- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Gastrointestinal Cancer Center, Peking University Cancer Hospital & Institute, Beijing, China
Background: Peritoneal carcinomatosis (PC) of gastric cancer indicates a poor outcome and is mainly diagnosed by staging laparoscopy (SL). This study was designed to develop a risk stratification model based on the number of risk factors to exempt low-risk patients from unnecessary SL.
Methods: This was a retrospective cohort study based on a single institution between January 2015 and December 2019. SL is indicated for patients of advanced locoregional stage, and clinicopathologic characteristics of 535 consecutive patients were included. PC-associated variables were identified by logistic regression analysis. A risk stratification model based on the number of risk factors was constructed, and we defined its predictive value with a receiver operating characteristic (ROC) curve and negative predictive value.
Results: In total, 15.9% of included patients were found to have PC during SL. Borrmann type IV, elevated CA125, and tumour diameter ≥5 cm were independent risk factors of PC. These three factors combined with cT4 were selected as predictive factors, and the number of predictive variables was significantly related to the possibility of PC (2.0%, 12.8%, 20.0%, 54.2%, and 100%, respectively). When the cutoff value is more than one predictive factor, the negative predictive value is 98.0%, with an area under the curve of 0.780. This model could exempt 29.8% of unnecessary SL compared to the indication of the current NCCN guideline.
Conclusions: We constructed a simple model to predict the probability of PC using the number of predictive factors. It is recommended that patients without any of these factors should be exempt from SL.
Introduction
The incidence of gastric cancer ranks fifth among malignant tumours, and the mortality rate ranks fourth (1). Peritoneal carcinomatosis (PC), including macroscopic carcinomatosis (P1) and positive cytology (CY1), is common among gastric cancer patients and tends to have poor outcomes regardless of the treatment approach (2, 3). Radiographic modalities, such as CT and MRI, have poor sensitivity for diagnosing PC (4–6); thus, staging laparoscopy (SL) combined with peritoneal cytology as a minimally invasive approach has been recommended by therapeutic guidelines, aiming for more accurate M1 staging (7).
However, the indication of SL remains debatable. NCCN guideline recommends that all locoregional patients undergo SL (8), with a 12.5%–20.7% chance of detecting PC (9–12). The relatively low positive rate discourages clinicians from performing it and makes it less cost-effective (13). A population-based study reported that only 8% of patients diagnosed with gastric cancer underwent SL (14). The JGCA guideline recommends only limited patients with large Borrmann III and Borrmann IV tumours and/or Bulky N status require SL (7). However, such a strict indication would raise concerns about omitting PC patients.
Therefore, this study was designed to identify factors related to PC and construct a risk stratification model to predict PC non-invasively and determine who should and should not undergo staging laparoscopy before the treatment.
Methods
Study patients
A retrospective analysis of data from patients with gastric cancer admitted to the Gastrointestinal Cancer Center of Peking University Cancer Hospital from January 2015 to December 2019 was performed. As a result, 535 patients were selected according to the inclusion and exclusion criteria.
The inclusion criteria were as follows: (1) pathologically confirmed gastric cancer; (2) clinical stage cT ≥ 2M0 or cN + M0 (no signs of PC by CT or other imaging tools); and (3) received SL. The exclusion criteria were as follows: (1) recurrence of gastric cancer; (2) simultaneous presence of other tumours; (3) received radiotherapy, chemotherapy, immune, or targeted therapy before SL; and (4) unable to extract imaging or clinical data.
Staging laparoscopy
SL is mandatory for locally advanced stage patients in our center before gastrectomy or applied alone before neoadjuvant therapy, as described in the inclusion criteria. The SL technique was described in a previous study (12). Patients lay supine, and a 10-mm disposable trocar (observation hole) was inserted above or below the umbilicus using the open Hasson method. The abdominal cavity was examined sequentially by a 30° laparoscope. We instilled 500 ml of saline in the abdominal cavity to obtain at least 100 ml of specimens divided into two tubes for centrifugation. Then, one of the tubes was prepared as conventional smears stained with hematoxylin–eosin and as liquid-based cytology samples stained with Papanicolaou stain. A cell pellet was obtained from the other tube and stained with hematoxylin–eosin. Nodules suspected of macroscopic carcinomatosis (P1) during inspection of the abdominal cavity were biopsied. The biopsy results determined the status of macroscopic carcinomatosis (P1 or P0). The cytology status (CY1 or CY0) was based on the peritoneal lavage results, which were performed before gastric cancer resection. Patients with P1 and/or CY1 were defined as PC+; those with P0 and CY0 were defined as PC−.
