- 1Department of Gastrointestinal Surgery, Clinical Medical College and The First Affiliated Hospital of Chengdu Medical College, Chengdu, China
- 2Department of Gastrointestinal Surgery, BaZhong Central Hospital, Bazhong, China
- 3Department of Urology, People's Hospital Affiliated to Chongqing Three Gorges Medical College, Chongqing, China
Background and Aim: The effectiveness of total neoadjuvant therapy (TNT) on patients with locally advanced rectal cancer (LARC) is controversy. This study aims to compare the prognostic value of TNT with standard neoadjuvant chemoradiotherapy (CRT) for LARC.
Methods: We searched databases (Embase [Ovid], Medline [Ovid], PubMed, Cochrane Library, and Web of Science) for articles published between January 1, 2000, and March 10, 2022. Studies on evaluating the effects of TNT and standard CRT on the prognosis of LARC were included. The primary outcomes were overall survival (OS) and disease-free survival (DFS).
Results: 19 primary studies, involving 10 randomized controlled trials, 3 prospective studies and 6 retrospective studies, with data on 5,074 patients treated for LARC were included in the meta-analysis. Statistical analyses revealed that, compared with standard CRT, TNT significantly improved OS (hazard ratio [HR]=0.77, 95% confidence interval [CI]=0.65–0.90, I2 = 30%, P = 0.17), DFS (HR = 0.85, 95% CI = 0.74–0.97, I² = 11%, P = 0.35), distant metastases-free survival (DMFS, HR = 0.76, 95% CI = 0.65–0.90, I² = 0%, P = 0.50), pathological complete response rate (pCR, OR = 1.89, 95% CI = 1.61–2.22, I² = 0%, P = 0.47), and R0 resection rate (OR = 1.33, 95% CI = 1.07–1.67, I² = 16%, P = 0.28), but local recurrence-free survival (LRFS, HR = 1.12, 95% CI = 0.90–1.39, I² = 4%, P = 0.37).
Conclusions: Comprehensive literature research shows that TNT showed excellent short-term efficacy in terms of pCR and R0 resection rate while also improved the long-term outcomes of OS, DFS and DMFS, might become a new standard of treatment in patients with LARC. Even so, more studies and longer follow-up were still warranted.
Introduction
The treatment of locally advanced rectal cancer (LARC, cT3-T4 or N0 or node-positive and M0) is progressing and developing continuously. At present, the accepted standard strategy is 3–4 cycles of neoadjuvant chemoradiotherapy (CRT) before surgery, followed by total mesorectal excision (TME) with or without postoperative chemotherapy. Compared with TME alone or TME with postoperative adjuvant chemotherapy, this standard treatment method shows a better local tumor control rate, R0 resection rate and anal sphincter retention rate (1). Hence, in recent decades, this trimodal therapy has always been the standard of care for LARC (1, 2). However, compared with preoperative neoadjuvant chemoradiotherapy, postoperative adjuvant chemotherapy makes the chemotherapy tolerance and compliance of most patients worse because of factors such as surgical blows and worse nutritional status. Some patients cannot even complete standard postoperative adjuvant chemotherapy, which is a hidden danger for the recurrence and metastasis of LARC (3, 4). In addition, The long-term results of the MRC CR07 and NCIC-CTG C016 trials (5), the EORTC 22921 trial (6), the German CAO/ARO/AIO-94 trial (7) and the Dutch PROCTOR-SCRIPT trial (8) demonstrated that postoperative adjuvant chemotherapy failed to improve overall survival (OS) and disease-free survival (DFS) or had a worse prognosis than without it. Therefore, the goal of many recent studies has been to improve LARC outcomes by modifying treatment strategies.
