi S, Drighiciu C, Stănoiu B, et al. Giant retroperitoneal sarcomas. Rom J Morphol Embryol. (2007) 48:303–8.
9. Han HH, Choi KH, Kim DS, Jeong WJ, Yang SC, Jang SJ, et al. Retroperitoneal giant liposarcoma. Korean J Urol. (2010) 51:579–82. doi: 10.4111/kju.2010.51.8.579
10. Hashimoto Y, Hatakeyama S, Tachiwada T, Yoneyama T, Koie T, Kamimura N, et al. Surgical treatment of a giant liposarcoma in a Japanese man. Adv Urol. (2010) 2010:943073. doi: 10.1155/2010/943073
11. Caizzone A, Saladino E, Fleres F, Paviglianiti C, Iaropoli F, Mazzeo C, et al. Giant retroperitoneal liposarcoma: case report and review of the literature. Int J Surg Case Rep. (2015) 9:23–6. doi: 10.1016/j.ijscr.2015.02.019
12. Oh SD, Oh SJ, Suh BJ, Shin JY, Oh CK, Park JK, et al. A giant retroperitoneal liposarcoma encasing the entire left kidney and adherent to adjacent structures: a case report. Case Rep Oncol. (2016) 9:368–72. doi: 10.1159/000447488
13. Zeng XY, Liu WZ, Wu XL, Gao JB, Zhang P, Shuai XM, et al. Clinicopathological characteristics and experience in the treatment of giant retroperitoneal liposarcoma: a case report and review of the literature. Cancer Biol Ther. (2017) 18:660–5. doi: 10.1080/15384047.2017.1345388
14. Hazen B, Cocieru A. Giant retroperitoneal sarcoma. J Gastrointest Surg. (2017) 21:602–3. doi: 10.1007/s11605-016-3258-0
15. Herzberg J, Niehaus K, Holl-Ulrich K, Honarpisheh H, Guraya SY, Strate T. Giant retroperitoneal liposarcoma: a case report and literature review. J Taibah Univ Med Sci. (2019) 14:466–71. doi: 10.1016/j.jtumed.2019.08.005
16. Xu C, Ma Z, Zhang H, Yu J, Chen S. Giant retroperitoneal liposarcoma with a maximum diameter of 37 cm: a case report and review of the literature. Ann Transl Med. (2020) 8:1248. doi: 10.21037/atm-20-1714
17. El-Helou E, Alimoradi M, Sabra H, Naccour J, Haddad MM, Bitar H. Recurrent giant retroperitoneal liposarcoma with 10 years follow up. Case report and review of literature. Int J Surg Case Rep. (2020) 75:504–12. doi: 10.1016/j.ijscr.2020.09.143
18. Kuperus JM, Steensma MR, Khachaturov V, Lane BR. Surgical management of a large retroperitoneal liposarcoma: a case study. Urol Case Rep. (2020) 34:101502. doi: 10.1016/j.eucr.2020.101502
19. WHO Classification of Tumours Editorial Board. WHO classification of tumours of soft tissue and bone. 5th ed. Lyon, France: IARC Press (2020).