Clinical variables
The cTNM stage and Borrmann type were determined based on the enhanced CT images of the abdomen performed at our hospital before SL; the method was consistent with that described by Kim et al. (15). The gross appearance of CT of the tumour was categorized into Borrmann type I–IV: type I, nodular polypoid tumour without ulceration; type II, an ulcerative lesion but distinct borders; type III, an ulcerating tumour with infiltrating ulcer base; and type IV, diffuse thickening of the gastric wall without distinct ulceration. Tumour diameter was defined as the largest diameter in the transverse, sagittal, or coronal position in the same CT. According to the pathology report, Lauren's classification, degree of differentiation, and signet ring cell carcinoma were determined. The levels of plasma tumour markers were determined according to the test results obtained at our hospital before SL.
Statistical analysis
Associations between clinicopathologic variables and diagnosis of carcinomatosis or positive cytology were examined using chi-square and Fisher's exact tests. Multivariate logistic regression models were used to identify risk factors for PC. Receiver operating characteristic (ROC) curves and negative predictive values were used to determine the diagnostic power of the model. P < 0.05 was defined as statistically significant. Internal validation was trained in the form of 10-fold cross-validation over 1,000 iterations using the “caret” package in R (version 4.1.1). All other statistical analyses were conducted using IBM SPSS Statistics (version 26; IBM Corp., NY, USA).
Results
Clinical characteristics
The clinical characteristics of 535 patients with locally advanced gastric cancer who had SL are presented in Table 1. A total of 376 patients were males (70.3%), and 159 were females (29.7%). The average age was 59.1 ± 11 years. In total, 61.5% of patients were staged as cT4, while 82.2% were cN+. The