To achieve a better therapeutic effect, some doctors advocate moving forward with radiotherapy and chemotherapy and even performing surgery after completing all chemotherapy cycles, which referred to as total neoadjuvant therapy (TNT). TNT model includes induction chemotherapy, delivering postoperative adjuvant chemotherapy to pre-CRT, and consolidation, delivering postoperative adjuvant chemotherapy to post-CRT and preoperation. The rationale of TNT was based on the potential to eradicate occult micrometastases before surgery by intensifying neoadjuvant combination chemotherapy (9) and to select the best approach for a given patient by in vivo assessment of chemosensitivity (10). Several recent studies have reported that the superiority of TNT is mainly reflected in high rates of tumor pathological complete response (pCR), tumor clinical downstaging, and R0 resection (11–13). In addition, compared with postoperative adjuvant chemotherapy, TNT does not need postoperative chemotherapy, so it can be repaid in advance for patients with protective ileostomy, which shortens the waiting time for ileostomy (14). This results in better patient compliance. A recent meta-analysis reported that TNT remarkably increased the odds of pCR compared with standard CRT (29.9% vs. 14.9%) and DFS (77.6% vs. 67.6%), but there was no statistically significant difference in the proportion of sphincter-preserving surgery or ileostomy (15), However, other meta-analyses reported different pooled results (16, 17). Consequently, the TNT model has received more attention and recognition, but the value of it is still controversial, especially with regard to long-term survival.
Our study retrieved updated and more comprehensive research data and better quality prospective randomized controlled trials, with the purpose of comparing the prognostic effects of TNT and standard CRT for LARC. Once again we scientifically evaluated whether TNT had better clinical significance and value than traditional standard CRT.
Materials and methods
In the meta-analysis, searching and screening studies, extracting data and quality assessment were following the Meta-Analysis of Observational Studies guidelines (18) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (19).
Search strategy and selection criteria
A systematic search was performed based on the following databases: PubMed, Web of Science, Embase (Ovid), Medline (Ovid) and Cochrane Library from January 1, 2000, to March 10, 2022. We used “rectal cancer”, “total neoadjuvant therapy”, “TNT”, “neoadjuvant chemoradiotherapy”, “CRT” and all their relevant keyword variations to search for literatures from above databases (the search strategy is in Supplementary Table 1). We restricted our searches to reports published in English. The title and abstract of retrieved articles were screened by two independent reviewers (MZ and TL). All articles about TNT and standard CRT for patients with LARC were accepted for inclusion. All articles of single arm designs, systematic reviews, letters to the editor and publishers, or concerning non-human species, were excluded. Studies that met the inclusion criteria were selected for full-text review. In the event of disagreement, a third reviewer (LZL) was consulted, and the controversial articles were discussed until reached consensus. Eventually, high-quality original studies which compared the prognosis of TNT and standard CRT for LARC were eligible for inclusion.
Outcomes of interest
The primary outcomes of interest were OS and DFS. The secondary outcomes were LRFS, DMFS, pCR, and R0 resection rate. OS was defined as death from time from surgery to any cause after surgery. DFS was defined as time from surgery to any recurrence after surgery. LRFS was defined as time from surgery to any local recurrence after surgery. DMFS was defined as time from surgery to any distant recurrence. All of local and distant recurrence were confirmed by histological assessment, cytological assessment, or imaging in original studies.
Data extraction and quality assessment
The following relevant information was extracted from all the included publications: first author, year of publication, country, number of patients, tumor grade, TNT model, years of follow-up and outcome type. If available, the following data were extracted: hazard ratios (HRs), 95% confidence intervals (CIs) and P values of OS, DFS, DMFS, and LRFS and odds ratios (ORs), 95% confidence intervals (CIs) and P values of pCR and R0 resection. When the literature report OS, DFS, DMFS, and LRFS K-M curves without HRs, Engauge Digitizer (version 10.8) were used to determine the survival rate of the corresponding time points on the curve, followed by the HR calculation table (20). We took the countdown if the HR reported in the literature was TNT vs. standard CRT. All the data were independently extracted by two authors (MZ and TL) and compared for consistency. In the literature quality assessment, RCT literature was assessed based on Cochrane Collaboration's tool (21), and non-RCT literature was assessed based on the Newcastle-Ottawa Scale (NOS) (22). Publication bias was assessed by visual inspection of the symmetry of the funnel plot.
Statistical analysis
We used the R (version 4.1.0) Meta package for meta-analysis (23). The HRs or ORs with 95% confidence intervals (CIs) of included studies were pooled. Heterogeneity was assessed by using I² index and P value. Because of results without heterogeneity, we used fixed effects model for all pooled outcomes. We drew forest plots showing the variation of the study estimates among all studies together with the pooled measure. We assessed publication bias by Egger's regression test for funnel-plot asymmetry. P values less than 0.05 were described as significant.
