- 1Department of Liver Transplant, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Transplant Medical Research Center, The Second Xiangya Hospital of Central South University, Changsha, China
Background: A large spontaneous splenorenal shunt (SRS) will greatly impact portal inflow to the graft during liver transplantation (LT). Direct ligation of a large SRS is an uncommon surgical procedure and the hemodynamic consequences of this procedure are unknown.
Methods: In this retrospective study, we described our technique for direct ligation of a large SRS and the consequent hemodynamic changes during LT. 3-Dimensional computed tomography and Doppler ultrasonography were used to evaluate SRS and portal vein blood flow volume (PFV).
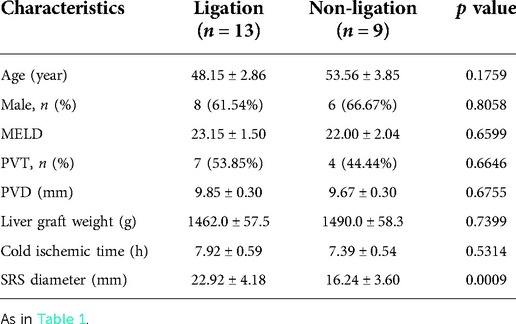
Results: A total of 22 recipients had large SRS including 13 with PFV <85 ml/min/100 g (ligation group) and 9 with PFV ≥85 ml/min/100 g (no ligation group). The diameter of SRS was significantly larger in the ligation group than in the non-ligation group (22.92 ± 4.18 vs. 16.24 ± 3.60 mm; p = 0.0009). In all ligation patients, the SRS was easily identified and isolated, it was located just below the distal pancreas and beside the inferior mesenteric vein. PV flow increased significantly from 68.74 ± 8.77 to 116.80 ± 16.50 ml/min/100 g (p < 0.0001) after ligation; this was followed by a reduction in peak systolic velocity of the hepatic artery from 58.17 ± 14.87 to 46.67 ± 13.28 cm/s (p = 0.0013).
Conclusions: Direct ligation of large SRS was an effective and safe surgical procedure to overcome the problem of portal hypoperfusion during LT.
Introduction
Whole liver transplantation (LT) requires a hepatopetal portal inflow of at least 1000 ml/min to ensure proper liver function (1, 2). The portal vein (PV) flow to the liver graft is mainly determined by graft weight and quality, the patency of the portomesenteric venous system, diameter of the recipient's PV, and presence of portosystemic shunt (3, 4). A spontaneous splenorenal shunt (SRS) was found in 20%–35% of LT candidates (4–6). The presence of a large portosystemic shunt (>10 mm in diameter) will greatly impact portal inflow to the graft during LT (4, 6), potentially causing portal flow steal and leading to PV thrombus, hepatic encephalopathy, and graft dysfunction if the shunt is left without intervention (7–9).
Surgical procedures used for SRS during LT include renoportal anastomosis (RPA) (4, 10, 11), left renal vein ligation (LRVL) (4, 11, 12), splenectomy (13), and splenic vein ligation (11). There have been relatively few reports of direct SRS ligation (6) and these studies only focused on the surgical technique, with no details on the hemodynamic consequences of the procedure. In this retrospective study, we described our technique for direct ligation of a large SRS during LT and the consequent hemodynamic changes.
Materials and methods
Study protocol
Three-dimensional computed tomography (3D CT) and Doppler ultrasonography (US) were used to evaluate the SRS. A large SRS was defined as a shunt with diameter >10 mm before transplantation. Between January 1, 2017 and December 31, 2020, 720 adults (age ≥18 years) underwent deceased donor LT at the Second Xiangya Hospital of Central South University. A total of 22 recipients (3.05%) who were found to have a large SRS (including 1 patient with a large gastrorenal shunt [GRS]) were retrospectively analyzed. The study protocol conformed to the ethics guidelines of the 1975 Declaration of Helsinki and was approved by Institutional Review Board of the Second Xiangya Hospital of Central South University (No. 2019–050). Written informed consent was obtained from all participants. The transplantations were performed according to the Declaration of Istanbul, and no executed prisoners were used as donors.