numbers of patients with Borrmann I–IV were 16 (3.0%), 70 (13.1%), 404 (75.5%), and 45 (8.4%), respectively. The average tumour diameter was 47.8 ± 22.5 mm. SL showed PC in 85 patients. Among them, 11 patients (13.0%) had P1 only, 45 patients (53.0%) had CY1 only, and 29 (34.1%) patient had both P1 and CY1.
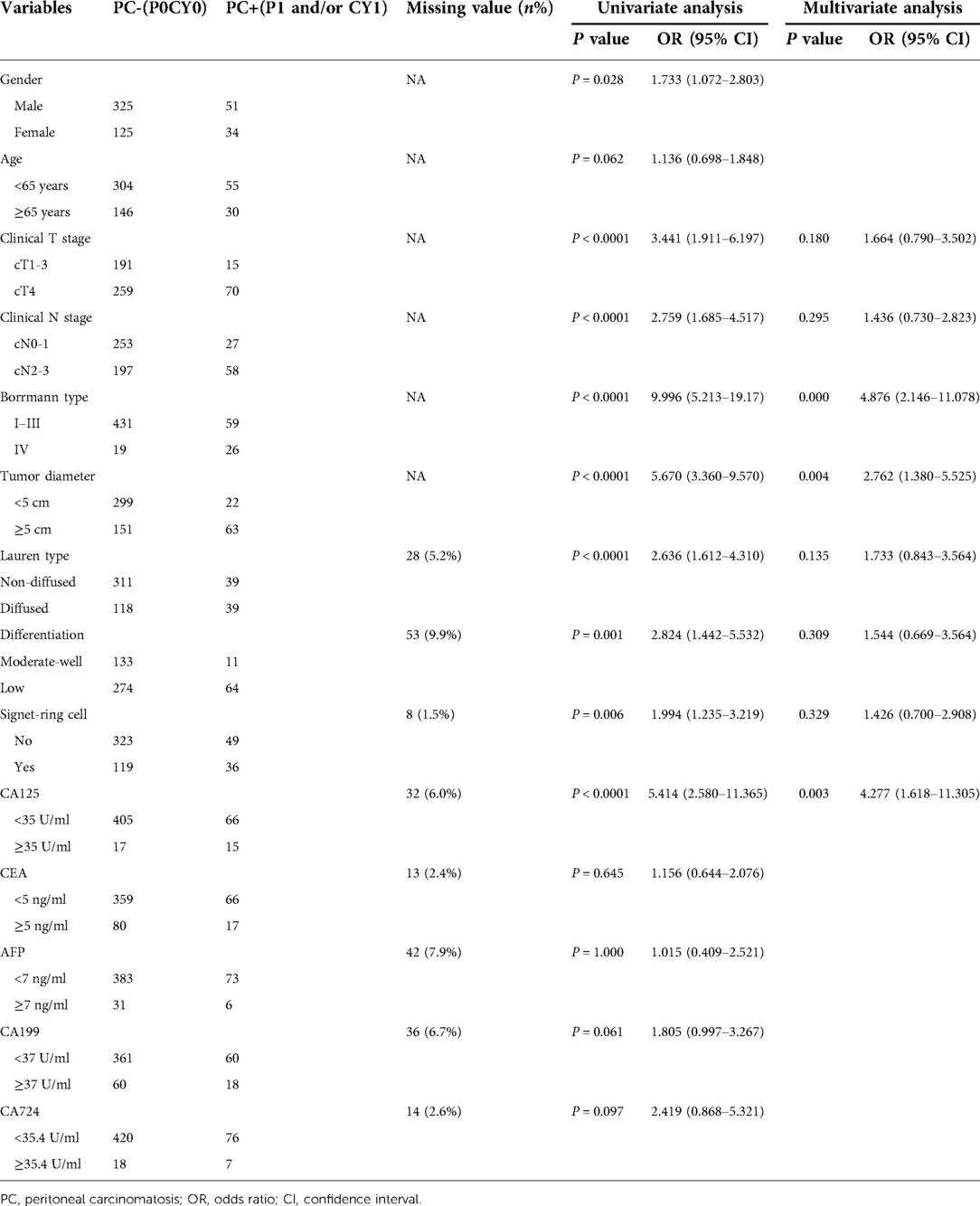
Table 1. Clinicopathologic features of patients and results of univariate analysis and multivariate analysis.
Results of univariate analysis and multivariate analysis
The relationships between the clinical factors and PC are shown in Table 1. In the univariate analysis, cT4, N2–3, tumour diameter ≥5 cm, Borrmann IV, Lauren diffused carcinoma, poorly differentiated carcinoma, signet ring cell carcinoma, and plasma CA125 ≥ 35 U/ml were significantly related to PC.
In the multivariate analysis of these factors among 444 patients, Borrmann type IV (OR 4.88; 95% CI, 2.15 to 11.08; P < 0.0001), CA125 ≥ 35 U/ml (OR 4.28; 95% CI, 1.62–11.30; P = 0.003), and tumour diameter ≥5 cm (OR 2.76; 95% CI, 1.38–5.52; P = 0.004) were independent risk factors of PC.
Predictive model and diagnostic performance
All three independent risk factors identified by logistic regression analysis, combined with cT4, were selected as predictive factors of PC. As shown in Table 2, the proportions of patients with PC who had 0–4 risk factors were 2.0% (3/150), 12.8% (23/180), 20.0% (24/120), 54.2% (26/48), and 100% (5/5), respectively.
The AUC value of the model was 0.780 with a 95% CI of 0.728–0.831 (Figure 1). The average AUC based on 1,000 tenfold validations was 0.784 with a 95% CI of 0.616–0.924 (Figure 2). The diagnostic sensitivity, specificity, positive predictive value, and negative predictive value of each cutoff value are presented in Table 3. Notably, the sensitivity and negative predictive values are 96.3% and 98.0%, respectively, when staging laparoscopy is to have at least one predictive factor.