Results
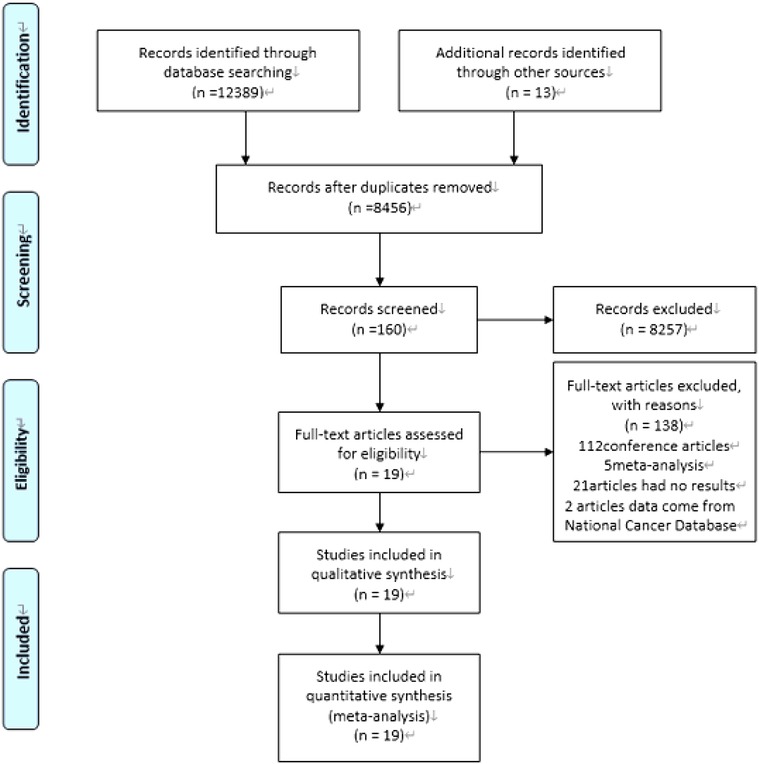
Our computer-aided search yielded 8,456 publications from PubMed, Medline (Ovid), Embase (Ovid), Web of Science and Cochrane Library after removing duplicate literature. By screening the titles and reading abstracts, we excluded another 8,298 obviously irrelevant documents. Further full-text screening of 158 publications was carried out, and 138 articles were excluded (Figure 1). Ultimately, this analysis contained 20 articles (14, 24–41), including 10 RCTs (24, 26–29, 31–35) and 3 prospective studies (30, 37, 41) and 6 retrospective studies (14, 25, 36, 38–40). In total, the included studies enrolled 5,074 patients treated for LARC. 2,751 patients received TNT while other 2,323 patients received standard CRT, followed by TME with or without adjuvant chemotherapy. The characteristics of the included studies (number of patients, tumor grade, TNT model, basic characteristics of the study population, etc.) are summarized in Table 1. The risk of bias and literature quality assessment of each included study in the meta-analysis are summarized in Supplementary Table 3. For RCTs, the risk of bias tool based on the Cochrane collaboration found that the quality of the included trials met the research standards. For non-RCTs, an NOS score of 7–9 indicates that the quality of the included trials meets the research standards.