Intraoperative hepatic flow measurement
Intraoperative and postoperative PV blood flow volume (PFV) was evaluated by color Doppler ultrasound (US). 3D CT was routinely performed before and after LT to assess portosystemic shunts. The Doppler US parameters for blood flow features were calculated using the Logiq P5 US System (GE Healthcare, Little Chalfont, UK). PFV was calculated as cross-sectional area × portal blood velocity (angle-corrected) (14). Donor livers were weighed after back-table preparation. PFV was indexed to the graft weight (ml/min/100 g). Hepatic artery (HA) flow was determined based on the inner diameter of the HA, angle-corrected peak systolic velocity (PSV), end diastolic velocity (EDV), and resistive index (RI). To better evaluate the perfusion of the liver grafts and minimize the influence of graft weight on the measured flows, PFV (ml/min/100 g) in the non-SRS cohort 100 deceased adult liver transplant donors were presented before the study of SRS ligation was done. The median PFV was 124 ml/min/100 g (range, 46∼238 ml/min/100 g); only 10% of patients had a value <85 ml/min/100 g before skin closure. Based on these data and those from previous studies (1, 3), 85 ml/min/100 g was used as the PFV threshold for direct ligation of large SRS in this study.
Surgical technique
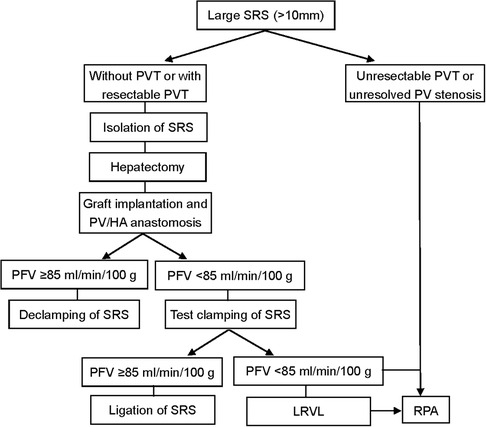
The operations were performed by the same surgical team with the same classical orthotopic technique or piggyback methods. The flowchart for the surgical management of large SRS is shown in Figure 1. After removing the recipient's diseased liver, the SRS was identified and isolated as previously described (6). The SRS was located just below the distal pancreas and beside the inferior mesenteric vein. If there was PV stenosis or thrombosis, we performed PV thrombectomy and/or angioplasty. With the recipient liver removed, the donor liver was anastomosed to the appropriate structures for placement in an orthotopic position. The new liver was then allowed to reperfusion and the HA was anastomosed. We then evaluated PV and HA flow by Doppler US, with test clamping of the SRS if PFV was <85 ml/min/100 g. When there was a marked increase in PFV (≥85 ml/min/100 g), the SRS was ligated proximally to the juncture with the left renal vein (LRV) using nonabsorbable sutures.
Liver biopsy evaluation and postoperative management
Liver biopsy routinely performed at the end of liver graft harvesting and LT. The same (blinded) pathologist evaluated all liver samples. Grafts were also evaluated for the presence of ischemia reperfusion injurys (IRI). IRI defined by the combination of apoptotic hepatocytes and inflammatory neutrophilic polynuclear's infiltrate throughout the lobule and around centrilobular veins. An IRI was graded as absent, mild, moderate, or severe. Severe IRI required centrocentrilobular necrosis (4). 3D CT was routinely performed on post-operative days (POD) 7 to assess the changes of collaterals and portal flow. Daily color Doppler US, biological tests and close clinical surveillance were conducted until POD 7, and then once every 3 days until discharge. After their discharge, all patients regularly visited our outpatient department: once a month for the first 6 months, and then once every 3 months.
Data analysis
Values were expressed as mean ± SD. Statistical analysis of hemodynamic consequences were performed with the Student's t test. SPSS 11.0 (IBM Corp., USA) statistical software were used for statistical analyses and differences with p < 0.05 were considered statistically significant.
Results
The preoperative characteristics and flow measurements of cases with large SRS are shown in Tables 1, 2. To illustrate the SRS ligation procedure and the impact on PVF during LT, each figure corresponds to a case listed in Table 1 (ie, Figure 2 correspond to case no. 5, Figures 3, 4 correspond to case no. 13 and Figure 5 correspond to case no. 7). The SRS was located just below the distal pancreas and beside the inferior mesenteric vein (Figures 2B, 3A, 5C). They were easily identified and isolated, and no procedure-related complications were found in patients who underwent direct SRS ligation.