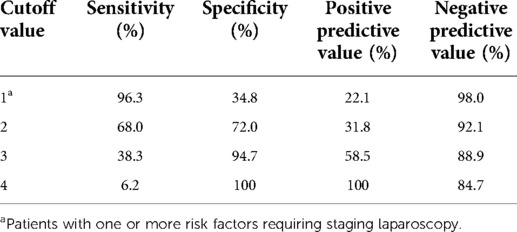
Table 3. Sensitivity, specificity, positive predictive value, and negative predictive value of the model with different cutoff values.
Comparison with the indication for staging laparoscopy in guidelines
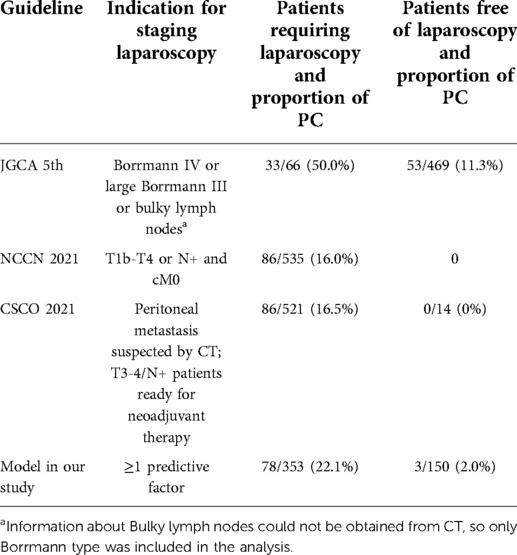
In addition, we compare the diagnostic performance of different indications for SL by using two parameters: yields of staging laparoscopy and omitted PC from patients exempted from staging laparoscopy (Table 4). According to the NCCN guideline, which was an indication for SL in our center, all patients were recommended to have SL, of which the positive rate was 16%. Although, according to the JGCA guideline, most patients were exempted from SL, 53 of these 469 patients (11.3%) had PC. According to our study, 150 patients without risk factors, accounting for 29.8% of all patients, could be exempt from staging laparoscopy, and only 3 (2%) patients with PC would be missed.
Discussion
This study proposed a risk stratification model of peritoneal carcinomatosis using four widely applied factors: clinical T stage, Borrmann type, tumour diameter, and CA125. The percentages of PC in patients with 0–4 risk factors were 2.0%, 12.8%, 20.0%, 54.2%, and 100%, respectively. The model could spare 29.8% of patients from SL compared with the current NCCN guideline, improving medical efficiency and reducing medical costs for patients without any risk factors.
Guidelines provide different indications for SL in patients with advanced gastric cancer. Japanese investigators apply indication for SL the same as neoadjuvant chemotherapy: gastric cancer with “bulky N” status and Borrmann type IV and large type III gastric cancer. The yield of this indication is reported to be 53.4%–56.8% (16), consistent with the result (50%) of our research. However, strict indications might omit potential PC patients from accurate staging. In our study, patients who do not meet the indication of JGCA guidelines still have an 11.3% chance of PC.
On the other hand, western countries chose indication for SL as “resectable GC and EGJ without definite distant metastases” (8), but a population-based study reported only 8% of patients diagnosed with gastric cancer underwent SL (14). Furthermore, too broad an indication introduces the problems of “many negative SL” and “cost-effectiveness” (17). Thus, patient selection for SL is still controversial, and the population that does not need SL must be carefully inspected.
Studies attempted to develop prediction models by constructing nomograms or summarizing high-risk factors by multivariate analysis. For example, Yang et al. constructed a nomogram based on the tumour size, degree of differentiation, and Glasgow prognosis score (18). Similarly, Zhao et al. used five factors, including weight loss and level of H5N5 (a kind of N-glycan), for his nomogram (19). Dong et al. used the Lauren classification and radiomics features from abdominal CT imaging to build a nomogram (20). A nomogram might result in a high AUC value, but it requires detailed calculations and often involve factors not commonly used in the clinical scenario. Thus, we had no intention of building a new one in this study. Studies also used the number of risk factors as an indication for SL; for example, Hu et al. used tumour size, cT4b, and Borrmann type III or IV as their risk factors (21). However, the missing CA125 might weaken their conclusion and lacks direct comparison with the current guideline.
