- 1Department of General Surgery, Xingtai People Hospital, Xingtai, China
- 2Department of Thyroid and Breast Surgery, the Second Hospital of Hebei Medical University, Shijiazhuang, China
Objective: To establish the criteria for a risk factor score (RFS) for predicting the probability of central compartment lymph node metastasis (LNM) in papillary thyroid carcinoma (PTC) and to explore the clinical significance of the RFS.
Methods: The data of 412 patients with PTC who underwent surgical resection between May 2013 and July 2016 were retrospectively analysed and divided into two groups: a central LNM group and a non-central LNM group. In each group, the frequency of six risk factors was documented: sex, age, tumour size, extracapsular spread (ECS), tumour multifocality, and tumour location. The maximum likelihood method of discriminant analysis was adopted to calculate patient scores for the six risk indicators. In addition, the data of 104 patients with PTC admitted between July 2016 and December 2016 were prospectively analysed using this method and these six risk factors. A higher score represented one certain possibility that was the more appropriate for one patient.
Results: In the retrospective group, the result was as follows: 129 patients with positive (+) lymph nodes in the central compartment and 168 patients with negative (−) lymph nodes in the central compartment, which was in line with the actual results. In the prospective group, there were 28 patients with positive lymph nodes in the central compartment and 48 patients with negative lymph nodes in the central compartment. The coincidence rates using the RFS were 71.9% for the retrospective group and 73.1% for the prospective group.
Conclusion: By simple and quantitative analyses of the presence of central LNM, the RFS is of great significance when choosing surgical approaches and postoperative individual-based treatment plans, as well as when determining the prognosis of central compartment LNM in patients with PTC.
Introduction
Thyroid carcinoma is the most common endocrine malignancy and a common head and neck malignancy. Papillary thyroid carcinoma (PTC), specifically, is a malignant tumour derived from the thyroid follicular epithelial cells that accounts for about 85% to 90% of thyroid cancers (1). Although PTC has a low degree of malignancy and a good prognosis, it is prone to either cervical lymph node metastases (LNM) or distant metastases at the time of diagnosis, and the central lymph node metastasis rate can be as high as 80% (2–4). The most fundamental and effective method of treating PTC primary tumours and lymph node metastases is surgery. In terms of patients with thyroid cancer who have suspected or confirmed central compartment LNM, therapeutic central lymph node dissection (CLND) is the mainstay of treatment, but one controversial issue is whether routine prophylactic dissection should be performed in patients without evident cervical LNM (5). If CLND is not performed in carcinoma cases, residual tumours are induced. When reoperation is required, it will inevitably lead to an increased risk of surgical complications due to the formation of surgical scars and the disruption of the anatomical hierarchy. Currently, ultrasonography (US) is the optimal imaging method in thyroid and regional lymph node examinations, and it has a high sensitivity to benign and malignant thyroid nodules and high accuracy for identifying them (6). However, due to the anatomic location of the central lymph nodes and for certain technical reasons, the diagnostic sensitivity is low (merely 31.3%) (7–12). For this paper, data of patients with PTC were retrospectively analysed using the maximum likelihood method to obtain the scores of factors associated with central LNM. The factors are sex, age, tumour diameter, extracapsular spread (ECS), multifocality, and tumour location. Sex: In thyroid cancer, female patients are more of a concern because of a higher incidence rate. However, several studies suggest that the rate of cervical LNM in men is higher than in women (3, 13, 14). Age: This has been an important factor in various staging systems for differentiated PTC (13, 15, 16). Tumour diameter and ECS: Both have always been considered to be important factors in the progression of PTC and are important criteria for evaluating treatment options and surgical scope, and larger tumour diameters are associated with higher cervical LNM rates and T staging (17). Multifocality: PTC often leads to intraglandular metastasis, and multifocality is one of its prominent features. According to literature reports, multifocal carcinoma accounts for 20.3%–33.5% of cases of PTC (14, 16, 18). Tumour location: In some digestive tract tumours, such as gastric cancer and colon cancer, LNM is closely related to the lymphatic flow path in the region where the tumour is located (19, 20). Therefore, we speculate that PTC LNM is related to the lymphatic flow path of the thyroid region where the tumour is located. The criteria for the risk factor score (RFS) were used to prospectively evaluate another group of 104 patients diagnosed with PTC, as well as the clinical significance of the RFS.