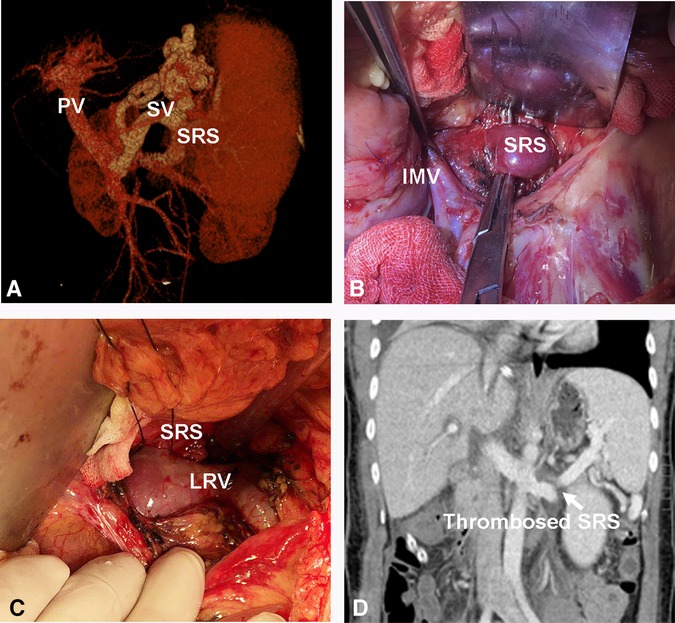
Figure 2. Direct ligation of a large SRS. (A) Large SRS before transplantation. (B, C) Isolation and ligation of large SRS. (D) Thrombosed SRS on day 7 post transplantation. SV, Splenic vein; IMV, inferior mesenteric vein.
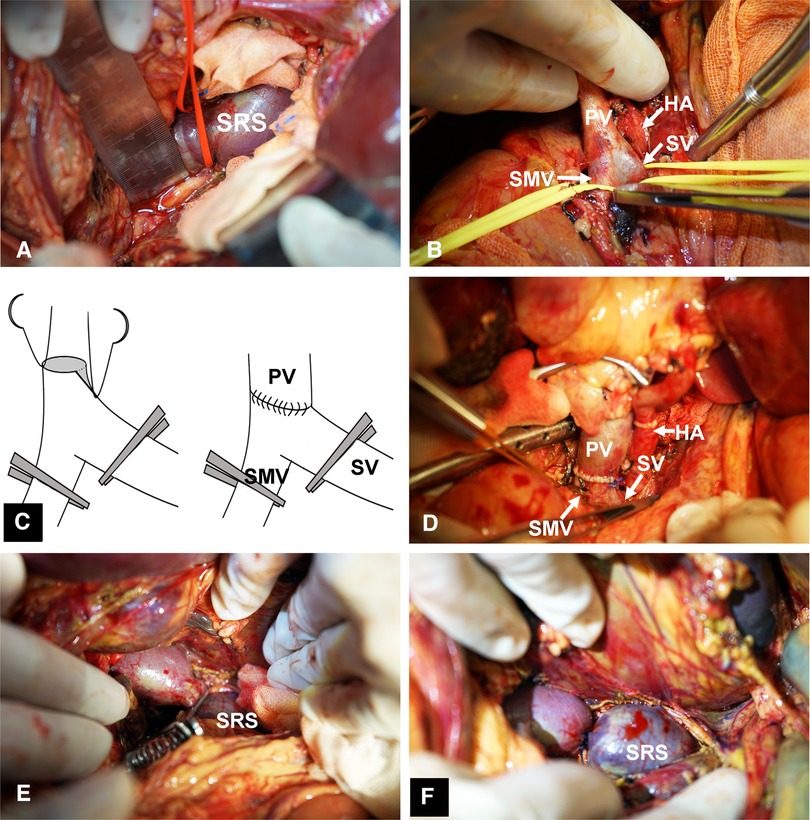
Figure 3. Surgical procedure for SRS ligation. (A) Identification and isolation of the large SRS. (B) Small diameter of PV,the isolation of SMV, and SV behind the neck of pancreas. (C) Angioplasty of the PV. (D) Portal vein anastomosis to the confluence of SV and SMV with satisfactory results. (E) Test clamping of SRS. (F) Ligation of SRS. SMV, superior mesenteric vein.