In our study, clinical stage T4, Borrmann type IV, tumour diameter ≥5 cm, and CA125 ≥ 35 U/ml were selected as risk factors, which have a theoretical basis. The cT4 stage complies with studies before and with Paget’s “seed and soil” hypothesis, which suggests that as the primary tumour penetrates the serosa, more cancer cells will be shed into the abdominal cavity. Thus, the frequency of PC will also increase (22). Borrmann type IV cancer is often present with the peritoneal disease and selected as one of the indications for SL in Japan; the incidence of PC in B-IV cancer was reported to be as high as 58%–78% in Japanese studies (23, 24). Tumour diameter is not recommended as indicated in western guidelines, but multiple studies have shown that tumour diameter was an independent risk factor for PC (11, 21, 25–27). Previous studies less mentioned CA0, maybe because the antigen CA125 is produced by normal epithelia and not tumour-specific (28, 29). However, our data indicate that nearly half of patients with elevated CA125 were confirmed to have PC in the following SL, which was reported by previous articles (28, 30, 31). Up to 58% of patients with Borrmann IV had PC; the proportion also reached 47% in patients with elevated CA125. These four factors are significantly related to PC, while common staging modalities can obtain them.
We also made a comparison with other combinations of risk factors. Removal of any of the elements from the model resulted in a significant decrease in its diagnostic efficacy, and the addition of other factors did not increase its diagnostic effectiveness. Comparisons between the different models are detailed in the Supplementary Materials, including a comparison with the model consisting of the three factors statistically associated with PC in the multivariate analysis. Unlike the current guideline, the N stage is not included in our model because the statistical correlation in our study is not significant and the N stage fails to show independent correlations in multiple previous studies (12, 32–35). As shown in Table 4, the negative predictive value was 98.0%, with a cutoff value of 1. However, the results for the other cutoff values were not satisfactory.
There are some limitations to the study. First, we did not separate macroscopic carcinomatosis from positive cytology in the analysis. Second, there were missing values in the multivariate analysis, and the efficiency of the predictive model was also not verified by external data. Finally, the staging laparoscopy strategy for patients with one or more risk factors requires further investigation and clarification.
In conclusion, we propose a model consisting of four factors, including Borrmann type IV, tumour diameter ≥5 cm, CA125 ≥ 35 U/ml, and cT4, for predicting PC in patients with advanced gastric cancer. We recommend that patients without any of these risk factors could be exempt from staging laparoscopy before treatment and the others, especially ones with Borrmann IV gastric cancer and/or elevated CA125, should undergo staging laparoscopy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Beijing Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZL, GG, FS, and ZL were responsible for the study conception and design. ZL, GG, ZL, XY, and JL contributed to data acquisition. ZL and GG analyzed the data and wrote the initial draft. ZL critically reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Beijing Municipal Administration of Hospitals Incubating Program (PX2022047), Beijing Municipal Health Commission (DFL20181103 and ZYLX201701), and Clinical Medicine Plus X – Young Scholars Project, Peking University.