Materials and methods
General information
Retrospective data
We enrolled 412 patients admitted to our hospital between May 2013 and July 2016 who were diagnosed by postoperative paraffin pathology as having PTC. There were 90 males and 322 females in the study, with a sex ratio of 1:3.6 and a mean age of 44.5 (range 14–81 years old). Tumour diameters ranged from 0.2 to 3.8 cm, with an average diameter of 1.22 cm. A total of 81 patients had ECS (19.6%) and 331 patients did not have ECS. In addition, 169 patients (41%) had papillary thyroid microcarcinoma (PTMC), and 197 patients had central LNM, with a metastasis rate of 47.9%.
Prospective data
A total of 104 patients admitted to our department between July 2016 and December 2016 were diagnosed by postoperative paraffin pathology as having PTC. There were 24 males and 80 females with a mean age of 46.6 (range 20–73 years old). The mean tumour diameter was 1.19 cm (range 0.3–3.8 cm). The number of patients with and without ECS was 9 (8.7%) and 95 (91.3%), respectively. A total of 48 patients had PTMC (46%) and 41 patients had central LNM (39.4%).
Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) neck surgery for the first time; (2) ipsilateral CLND performed; and (3) complete medical records, including a B-ultrasound examination and postoperative paraffin pathology. Patients with the following diagnoses were excluded: (1) metastatic thyroid cancer; (2) thyroid cancer combined with other types of undifferentiated carcinoma; and (3) multiple foci in two or more different parts (the upper, middle, and lower poles, as well as the isthmus).
Clinical observation criteria
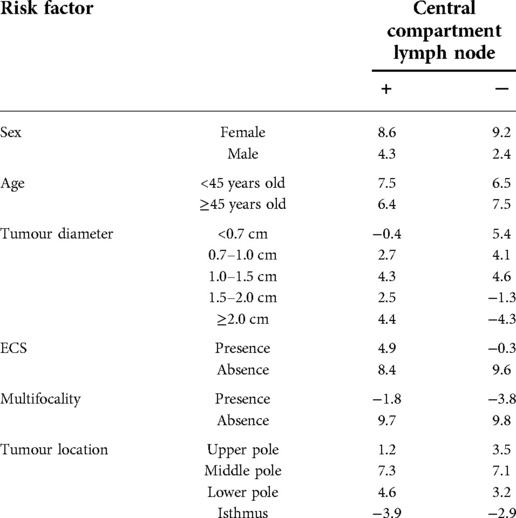
Six factors predictive of central LNM were described: (1) sex: both male and female; (2) age: <45 years old and ≥45 years old; and (3) tumour diameter: <0.7 cm, 0.7–1.0 cm, 1.0–1.5 cm, 1.5–2.0 cm, and ≥2.0 cm; (4) ECS: presence or absence; (5) multifocality: presence or absence; and (6) tumour location: the upper (upper 1/3 of the gland), the middle (middle 1/3 of the gland) and the lower pole (lower 1/3 of the gland), as well as the isthmus. A frequency table of count data was constructed in accordance with these six indicators based on data collected from the 412 patients in the retrospective study (Table 1).
Treatment
Ultrasonography was performed by two sonographers with more than 5 years of experience. The central lymph node status was recorded. The extent of thyroidectomy was determined according to the clinical examination results or preoperative biopsy results. At least unilateral gland lobe plus isthmus resection and total thyroidectomy or near-total thyroidectomy should be performed if any of the following conditions are met: distant metastasis, the primary tumour is larger than 4 cm, the primary tumour invades the surrounding tissue, or a multifocal tumour or cervical LNM can be seen with the naked eye. The histology of frozen sections further helps surgeons determine the extent of surgery required.