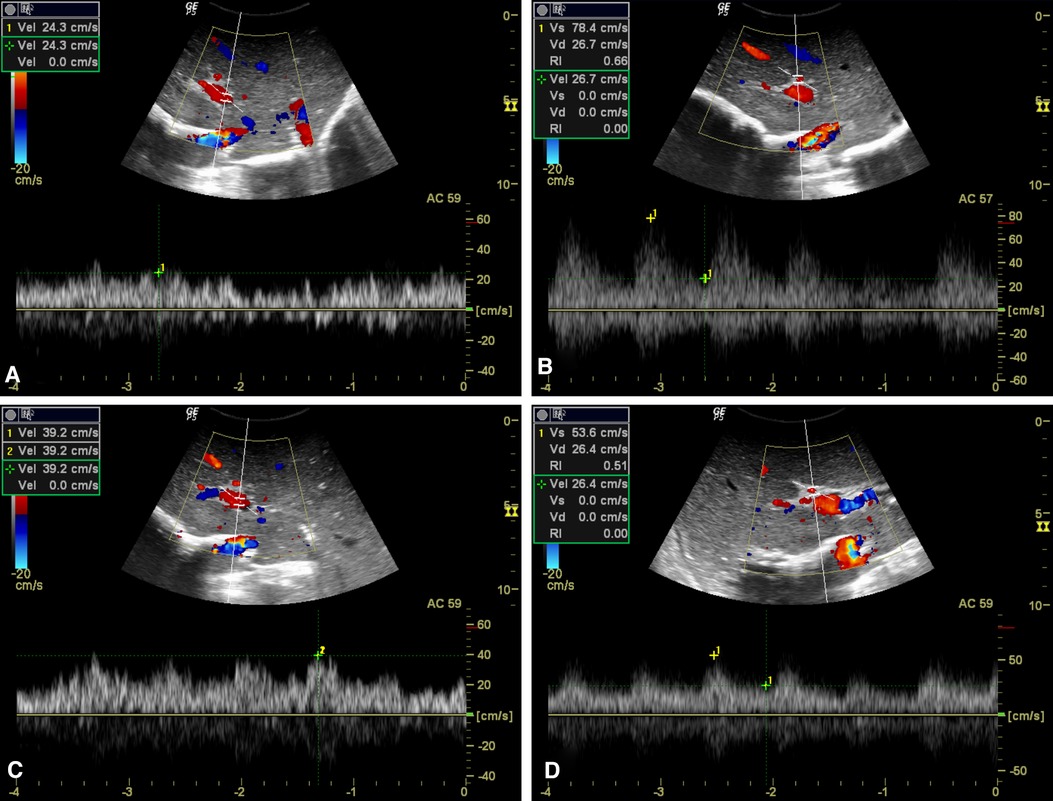
Figure 4. Hemodynamic consequences of test clamping of SRS. (A, C) PV flow increased markedly after test clamping of the SRS, from 24.3 cm/s (A) to 39.2 cm/s (C). (B, D) Decreased PSV of HA in response to SRS blocking from 78.4 cm/s (B) to 53.6 cm/s (D).
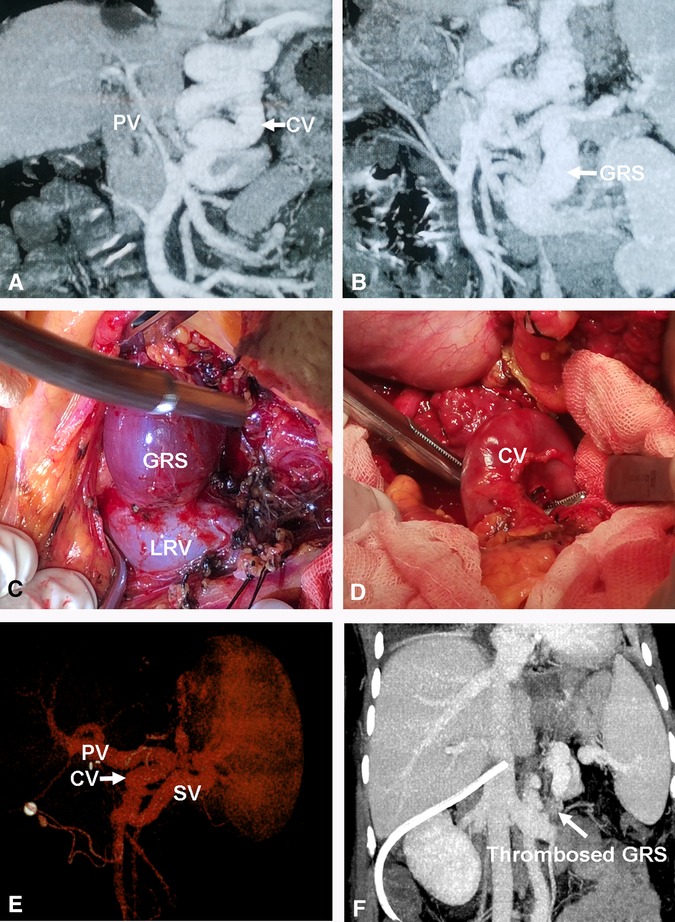
Figure 5. Direct ligation of a large GRS and reconstruction of gastric CV with PV. (A) Small diameter PV or cavernous transformation of PV. (B) Large GRS before transplantation. (C) Isolation of large GRS. (D) Isolation of gastric CV. (E) The images of the PV reconstruction with recipient gastric CV and graft PV on 3D CT. (F) Thrombosed GRS on day 7 post transplantation. CV, coronary vein; GRS, gastrorenal shunt.