Primary outcomes
OS for TNT vs. standard CRT
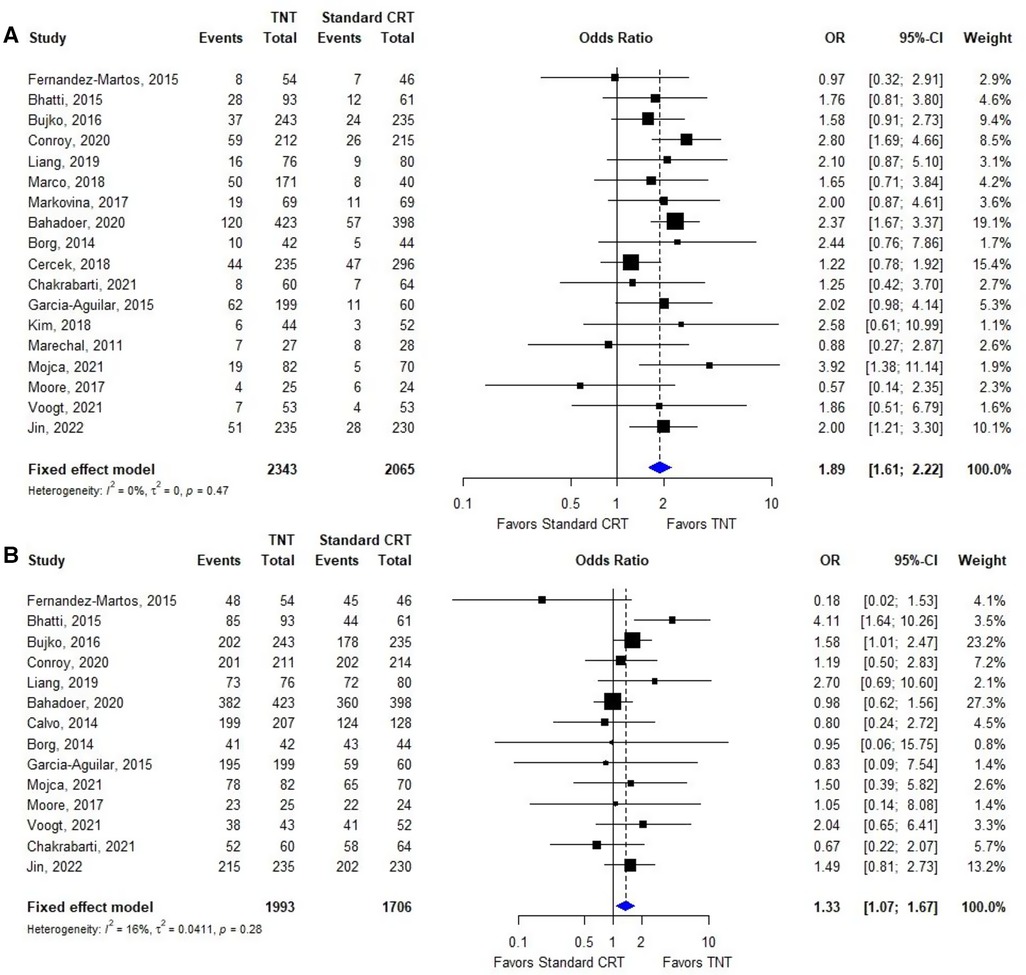
Nine (24, 25, 28, 29, 31, 32, 36, 38, 41) of the 19 included studies reported OS data based on TNT and standard CRT; the HRs and 95% CIs of these studies are summarized in Figure 3A. The overall HR was 0.77 (95% CI: 0.65–0.90). The heterogeneity test showed that these trials were not heterogeneous (I2 = 30%, P = 0.17).
DFS for TNT vs. standard CRT
Eight (24, 25, 28, 29, 32, 36, 38, 41) of the 19 included studies reported DFS data based on TNT and standard CRT; the HRs and 95% CIs of these studies are summarized in Figure 3B. The overall HR was 0.85 (95% CI: 0.74–0.97). The heterogeneity test showed that these trials were not heterogeneous (I2 = 11%, P = 0.35).
Secondary outcomes
pCR for TNT vs. standard CRT
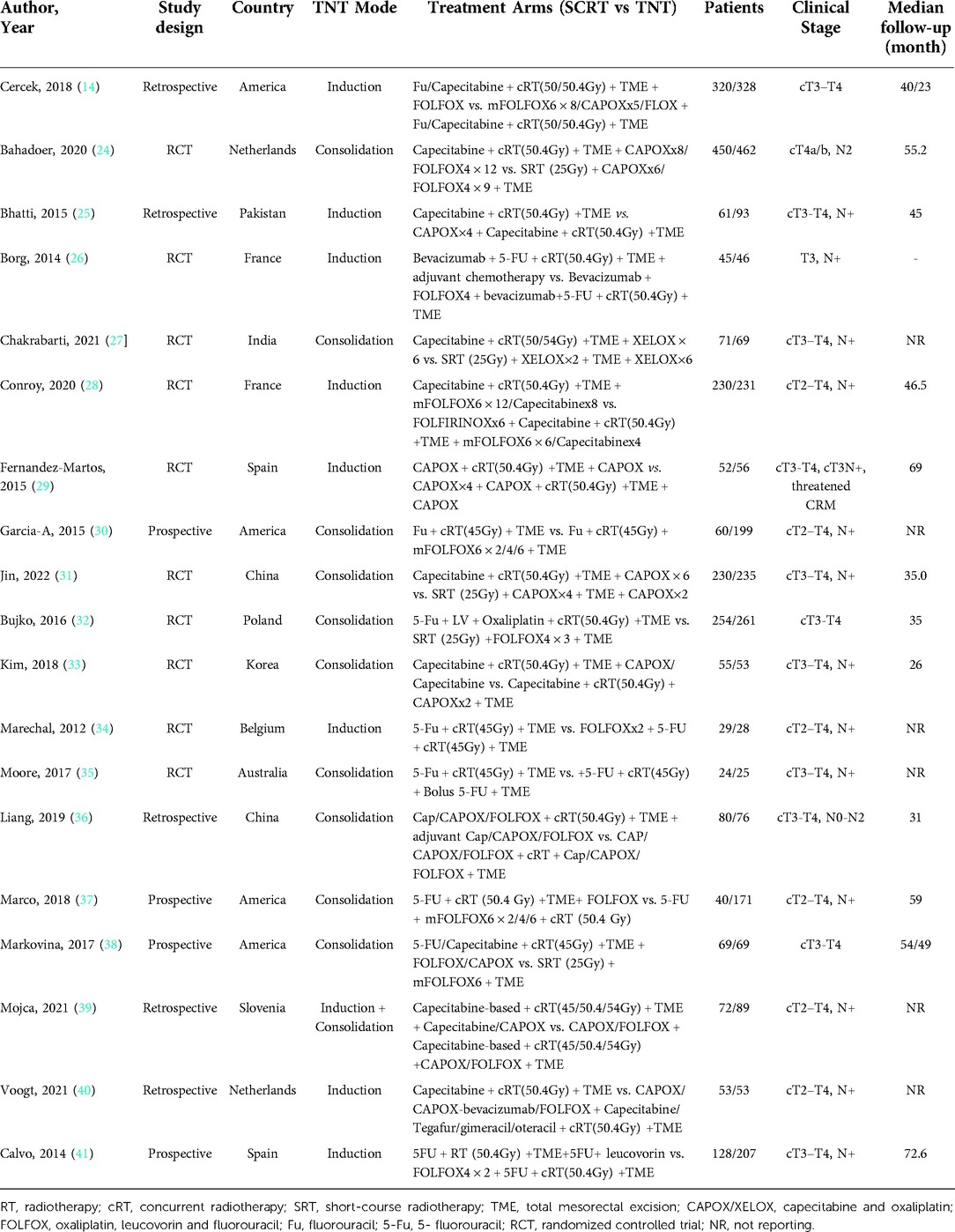
Nineteen (14, 24–40) of the 19 included studies reported pCR data based on TNT and standard CRT; the ORs and 95% CIs of these studies are summarized in Figure 2A. The overall OR was 1.89 (95% CI: 1.61–2.22). The heterogeneity test showed that these trials were not heterogeneous (I2 = 0%, P = 0.47).
R0 resection for TNT vs. standard CRT
Fourteen (24–32, 35, 36, 39–41) of the 19 included studies reporting R0 data based on TNT and standard CRT; the ORs and 95% CIs of these studies are summarized in Figure 2B. The overall OR was 1.33 (95% CI: 1.07–1.67). The heterogeneity test showed that these trials were not heterogeneous (I2 = 16%, P = 0.28).
LRFS for TNT vs. standard CRT
Three (24, 29, 31, 32) of the 21 included studies reported LRFS data based on TNT and standard CRT; the HRs and 95% CIs of these studies are summarized in Figure 3C. The overall HR was 1.12 (95% CI: 0.90–1.39). The heterogeneity test showed that these trials were not heterogeneous (I2 = 4%, P = 0.37).
DMFS for TNT vs. standard CRT
Five (24, 28, 29, 31, 36, 41) of the 20 included studies reported DMFS data based on TNT and standard CRT; the HRs and 95% CIs of these studies are summarized in Figure 3D. The overall HR was 0.76 (95% CI: 0.65–0.90). The heterogeneity test showed that these trials were not heterogeneous (I2 = 0%, P = 0.50).
Publication bias
Publication bias was assessed by visual examination of the symmetry of the funnel plot. Our funnel plot showed no publication bias (Figure 4).