A total of 412 patients in the retrospective group underwent standardised surgical treatment: thyroid lobectomy, isthmus resection, and ipsilateral CLND (n = 168); total thyroidectomy and ipsilateral CLND (n = 190); and total thyroidectomy plus bilateral CLND (n = 54). In the prospective study of 104 patients, the above operation methods were used in 46 cases, 28 cases, and 30 cases, respectively. All patients who were given oral levothyroxine sodium tablets underwent thyroid-stimulating hormone (TSH) inhibition treatment. One month later, thyroid function tests were performed, and the dosage of Euthyrox was tailored according to the TSH level. During this period, thyroid function tests were reperformed every 1 and a half months. B-ultrasound examinations of the thyroid and neck lymph nodes were conducted half a year after surgery and every half a year thereafter, as well as other tests such as physical examinations, thyroid function tests, ECGs, and chest x-rays. All patients were advised to see a doctor if necessary.
Postoperative follow-up
All patients were followed up for one to 4 years, with an average of 28.3 months, apart from three who were lost to the study due to changes in telephone numbers. Some patients had postoperative transient hypocalcaemia and hoarseness. Nonetheless, all of them recovered within 6 months of surgery. No other serious complications arose.
Statistical method
As given in Table 1, the frequency (P) of the six indicators for central LNM was calculated using the formula (Xkj/Yg), in which Y represents the type of the central lymph node: g = 1 indicates positive (+), and g = 2 indicates negative (−). Xkj (k = 1,2,3,4,5,6; j = 1,2) is the jth classification of the kth indicator. The maximum likelihood method of discriminant analysis for qualitative data was used to estimate the probability of type g central lymph node in Xkj patients: Pg = P (X1j/Yg)×P (X2j/Yg)…×P (X6j/Yg).
Take the logarithm of both sides: lgPg = lgP (X1j/Yg) + lgP (X2j/Yg)…+lgP (X6j/Yg). The score of each indicator was derived from [lgP (Xkj/Yg)+1]×10 (Table 2).
The scoring formula was as follows: Sg = {[lgP (X1j/Yg)+1] + [lgP (X2j/Yg)+1]…+[lgP (X6j/Yg)+1]}×10.
Statistical analysis
In this study, the above formula was used for discriminant analysis of the data from the 104 patients in the prospective group and for back substitution of the data from the 412 patients in the retrospective group. The Wilcoxon signed-rank test of two independent samples was performed for comparison between the retrospective and the prospective groups. The significance for all variables was set at α = 0.05. SPSS 21.0 software was used for statistical analyses.
Results
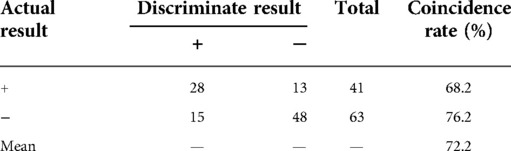
The age distribution and tumour diameter distribution of the two groups are shown in Figures 1, 2, and the score of each factor related to central LNM is given in Table 2. In the retrospective group, the discriminant analysis result was as follows: 129 patients had positive (+) lymph nodes in the central compartment and 168 patients had negative (−) lymph nodes in the central compartment, which was in line with actual results. The coincidence rates were 65.5% and 78.1%, respectively, and the overall coincidence rate was 71.8% (Table 3). However, in the prospective group, 28 patients had positive lymph nodes in the central compartment and 48 patients had negative lymph nodes in the central compartment. The coincidence rates were 68.2% and 76.2% respectively, with an overall coincidence rate of 72.2% (Tables 3, 4).
Discussion
PTC is a common disease in clinical settings. A B-ultrasound examination is currently an effective method of distinguishing between benign and malignant thyroid nodules. Dan et al. (21) reported that the diagnosis rate of PTC was as high as 87.64% by high-frequency colour ultrasound, which was, however, not sensitive in the detection of central LNM [approximately 31.3% (7–12)], much lower than the detection rate of lateral neck metastasis (93.8%) (22). This may be related to the anatomical location of the central lymph nodes (adjacent to thyroid and gas-containing trachea) and available technology.