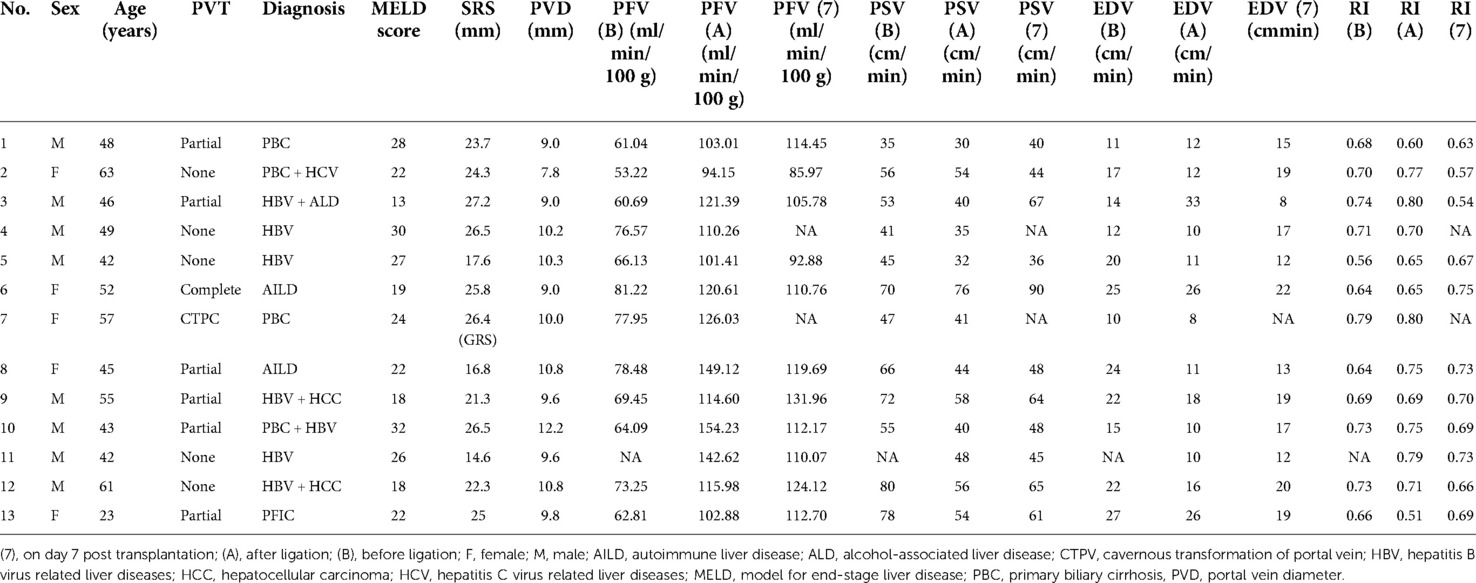
Table 1. Preoperative characteristics and flow measurements of liver transplant recipients with direct ligation of a large splenorenal shunt.
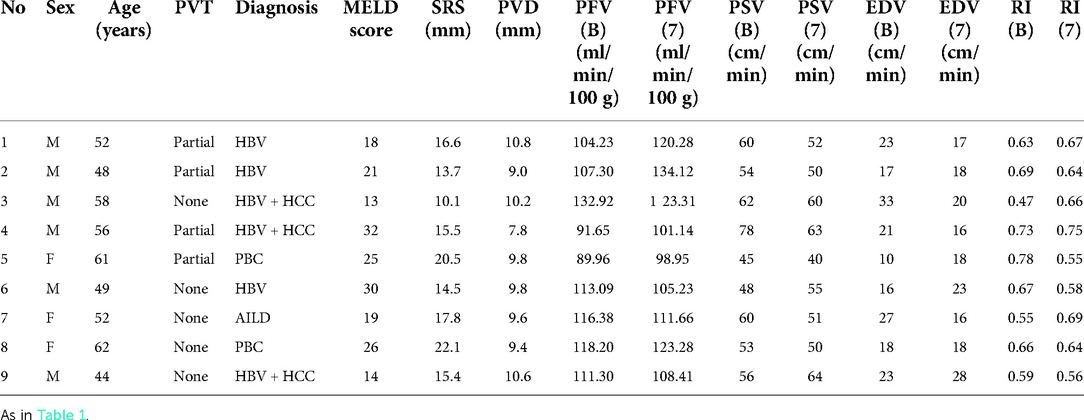
Table 2. Preoperative characteristics and flow measurements of liver transplant recipients without ligation of large splenorenal shunt.
There were 13 patients with PFV <85 ml/min/100 g (ligation group) and 9 with PFV ≥85 ml/min/100 g (non-ligation group). As shown in Table 3, recipients with ligation group and non-ligation group were similar in age, end-stage liver disease (MELD) score, the portal vein diameter (PVD), and the incidence of portal vein thrombosis (PVT). Donors to patients in the two recipient groups showed no differences in liver graft weight and cold ischemia time (Table 3). Liver grafts with mild-moderate steatosis were similar in the 2 groups, and grafts with severe steatosis did not included in the 2 groups. Only 1 liver graft was found severe IRI with centrocentrilobular necrosis, and this graft was in ligation group. However, SRS diameter was significantly larger in the ligation group than that in the non-ligation group (Table 3).