20. Yu JSE, Colborne S, Hughes CS, Morin GB, Nielsen TO. The FUS-DDIT3 interactome in myxoid liposarcoma. Neoplasia. (2019) 21:740–51. doi: 10.1016/j.neo.2019.05.004
21. Abaricia S, Hirbe AC. Diagnosis and treatment of myxoid liposarcomas: histology matters. Curr Treat Options Oncol. (2018) 19:64. doi: 10.1007/s11864-018-0590-5
22. Clark MA, Thomas JM. Portsite recurrence after laparoscopy for staging of retroperitoneal sarcoma. Surg Laparosc Endosc Percutan Tech. (2003) 13:290–1. doi: 10.1097/00129689-200308000-00015
23. Ikoma N, Torres KE, Somaiah N, Hunt KK, Cormier JN, Tseng W, et al. Accuracy of preoperative percutaneous biopsy for the diagnosis of retroperitoneal liposarcoma subtypes. Ann. Surg. Oncol. (2015) 22:1068–72. doi: 10.1245/s10434-014-4210-8
24. Vijay A, Ram L. Retroperitoneal liposarcoma: a comprehensive review. Am J Clin Oncol. (2015) 38:213–9. doi: 10.1097/COC.0b013e31829b5667
25. Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. (2014) 64:38–52. doi: 10.1111/his.12311
26. Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype andmargin of resection predictpattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. (2003) 238:358–71. doi: 10.1097/01.sla.0000086542.11899.38
27. Matthyssens LE, Creytens D, Ceelen WP. Retroperitoneal liposarcoma: current insights in diagnosis and treatment. Front Surg. (2015) 2:4. doi: 10.3389/fsurg.2015.00004
28. Fletcher C, Unni K, Mertens F. Pathology and genetics of tumors of soft tissue and bone. In: Kleihues P, editors. World health organization classifification of tumors. Lyon: International Agency for Research on Cancer Press (2002). p. 427.
29. van Houdt WJ, Zaidi S, Messiou C, Thway K, Strauss DC, Jones RL. Treatment of retroperitoneal sarcoma: current standards and new developments. Curr Opin Oncol. (2017) 29:260–7. doi: 10.1097/CCO.0000000000000377
30. Anaya DA, Lahat G, Liu J, Xing Y, Cormier JN, Pisters PW, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decisionmaking. Ann Surg. (2009) 249:137–42. doi: 10.1097/SLA.0b013e3181928f2f
31. Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. (2009) 250:977–82. doi: 10.1097/SLA.0b013e3181b2468b
32. Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. (2013) 31:1649–55. doi: 10.1200/JCO.2012.44.3747
33. Haas RLM, Bonvalot S, Miceli R, Strauss DC, Swallow CJ, Hohenberger P, et al. Radiotherapy for retroperitoneal liposarcoma: a report from the transatlantic retroperitoneal sarcoma working group. Cancer. (2019) 125:1290–300. doi: 10.1002/cncr.31927
34. Nussbaum DP, Speicher PJ, Gulack BC, Ganapathi AM, Englum BR, Kirsch DG, et al. Long-term oncologic outcomes after neoadjuvant radiation therapy for retroperitoneal sarcomas. Ann Surg. (2015) 262:163–70. doi: 10.1097/SLA.0000000000000840
35. Bachmann R, Eckert F, Gelfert D, Strohäker J, Beltzer C, Ladurner R. Perioperative strategy and outcome in giant retroperitoneal dedifferentiated liposarcoma-results of a retrospective cohort study. World J Surg Oncol. (2020) 18:296. doi: 10.1186/s12957-020-02069-2
36. Yokoyama Y, Nishida Y, Ikuta K, Nagino M. A case of retroperitoneal dedifferentiated liposarcoma successfully treated by neoadjuvant chemotherapy and subsequent surgery. Surg Case Rep. (2020) 6:105. doi: 10.1186/s40792-020-00865-2
Keywords: giant retroperitoneal liposarcoma, recurrence, dedifferentiated, surgery, prognosis, computed tomography, target genomic therapy
Citation: Xia H, Fang F, Yuan H and Tu Y (2022) Survival of a patient with multiple-recurrent giant retroperitoneal dedifferentiated liposarcoma for 15 years: A case report. Front. Surg. 9:916802. doi: 10.3389/fsurg.2022.916802
Received: 10 April 2022; Accepted: 10 October 2022;
Published: 7 November 2022.
Edited by:
Alessandro Inserra, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Simone Frediani, Bambino Gesù Children's Hospital (IRCCS), ItalyGiorgio Persano, Bambino Gesù Children's Hospital (IRCCS), Italy
© 2022 Xia, Fang, Yuan and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Corresponding Author: Hao Xia YW5kcnV4aWFAMTYzLmNvbQ==
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Hao Xia
Hao Xia Fang Fang
Fang Fang Haijuan Yuan
Haijuan Yuan Yimei Tu
Yimei Tu