Acknowledgment
The authors appreciate all support from the Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Gastrointestinal Cancer Center, Peking University Cancer Hospital, Beijing, China.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.916001/full#supplementary-material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Fujitani K, Yang H, Mizusawa J, Kim Y, Terashima M, Han S, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. (2016) 17(3):309–18. doi: 10.1016/S1470-2045(15)00553-7
3. Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol (EJSO). (2014) 40(1):12–26. doi: 10.1016/j.ejso.2013.10.019
4. Burbidge S, Mahady K, Naik K. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin radiol. (2013) 68(3):251–5. doi: 10.1016/j.crad.2012.07.015
5. Kim SJ, Kim HH, Kim YH, Hwang SH, Lee HS, Park DJ, et al. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology. (2009) 253(2):407–15. doi: 10.1148/radiol.2532082272
6. Yajima K, Kanda T, Ohashi M, Wakai T, Nakagawa S, Sasamoto R, et al. Clinical and diagnostic significance of preoperative computed tomography findings of ascites in patients with advanced gastric cancer. Am J Surg. (2006) 192(2):185–90. doi: 10.1016/j.amjsurg.2006.05.007
7. Japanese Gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. (2020) 24(1):1–21. doi: 10.1007/s10120-020-01042-y
8. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20(2):167–92. doi: 10.6004/jnccn.2022.0008
9. Yang C, Yang Y, Huang X, Li H, Cheng H, Tong S, et al. A nomogram based on clinicopathologic features and preoperative hematology parameters to predict occult peritoneal metastasis of gastric cancer: a single-center retrospective study. Dis Markers. (2020) 2020:1–10. doi: 10.1155/2020/1418978
10. Harris C, Ostwal V, Vallathol DH, Dusane R, Mandavkar S, Patkar S, et al. Calculation of a clinical predictive factors identifying peritoneal disease on a staging laparoscopy in gastric cancers. South Asian J Cancer. (2019) 8(3):166–7. doi: 10.4103/sajc.sajc_182_18
11. Huang J, Luo H, Zhou C, Zhan J, Rao X, Zhao G, et al. Yield of staging laparoscopy for incurable factors in Chinese patients with advanced gastric cancer. J Laparoendosc Adv S. (2018) 28(1):19–24. doi: 10.1089/lap.2017.0170
12. Li Z, Li Z, Jia S, Bu Z, Zhang L, Wu X, et al. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis. Chin J Cancer Res. (2017) 29(2):109–17. doi: 10.21147/j.issn.1000-9604.2017.02.03
13. Li K, Cannon J, Jiang SY, Sambare TD, Owens DK, Bendavid E, et al. Diagnostic staging laparoscopy in gastric cancer treatment: a cost-effectiveness analysis. J Surg Oncol. (2018) 117(6):1288–96. doi: 10.1002/jso.24942
14. Karanicolas PJ, Elkin EB, Jacks LM, Atoria CL, Strong VE, Brennan MF, et al. Staging laparoscopy in the management of gastric cancer: a population-based analysis. J Am Coll Surg. (2011) 213(5):644–51. doi: 10.1016/j.jamcollsurg.2011.07.018
15. Kim JW, Shin SS, Heo SH, Choi YD, Lim HS, Park YK, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol. (2012) 22(3):654–62. doi: 10.1007/s00330-011-2283-3
16. Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. (2019) 22(5):1044–52. doi: 10.1007/s10120-019-00941-z
17. Fukagawa T. Role of staging laparoscopy for gastric cancer patients. Ann Gastroenterol Surg. (2019) 3(5):496–505. doi: 10.1002/ags3.12283
18. Yang C, Yang Y, Huang X, Li H, Cheng H, Tong S, et al. A nomogram based on clinicopathologic features and preoperative hematology parameters to predict occult peritoneal metastasis of gastric cancer: a single-center retrospective study. Dis Markers. (2020) 2020:1–10. doi: 10.1155/2020/1418978
19. Zhao J, Qin R, Chen H, Yang Y, Qin W, Han J, et al. A nomogram based on glycomic biomarkers in serum and clinicopathological characteristics for evaluating the risk of peritoneal metastasis in gastric cancer. Clin Proteom. (2020) 17(1):34. doi: 10.1186/s12014-020-09297-4
20. Dong D, Tang L, Li ZY, Fang MJ, Gao JB, Shan XH, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol. (2019) 30(3):431–8. doi: 10.1093/annonc/mdz001
21. Hu YF, Deng ZW, Liu H, Mou TY, Chen T, Lu X, et al. Staging laparoscopy improves treatment decision-making for advanced gastric cancer. World J Gastroenterol. (2016) 22(5):1859–68. doi: 10.3748/wjg.v22.i5.1859
22. Akhtar M, Haider A, Rashid S, Al-Nabet A. Paget's “seed and soil” theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol. (2019) 26(1):69–74. doi: 10.1097/PAP.0000000000000219
23. Hosogi H, Shinohara H, Tsunoda S, Hisamori S, Sumida H, Hida K, et al. Staging laparoscopy for advanced gastric cancer: significance of preoperative clinicopathological factors. Langenbecks Arch Surg. (2017) 402(1):33–9. doi: 10.1007/s00423-016-1536-7
24. Irino T, Sano T, Hiki N, Ohashi M, Nunobe S, Kumagai K, et al. Diagnostic staging laparoscopy in gastric cancer: a prospective cohort at a cancer institute in Japan. Surg Endosc. (2018) 32(1):268–75. doi: 10.1007/s00464-017-5673-z
25. Hur H, Lee HH, Jung H, Song KY, Jeon HM, Park CH. Predicting factors of unexpected peritoneal seeding in locally advanced gastric cancer: indications for staging laparoscopy. J Surg Oncol. (2010) 102(7):753–7. doi: 10.1002/jso.21685
26. Kurita N, Shimada M, Utsunomiya T, Iwata T, Nishioka M, Yoshikawa K, et al. Predictive factors of peritoneal metastasis in gastric cancer. Hepatogastroenterology. (2010) 57(101):980–3.