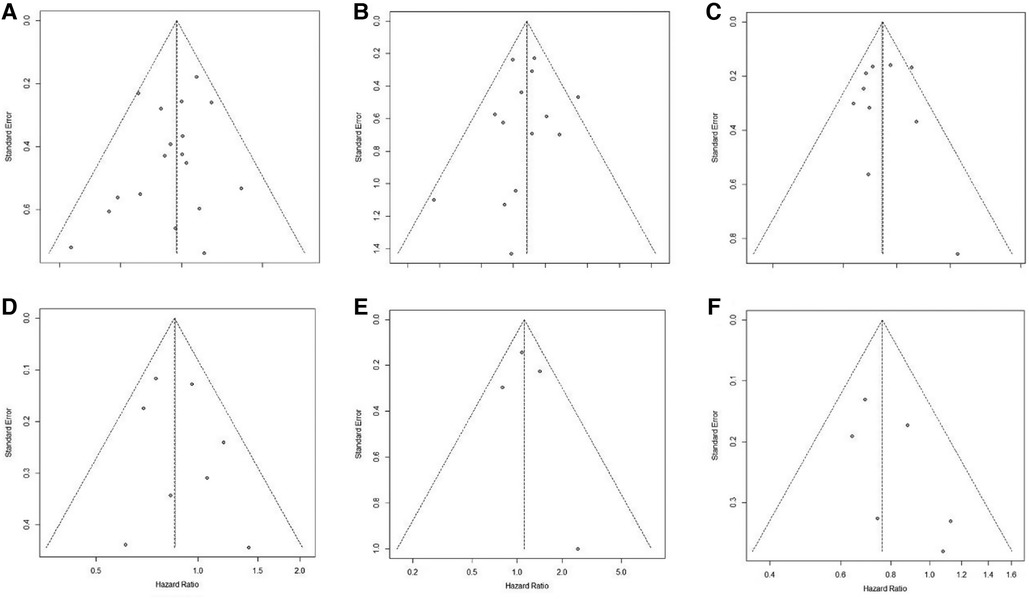
Figure 4. Funnel plot of publication bias in the meta-analysis. (A): pCR; (B): R0 resection; (C): OS; (D): DFS; (E): LRFS; (F): DMFS.
Discussion
This meta-analysis and systematic review retrieved the latest and most comprehensive literature data to compare the efficacy of TNT with standard CRT. In this summary analysis, our research table shows that TNT improves OS, DFS, pCR, R0 resection rate and DMFS in advanced rectal cancer. Unfortunately, TNT does not improve LRFS in advanced rectal cancer.
The standard treatment plan for stage II and III rectal cancer includes neoadjuvant radiotherapy and chemotherapy, followed by radical surgical resection. In addition, some milestone studies have demonstrated the benefits of preoperative CRT. According to reports, the failure rate of local area treatment is <10%. It can significantly improve the radical resection rate of local tumors and the chance of preserving anal sphincter function (5, 42). Therefore, neoadjuvant CRT followed by radical surgical resection has become the standard treatment for rectal cancer. The long-term follow-up results after this treatment showed that the 5-year survival rate for a specific stage was 63% to 77.4% (43, 44). Radical surgery combined with perioperative adjuvant radiotherapy and chemotherapy has reduced the local recurrence rate from 25% to 40% to less than 10%, but the remote recurrence rate was still as high as 25% to 38% (4, 45). The traditional "sandwich" treatment model reduces the local recurrence rate but at the same time highlights the risk of distant metastasis, which affects the long-term survival of patients. In recent years, many clinical studies have completed adjuvant radiotherapy and chemotherapy before surgery, which is called TNT. An increasing number of studies have shown that the TNT model has more advantages than the traditional "sandwich” treatment model. Cercek et al. (14). reported a study of 628 patients with LARC. Among them, 320 patients were treated with traditional concurrent CRT + TME + postoperative adjuvant chemotherapy (CRT + TME + ACT), and 308 patients received TNT. The chemotherapy regimen was FOLFOX or CAPOX. The results showed that the completion rate of radiotherapy and chemotherapy in the TNT group was significantly higher than that of the traditional treatment group (P < 0.001), and the distant recurrence rate was also significantly lower in the TNT group. The incidence of complete remission (complete response), including pCR and clinical complete remission (clinical complete response, cCR), was higher than that in the traditional treatment group. Previous studies have shown that increasing pCR may help reduce the risk of recurrence and death. Although not sufficient to replace OS, pCR is considered an important prognostic parameter for the long-term outcome of LARC (46). Recent studies have shown that TNT increases the pCR of LARC, especially for high-risk patients, such as T4 and peripheral resection margin involvement. Fokas et al. (47). showed that, compared with adverse pathological reactions, the cumulative incidence of distant metastases in the pCR of patients was significantly reduced (10.5% vs. 39.6%) and DFS was higher (89.5% vs. 63%). Perhaps pCR can truly be an important indicator of long-term survival for advanced rectal cancer.