PFV was elevated in all cases with SRS occlusion and increased significantly from 68.74 ± 8.77 to 116.80 ± 16.50 ml/min/100 g (p < 0.0001) after ligation (Table 1 and Figure 4). On day 7 after transplantation, PFV was decreased compared to immediately after SRS ligation, although the difference was not statistically significant (Table 1). The PSV of the HA decreased immediately after SRS ligation from 58.17 ± 14.87 to 46.67 ± 13.28 cm/s (p = 0.0013), with no significant difference in PSV immediately after ligation as compared to day 7 post transplantation (Table 1). In contrast, SRS clamping had no effect on EDV and RI during LT (Table 1). As shown in Table 2, there was no difference between the intraoperative PFV and the PFV on day 7 post transplantation (107.90 ± 14.86 vs. 113.50 ± 12.93 ml/min/100 g; p = 0.3173) in the non-ligation group.
One patient had a large spontaneous GRS, and the recipient gastric coronary vein was end-to-end anastomosed with the graft PV; after clamping the GRS, PFV increased from 77.95 to 126.03 ml/min/100 g (Figure 5).
The median follow-up was 31.36 months (range: 10–54 months); at the time of writing, no HA flow-related biliary complications or SRS ligation-related kidney injury occurred in 13 patients, 21 of the 22 patients were alive with no evidence of PV complications. One patient (ligation group) died of severe infection during the perioperative period but this was unrelated to the procedure of SRS ligation.
Discussion
Spontaneous portosystemic shunts such as SRS are portocaval communications in patients with chronic liver disease and portal hypertension (15). A large SRS was observed in 3.05% of liver transplant recipients in our study, although a much higher incidence has been reported in patients with chronic liver disease (16). In the present study, the diameter of SRS was larger in the ligation group than that in the non-ligation group, suggesting that SRS diameter is associated with PFV reduction during LT. PFV increased nearly 2 folds when the shunt was occluded by SRS clamping, an effect that persisted to the day 7 post transplantation. When the SRS was clamped, the increase in PV flow was followed by a reduction in the PSV of the HA, consistent with the previously reported hepatic arterial buffer response to PV flow (17).
In the present study, no SRS ligation-related kidney injury or HA flow-related biliary complications were occurred in 13 patients. For patients without PVT or with resectable PVT, LRVL is the recommended surgical treatment for SRS in LT, however, previous studies have shown that ligation of the left RV can lead to sustained and elevated serum creatinine levels and decreased kidney size in recipients (12, 18). RPA, in which the donor PV is anastomosed end-to-end with the left renal vein, is another effective treatment for SRS, especially in patients with PVT. However, the procedure is complicated and may require an additional interposed vein graft to connect the left RV to the PV. Additionally, because PV flow is derived from both a large SRS and the left renal vein, portal hyperperfusion can occur during living donor LT (PFV >250 ml/100 g/min) (11, 19). A recent study reported a 31.3% (5/16) incidence of upper gastrointestinal bleeding and 43.8% (7/16) incidence of postoperative ascites when portal hypertension was not fully relieved after RPA (20). In our patients, direct ligation of a large SRS was performed safely and with satisfactory PV flow and no instance of either portal hypoperfusion or PV hyperinflow.
The hepatic arterial buffer response is an intrinsic mechanism of the liver to maintain total hepatic blood flow when portal perfusion decreases (21). Adenosine is a potent vasodilator that acts directly on the HA but not the PV; it is secreted at a constant rate and is washed away by PV flow. A reduction in PV flow leads to accumulation of adenosine and dilation of the HA (22). The occurrence and degree of the arterial buffer response should be considered when deciding whether the SRS should be ligated, as a significant reduction in HA flow can increase the risk of biliary complications during LT (23).
In all of our patients, the SRS was located below the distal pancreas and beside the inferior mesenteric vein, it was easily identified by locating and following the LRV. The retroperitoneal tissue was relatively loose, and the SRS was always free without extra branches, thus, the large SRS was not technically difficult to identify, nor was it dangerous to isolate. In the present study, no procedure-related complications occurred in patients who underwent direct SRS ligation. However, a patent PV is mandatory for direct ligation of the SRS. Additionally, SRS ligation should not be attempted in patients with unresectable PVT or in whom angioplasty of PV stenosis has failed.