27. Yang C, Yang Y, Huang X, Li H, Cheng H, Tong S, et al. A nomogram based on clinicopathologic features and preoperative hematology parameters to predict occult peritoneal metastasis of gastric cancer: a single-center retrospective study. Dis Markers. (2020) 2020:1418978. doi: 10.1155/2020/1418978
28. Zhao J, Qin R, Chen H, Yang Y, Qin W, Han J, et al. A nomogram based on glycomic biomarkers in serum and clinicopathological characteristics for evaluating the risk of peritoneal metastasis in gastric cancer. Clin Proteomics. (2020) 17:34. doi: 10.1186/s12014-020-09297-4
29. Miralles C, Orea M, España P, Provencio M, Sánchez A, Cantos B, et al. Cancer antigen 125 associated with multiple benign and malignant pathologies. Ann Surg Oncol. (2003) 10(2):150–4. doi: 10.1245/aso.2003.05.015
30. Hwang GI, Yoo CH, Sohn BH, Shin JH, Park YL, Kim HD, et al. Predictive value of preoperative serum CEA, CA19-9 and CA125 levels for peritoneal metastasis in patients with gastric carcinoma. Cancer Res Treat. (2004) 36(3):178–81. doi: 10.4143/crt.2004.36.3.178
31. Fujimura T, Kinami S, Ninomiya I, Kitagawa H, Fushida S, Nishimura G, et al. Diagnostic laparoscopy, serum CA125, and peritoneal metastasis in gastric cancer. Endoscopy. (2002) 34(7):569–74. doi: 10.1055/s-2002-33228
32. Badgwell B, Cormier JN, Krishnan S, Yao J, Staerkel GA, Lupo PJ, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. (2008) 15(10):2684–91. doi: 10.1245/s10434-008-0055-3
33. Ikoma N, Blum M, Chiang YJ, Estrella JS, Roy-Chowdhuri S, Fournier K, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. (2016) 23(13):4332–7. doi: 10.1245/s10434-016-5409-7
34. Rawicz-Pruszyński K, Mielko J, Pudło K, Lisiecki R, Skoczylas T, Murawa D, et al. Yield of staging laparoscopy in gastric cancer is influenced by Laurén histologic subtype. J Surg Oncol. (2019) 120(7):1148–53. doi: 10.1002/jso.25711
Keywords: gastric cancer, peritoneal carcinomatosis, risk factor, prediction model, staging laparoscopy
Citation: Li Z, Guan G, Liu Z, Li J, Ying X, Shan F and Li Z (2022) Predicting peritoneal carcinomatosis of gastric cancer: A simple model to exempt low-risk patients from unnecessary staging laparoscopy. Front. Surg. 9:916001. doi: 10.3389/fsurg.2022.916001
Received: 8 April 2022; Accepted: 28 June 2022;
Published: 21 July 2022.
Edited by:
Sungsoo Park, Korea University, South KoreaReviewed by:
Ahmad Alromi, Ministry of Health (Jordan), JordanHee Sung Kim, Chung-AnG University, South Korea
© 2022 Li, Guan, Liu, Li, Ying, Shan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziyu Li eml5dV9saUBoc2MucGt1LmVkdS5jbg==
†These authors have contributed equally to this work.
Specialty Section This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Zhemin Li
Zhemin Li Guangmin Guan
Guangmin Guan Zining Liu
Zining Liu Jiazheng Li
Jiazheng Li Ziyu Li
Ziyu Li