Previous studies have shown that TNT can improve the pCR of LARC, further improve the patient's OS and DFS, and reduce distant metastasis (24, 28, 38). Similar results were obtained in our meta-analysis. However, there was no significant difference between TNT and standard CRT in terms of local recurrence-free survival (LRFS). We speculate that it may be due to extension of the preoperative treatment period, some nonresponsive patients have local tumor progression, and the risk of residual tumor is increased. On the other hand, although the proportion of patients with pCR was increased by TNT, the tissue cell microenvironment after tumor regression might differ widely from normal tissues, which was difficult to identify and resect intraoperatively and induced identical local relapse risks between TNT and standard CRT.
Inspiringly, our meta-analysis indicated that TNT had favorable outcomes regarding DFS and DMFS. The primary inadequacy of standard CRT was the difficulty of decreasing the distant relapse rate (48), suggesting that the metastatic potential was not acquired by nongenomic factors but specialized tumor cells that present cancer stem cell properties (49). Cancer stem cells existing in the hematologic and lymphatic systems might lead to high distant relapse for LARC. The reason that TNT significantly increased DMFS might be explained by the ability to eradicate cancer stem cells in the hematologic and lymphatic systems. TNT can intervene in micrometastasis at an earlier stage, reduce tumor cell activity, reduce free tumor cells to a certain extent, and reduce the risk of metastasis and implantation caused by radiotherapy and surgery. Perhaps because of this increased DMFS, DFS was significantly increased by TNT. Meanwhile, OS in the TNT group showed an obvious advantage compared with that in the standard CRT group in our meta-analysis. Reported evidence has indicated that TNT possesses many advantages, such as better compliance with treatment, pCR rate, R0 resection rate, DMFS and DFS. Hence, TNT improved OS logically. However, at the same time, because the chemotherapy time of TNT is longer and the cumulative effect of radiotherapy and chemotherapy toxicity is more obvious, the grade 3–4 toxicity of TNT is usually higher than that of standard CRT (24). The increased grade 3–4 toxicity seems to have no effect on the excellent compliance rate and long-term survival results of the TNT group, but it also needs to attract more attention.
In the TNT model, is the chemotherapy regimen before radiotherapy? Or after? It is controversial and may have a different effect on the patient's prognosis. There are two regimens of induction and consolidation chemotherapy for the TNT regimen of LARC. To date, the two regimens are still controversial. The Spanish GCR-3 trial took 4 cycles of COPAX before CRT as an induction regimen because CAPOX-based CRT achieved pCR rates of 10% to 19% in some studies (29, 50, 51). The results of the Spanish GCR-3 trial showed that TNT had a similar effect on pCR, 5-DFS and 5-OS compared with standard CRT (29). Nevertheless, as an induction chemotherapy regimen in the UNICANCER-PRODIGE 23 trial, 6 cycles of FOLFIRINOX before CRT took full advantage of significantly improved pCR, 3-DFS and 3-year metastasis-free survival rates (28). In the RAPIDO trial (24), the consolidation chemotherapy regimen after short-course radiotherapy consisted of 6 cycles of CAPOX or 9 cycles of FOLFOX4. Except for pCR, the cumulative incidence of disease-related treatment failure and distant metastases were lower with TNT. In a randomized clinical trial in Spain, neoadjuvant short-course radiotherapy followed by 2 cycles of XELOX achieved indistinctive pCR compared with standard CRT (27). Our study conducted a subgroup analysis of induction and consolidation chemotherapy for TNT. The results showed that the induction and consolidation regimens of TNT compared with those of standardized radiotherapy can significantly improve pCR, and the consolidation regimen of TNT, compared with standardized CRT, can improve OS. However, there was no difference in OS between the induction regimen for TNT and standardized CRT (Supplementary Figure 1). Therefore, it is necessary to further study the prognostic difference between the induction regimen TNT and the consolidation regimen TNT of LARC. Recently, adding targeted drugs and immunotherapy to induction chemotherapy has been widely studied. Studies have shown that the pCR rate of TNT combined with pembrolizumab is higher than that of TNT alone (31.9% vs. 29.4%) (52), and TNT and cetuximab significantly improved OS (53). Immunotherapy might activate T cells to reach tumors by limiting the interaction of programmed cell death 1 (PD-1) with its ligand PD-L1 and has shown clinical efficacy in patients with tumors. The addition of targeted therapies might further increase pCR rates or reduce metastatic progression for LARC (54). Whether adding immunotherapeutic drugs or targeted drugs will improve and optimize the therapeutic effect during TNT is still inconclusive. More convincing evidence is needed to clarify the clinical application value of the above drugs. Meanwhile, it is necessary to study the molecular mechanism of TNT to achieve personalized treatment for LARC.