Central LNM is associated with a number of factors. The relevant factors involved in this study were as follows: (1) Sex: Male patients are more likely to experience LNM than female patients (23). Of the 516 patients enrolled, 66 of 114 males developed central LNM and 172 of 402 females. The metastasis rates were 57.9% and 42.8%, respectively. (2) Age: The cut-off age of 45 is widely used as a clinical marker in the American Joint Committee on Cancer TNM staging system guideline (2012 Chinese version) (24). In Western literature, an age of >45 was referred to as a risk factor for thyroid cancer. It is noteworthy that Ito et al. (25) demonstrated an association between a younger age and a higher rate of LNM. In addition, patients 20 years old or younger had a metastasis rate of up to 50%. (3) Tumour diameter: According to National Comprehensive Cancer Network guidelines, a tumour diameter of >1 cm is a risk factor for cervical LNM (26), and patients with PTC with tumour diameters of >2 cm had a higher LNM rate in the central region and the lateral neck than those with tumour diameters of ≤2 cm (27). 0.5 or 0.6 cm has been recognised as the cut-off value of central LNM in PTC. In our previous studies (28), a tumour diameter of ≥0.7 cm was of statistical significance in the prediction of central LNM in PTMC. (4) ECS: Based on studies by Radowsky et al. (29), ECS is an important factor affecting the prognosis of PTC. Extrathyroidal extension contributed to increased invasion associated with a weakened inhibitory effect of extracellular matrix on LNM in PTC and capsular invasion of the dense network in the thyroid (30). (5) In terms of multifocality, Kuo et al. (31) found that multifocal primary PTMC had a significantly increased metastasis rate compared with unifocal PTMC, consistent with the findings in our previous studies. (6) Tumour location: Zhang et al. (14) reported that a tumour located in the upper pole of the gland was associated with an increased risk of LNM in the lateral neck but a decreased risk of central LNM. Wang et al. (32) found that patients with tumours located in the middle and lower poles are more likely to experience central LNM. Based on the data in Table 1, the rates of central LNM in tumours located in the upper, middle, and lower poles and the isthmus were 35.1%, 48.8%, 55.9%, and 42.1%, respectively.
There has been no international consensus regarding prophylactic CLND in patients with cN0 PTC. In this study, the frequency of each relevant factor was measured based on retrospective data. Likelihood function discriminant analysis was used in the prospective group, and samples were subject to back substitution to evaluate the method's effectiveness. Given unchanged discriminant rules in the likelihood function after logarithm transformation, scoring criteria were developed and applied as follows: With respect to a 35-year-old female PTC patient with a tumour diameter of 1.6 cm, single lesions and no extracapsular spread, and with the tumour located in the lower pole of the lobes, the score was Spositive = 41.3, Snegative = 37, and Spositive > Snegative, indicating the presence of central LNM.
In this study, statistical methods were used for specific and quantitative discriminant analysis of central LNM and benign lesions in patients with PTC. The accuracy (approximately 72%) was superior to the current discriminant analysis of B-ultrasound measurements, which is of clinical significance. Several studies have reported that central LNM is also associated with the coexistence of Hashimoto's thyroiditis and higher TSH levels in patients.