There were several limitations to the study. Firstly, it was designed as a retrospective analysis of a relatively small number of patients. Secondly, the judgment of portal vein blood flow by Doppler US is not very precise, while measuring PFV by ultrasonic flowmetry is more objective, this was not always possible at our center. Thirdly, the PFV reference value of 85 ml/min/100 g of our center was calculated from a historical non-SRS cohort of 100 deceased adult liver transplant donors. However, a previous study used PFV <1200 ml/min after reconstruction as a reference value to determine whether the SRS was disconnected by LRVL (17). Prospective studies with a larger sample size are needed to establish more reasonable reference values.
In conclusion, we demonstrated that direct ligation of a large SRS was effective in achieving nearly 2 folds portal inflow. The large SRS was easily identified and isolated, and no SRS ligation-related complications injury were found in the ligation group. Additionally, the surgical procedure for SRS ligation is in line with normal physiology and minimally invasive. Direct ligation of large SRS could be an effective and safe surgical procedure to overcome the problem of portal hypoperfusion during LT.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Second Xiangya Hospital of Central South University (IRB no: 2019–050). The patients/participants provided their written informed consent to participate in this study.
Author contributions
GC and QL: design and performance of experiments, data collection and analysis, manuscript preparation. ZQ, JL and BX: conduct of some experiments and data analysis. ZS: general guidance, concept development. JL: overall supervision, design, data analysis, funding support, and manuscript preparation and finalization. All authors contributed to the article and approved the submitted version.
Fundings
This work was supported by Hunan Provincial Key Research and Development Programme (Grant No. 2022SK2040), National Natural Science Foundation of China (Grant No. 82070679) and Natural Science Foundation of Hunan Province, China (Grant No. 2019JJ50870).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Spitzer AL, Dick AA, Bakthavatsalam R, Halldorson JB, Salvalaggio PR, Reyes JD, et al. Intraoperative portal vein blood flow predicts allograft and patient survival following liver transplantation. HPB (Oxford). (2010) 12:166–73. doi: 10.1111/j.1477-2574.2009.00137.x
2. Aucejo FN, Hashimoto K, Quintini C, Kelly D, Vogt D, Winans C, et al. Triple-phase computed tomography and intraoperative flow measurements improve the management of portosystemic shunts during liver transplantation. Liver Transpl. (2008) 14:96–9. doi: 10.1002/lt.21377
3. Sainz-Barriga M, Reyntjens K, Costa MG, Scudeller L, Rogiers X, Wouters P, et al. Prospective evaluation of intraoperative hemodynamics in liver transplantation with whole, partial and DCD grafts. Am J Transplant. (2010) 10:1850–60. doi: 10.1111/j.1600-6143.2010.03207.x
4. Golse N, Bucur PO, Faitot F, Bekheit M, Pittau G, Ciacio O, et al. Spontaneous splenorenal shunt in liver transplantation: results of left renal vein ligation versus renoportal anastomosis. Transplantation. (2015) 99:2576–85. doi: 10.1097/TP.0000000000000766
5. Awad N, Horrow MM, Parsikia A, Brady P, Zaki R, Fishman MD, et al. Perioperative management of spontaneous splenorenal shunts in orthotopic liver transplant patients. Exp Clin Transplant. (2012) 10:475–81. doi: 10.6002/ect.2011.0201
6. Kim H, Yoon KC, Lee KW, Yi NJ, Lee HW, Choi Y, et al. Tips and pitfalls in direct ligation of large spontaneous splenorenal shunt during liver transplantation. Liver Transpl. (2017) 23:899–906. doi: 10.1002/lt.24783
7. Braun MM, Bar-Nathan N, Shaharabani E, Aizner S, Tur-Kaspa R, Belenky A, et al. Postshunt hepatic encephalopathy in liver transplant recipients. Transplantation. (2009) 87:734–9. doi: 10.1097/TP.0b013e318196340d
8. De Carlis L, Del Favero E, Rondinara G, Belli LS, Sansalone CV, Zani B, et al. The role of spontaneous portosystemic shunts in the course of orthotopic liver transplantation. Transpl Int. (1992) 5:9–14. doi: 10.1007/BF00337182