The present study has several limitations. First, the above factors have not been included, and a multicentre study has not been conducted, leading to a certain selection bias, which should ideally be avoided. Further study is, therefore, needed to standardise the scoring criteria. Second, the current follow-up timeframe has not been long enough compared with disease development. We will continue to follow up on these patients. Another limitation of the current study is the small number of patients, and further studies with larger samples are needed. Furthermore, the time of the study has a certain bias, and the results need to be further verified in a subsequent study at a different time. Last, we have not analysed the relationship between Hashimoto's thyroiditis and PTC, and we will add this feature as a part of the risk factor score.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Second Hospital of Hebei Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XP conceptualized and designed the article; QL provided administrative support; all authors provided study materials or patients all authors collected and assembled data; all authors conducted data analysis and interpretation; all authors wrote the manuscript; all authors gave final approval of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Seethala RR, Chiosea SI, Liu CZ, Nikiforova M, Nikiforov YE. Clinical and morphologic features of ETV6-NTRK3 translocated papillary thyroid carcinoma in an adult population without radiation exposure. Am J Surg Pathol. (2017) 41(4):446–57. doi: 10.1097/PAS.0000000000000814
2. Zhu YX, Wang HS, Wu Y, Ji QH, Huang CP. Whether VI region lymph nodes belong to primary site of the thyroid carcinoma or lateral cervical lymph node metastases. Chin J Surg. (2004) 42(14):867–9. doi: 10.3760/j:issn:0529-5815.2004.14.011
3. Nam IC, Park JO, Joo YH, Cho KJ, Kim MS. Pattern and predictive factors of regional lymph node metastasis in papillary thyroid carcinoma: a prospective study. Head Neck. (2013) 35(1):40–5. doi: 10.1002/hed.22903
4. Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol. (2009) 20(10):1728–35. doi: 10.1093/annonc/mdp050
5. Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol). (2010) 22(6):395–404. doi: 10.1016/j.clon.2010.05.004
6. Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, et al. American thyroid association statement on preoperative imaging for thyroid cancer surgery. Thyroid. (2015) 25(1):3–14. doi: 10.1089/thy.2014.0096
7. Zhang L, Yang J, Sun Q, Liu Y, Liang F, Liu Z, et al. Risk factors for lymph node metastasis in papillary thyroid microcarcinoma: older patients with fewer lymph node metastases. Eur J Surg Oncol. (2016) 42(10):1478–82. doi: 10.1016/j.ejso.2016.07.002
8. Cho SY, Lee TH, Ku YH, Kim H, Lee GH, Kim MJ. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery. (2015) 157(1):111–8. doi: 10.1016/j.surg.2014.05.023
9. Xu SY, Yao JJ, Zhou W, Chen L, Zhan WW. Clinical characteristics and ultrasonographic features for predicting central lymph node metastasis in clinically node-negative papillary thyroid carcinoma without capsule invasion. Head Neck. (2019) 41(11):3984–91. doi: 10.1002/hed.25941
10. Jian ML, Ji BL, Lin XQ. Suspicious ultrasound characteristics correlate with multiple factors that predict central lymph node metastasis of papillary thyroid carcinoma: significant role of HBME-1. Eur J Radiol. (2020) 123:108801. doi: 10.1016/j.ejrad.2019.108801
11. Xu JM, Xu XH, Xu HX, Zhang YF, Guo LH, Liu LN, et al. Prediction of cervical lymph node metastasis in patients with papillary thyroid cancer using combined conventional ultrasound, strain elastography, and acoustic radiation force impulse (ARFI) elastography. Eur Radiol. (2016) 26(8):2611–22. doi: 10.1007/s00330-015-4088-2
12. Zhan J, Diao X, Chen Y, Wang W, Ding H. Predicting cervical lymph node metastasis in patients with papillary thyroid cancer (PTC)—why contrast enhanced ultrasound (CEUS) was performed before thyroidectomy. Clin Hemorheol Microcirc. (2019) 72(1):61–73. doi: 10.3233/CH-180454
13. Mao LN, Wang P, Li ZY, Wang Y, Song ZY. Risk factor analysis for central nodal metastasis in papillary thyroid carcinoma. Oncol Lett. (2015) 9(1):103–7. doi: 10.3892/ol.2014.2667
14. Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab. (2012) 97(4):1250–7. doi: 10.1210/jc.2011-1546
15. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. (2006) 106(3):524–31. doi: 10.1002/cncr.21653
16. Malandrino P, Pellegriti G, Attard M, et al. Papillary thyroid microcarcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registries. J Clin Endocrinol Metab. (2013) 98(4):1427–34. doi: 10.1210/jc.2012-3728
17. Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
18. Kim HJ, Park HK, Byun DW, et al. Number of tumor foci as predictor of lateral lymph node metastasis in papillary thyroid carcinoma. Head Neck. (2015) 37(5):650–4. doi: 10.1002/hed.23650
19. Zhang LY, Liu ZW, Liu YW, Gao WS, Zheng CJ. Risk factors for nodal metastasis in cN0 papillary thyroid microcarcinoma. Asian Pac J Cancer Prev. (2015) 16(8):3361–3. doi: 10.7314/apjcp.2015.16.8.3361
20. Xiang D, Xie L, Xu Y, Li Z, Hong Y, Wang P. Papillary thyroid microcarcinomas located at the middle part of the middle third of the thyroid gland correlates with the presence of neck metastasis. Surgery. (2015) 157(3):526–33. doi: 10.1016/j.surg.2014.10.020
21. Dan HJ, Zhang ZY, Wu YE, Chen Z, Zhai SM. High frequency color ultrasound diagnosis of thyroid micropapillary carcinoma. J Hebei Med Univ. (2014) 35(12):1444–6. doi: 10.3969/j.issn.1007-3205.2014.12.030
22. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. (2011) 121(3):487–91. doi: 10.1002/lary.21227
23. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. (2006) 30(1):91–9. doi: 10.1007/s00268-005-0113-y
24. Verburg FA, Mader U, Tanase K, Thies ED, Diessl S, Buck AK, et al. Life expectancy is reduced in differentiated thyroid cancer patients ≥45 years old with extensive local tumor invasion, lateral lymph node, or distant metastases at diagnosis and normal in all other DTC patients. J Clin Endocrinol Metab. (2013) 98(1):172–80. doi: 10.1210/jc.2012-2458
25. Ito Y, Miyauchi A, Kihara M, Takamura Y, Kobayashi K, Miya A. Relationship between prognosis of papillary thyroid carcinoma patient and age: a retrospective single-institution study. Endocr J. (2012) 59(5):399–405. doi: 10.1507/endocrj.ej12-0044
26. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: thyroid carcinoma. Version 2.2013.
27. Xiang J, Wu Y. New clinical characteristics of thyroid cancer: clinic research on 572 cases. ChinJ Pract Surg. (2008) 28(5):365–7. doi: 10.3321/j.issn:1005-2208.2008.05.015
28. Yan L, Ji H, Li QH, Dan H, Hao F. Clinical factors related to central lymph node metastasis in papillary thyroid icrocarinoma. Chin J Exp Surg. (2016) 33(3):765–7. doi: 10.3760/cma.j.issn.1001-9030.2016.03.065
29. Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroid extension of disease on papillary thyroid cancer outcome. Thyroid. (2014) 24(2):241–4. doi: 10.1089/thy.2012.0567
30. Wu HS, Gu WL, Liang LS. Research progress of extracellular matrix in the mechanism of thyroid cancer metastasis. J Endocr Surg. (2009) 3(2):126–8. doi: 10.3760/cma.j.issn.1674-6090.2009.02.018
31. Kuo SF, Lin SF, Chao TC, Hsueh C, Lin KJ, Lin JD. Prognosis of multifocal papillary thyroid carcinoma. Int J Endocrinol. (2013) 2013:809382. doi: 10.1155/2013/809382
Keywords: thyroid carcinoma, central compartment lymph node, lymph node metastasis, risk factor score, surgery
Citation: Pan X and Li Q (2022) Risk factor score for the prediction of central compartment lymph node metastasis in papillary thyroid carcinoma and its clinical significance. Front. Surg. 9:914696. doi: 10.3389/fsurg.2022.914696
Received: 7 April 2022; Accepted: 28 June 2022;
Published: 7 November 2022.
Edited by:
Pasquale Cianci, Azienda Sanitaria Localedella Provincia di Barletta Andri Trani (ASL BT), ItalyReviewed by:
Vincenzo Neri, University of Foggia, ItalyMaria Rosa Pelizzo, University of Padua, Italy
Eleonora M.C. Trecca, IRCCS Casa Sollievo della Sofferenza Ospedale di San Pio da Pietrelcina, Italy
© 2022 Pan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghuai Li cWluZ2h1YWxpMTU3NEAxNjMuY29t
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Xiaojia Pan1
Xiaojia Pan1 Qinghuai Li
Qinghuai Li