9. Margarit C, Lazaro JL, Charco R, Hidalgo E, Revhaug A, Murio E. Liver transplantation in patients with splenorenal shunts: intraoperative flow measurements to indicate shunt occlusion. Liver Transpl Surg. (1999) 5:35–9. doi: 10.1002/lt.500050114
10. Marubashi S, Dono K, Nagano H, Gotoh K, Takahashi H, Hashimoto K, et al. Living-donor liver transplantation with renoportal anastomosis for patients with large spontaneous splenorenal shunts. Transplantation. (2005) 80:1671–5. doi: 10.1097/01.tp.0000185087.93572.1d
11. Kisaoglu A, Dandin O, Demiryilmaz I, Dinc B, Adanir H, Yilmaz VT, et al. A single-center experience in portal flow augmentation in liver transplantation with prior large spontaneous splenorenal shunt. Transplant Proc. (2021) 53:54–64. doi: 10.1016/j.transproceed.2020.05.015
12. Lee SG, Moon DB, Ahn CS, Kim KH, Hwang S, Park KM, et al. Ligation of left renal vein for large spontaneous splenorenal shunt to prevent portal flow steal in adult living donor liver transplantation. Transpl Int. (2007) 20:45–50. doi: 10.1111/j.1432-2277.2006.00392.x
13. Golse N, Mohkam K, Rode A, Mezoughi S, Demian H, Ducerf C, et al. Surgical management of large spontaneous portosystemic splenorenal shunts during liver transplantation: splenectomy or left renal vein ligation? Transplant Proc. (2015) 47:1866–76. doi: 10.1016/j.transproceed.2015.06.019
14. Brown HS, Halliwell M, Qamar M, Read AE, Evans JM, Wells PN. Measurement of Normal portal venous blood flow by Doppler ultrasound. Gut. (1989) 30:503–9. doi: 10.1136/gut.30.4.503
15. Nardelli S, Riggio O, Gioia S, Puzzono M, Pelle G, Ridola L. Spontaneous porto-systemic shunts in liver cirrhosis: clinical and therapeutical aspects. World J Gastroenterol. (2020) 26:1726–32. doi: 10.3748/wjg.v26.i15.1726
16. Achiwa S, Hirota S, Kako Y, Takaki H, Kobayashi K, Yamakado K. Radiological anatomy of spontaneous splenorenal shunts in patients with chronic liver disease. Jpn J Radiol. (2017) 35:206–14. doi: 10.1007/s11604-017-0623-1
17. Castillo-Suescun F, Oniscu GC, Hidalgo E. Hemodynamic consequences of spontaneous splenorenal shunts in deceased donor liver transplantation. Liver Transpl. (2011) 17:891–5. doi: 10.1002/lt.22304
18. Ikegami T, Shirabe K, Nakagawara H, Yoshizumi T, Toshima T, Soejima Y, et al. Obstructing spontaneous major shunt vessels is mandatory to keep adequate portal inflow in living-donor liver transplantation. Transplantation. (2013) 95:1270–7. doi: 10.1097/TP.0b013e318288cadc
19. Troisi R, de Hemptinne B. Clinical relevance of adapting portal vein flow in living donor liver transplantation in adult patients. Liver Transpl. (2003) 9:S36–41. doi: 10.1053/jlts.2003.50200
20. D'Amico G, Matsushima H, Diago Uso T, Armanyous SR, Hashimoto K, Eghtesad B, et al. Long term outcomes and complications of reno-portal anastomosis in liver transplantation: results from a propensity score-based outcome analysis. Transpl Int. (2021) 34:1938–47. doi: 10.1111/tri.13920
21. Gulberg V, Haag K, Rossle M, Gerbes AL. Hepatic arterial buffer response in patients with advanced cirrhosis. Hepatol. (2002) 35:630–4. doi: 10.1053/jhep.2002.31722
22. Lautt WW. The 1995 ciba-geigy award lecture. Intrinsic regulation of hepatic blood flow. Can J Physiol Pharmacol. (1996) 74:223–33. doi: 10.1139/y96-029
Keywords: portal blood flow volume, liver transplantation, hemodynamic consequences, spontaneous splenorenal shunts, portal vein thrombus
Citation: Chen G, Li Q, Zhang Z, Xie B, Luo J, Si Z and Li J (2022) Hemodynamic alterations with large spontaneous splenorenal shunt ligation during adult deceased donor liver transplantation. Front. Surg. 9:916327. doi: 10.3389/fsurg.2022.916327
Received: 9 April 2022; Accepted: 27 September 2022;
Published: 17 October 2022.
Edited by:
Stefano Olmi, Policlinico San Marco, ItalyReviewed by:
Xiaofeng Zhu, The First Affiliated Hospital of Sun Yat-sen University, ChinaAfshin Parsikia, Einstein Healthcare Network, United States
Jinyang Gu, Shanghai Jiao Tong University, China
Lai Wei, Huazhong University of Science and Technology, China
© 2022 Chen, Li, Zhang, Xie, Luo, Si and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiequn Li bGVlamllcXVuQGNzdS5lZHUuY24=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Guangshun Chen1,2,†
Guangshun Chen1,2,† Zhongqiang Zhang
Zhongqiang Zhang Jiequn Li
Jiequn Li