TNT has the following advantages over traditional CRT: (1) it can intervene in micrometastasis at an earlier stage; (2) it can reduce tumor burden and staging, as well as increase the R0 resection rate; (3) Avoid it radiotherapy and surgery destroy the original structure of the tumor structure, use the original blood supply of the tumor, increase the drug perfusion rate, and increase the effect of chemotherapy; (4) compared with postoperative radiotherapy and chemotherapy, TNT can control symptoms early and has higher patient compliance and tolerance, ensuring the implementation of a complete course of chemotherapy; (5) it inhibits the stimulation of tumor proliferation caused by surgery; (6) Avoid chemotherapy delays (such as anastomotic leakage) caused by poor recovery of some postoperative patients, and significantly shorten prevention The fistula period of patients with ileostomy improves the quality of life of patients; (7) it reduces tumor cell activity, reduces free tumor cells (to a certain extent), and reduces the risk of metastasis and implantation caused by radiotherapy and surgery; and (8) it does not significantly increase the cost, while the shortening of the chemotherapy cycle can also reduce costs (to a certain extent). However, in theory, TNT has some of the following shortcomings: (1) the preoperative treatment period is prolonged, which increases the short-term potential risk of progression in nonresponsive patients; (2) it affects the body's immune status and reduces the patient's surgical tolerance; and (3) it increases the risk of adverse events during the perioperative period. Of course, there are still many unresolved problems in the TNT model. For example, is it better to use induction chemotherapy or consolidation chemotherapy in the TNT model? What are the reasons for their differences? After the whole course of chemotherapy, the patient's physical condition will be weaker, and the ability to withstand surgical shocks will be worse. How do you balance the choices? After TNT, some patients can achieve pCR. Does this type of patient need surgery? If surgery is not possible, how can this type of patient be effectively screened out? During TNT, do immunotherapy drugs or targeted drugs be added, and what role do they play? These are issues worthy of further in-depth study and discussion.
This meta-analysis and systematic review showed that TNT can improve the OS, DFS, PCR, R0 resection and DMFS of LARC. At the same time, this study has some limitations. First, although 10 randomized trials were included, only 5 randomized trials had long-term follow-up results. Additionally, other phase 1 and phase 2 randomized trials are ongoing. Second, the current research lacks a direct comparison between induction and consolidation programs, and more relevant data should be collected in current ongoing trials in the future.
Conclusion
Our study has demonstrated that, compared with standard CRT, TNT therapy has excellent short-term efficacy in terms of pCR and R0 resection rate, while also improving OS, DFS, and DMFS for long-term results. However, The safety, effectiveness and clinical economics of the TNT model still require a large number of clinical high-quality research evaluations. Similar to the traditional treatment model, the TNT model is a combination of challenges and opportunities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
MZ and TL acquisition of data, analysis and interpretation of data, drafting the article, final approval; LZ interpretation of data, revising the article, final approval; JX conception and design of the study, critical revision, final approval. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China, No. 81070378 and 81270561; and High-level Talents Introduction Fund for the First Affiliated Hospital of Chengdu Medical College, NO. CYFY2018GQ17.
Acknowledgments
We are very grateful to Ni Ran for providing guidance on search strategies for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.911538/full#supplementary-material.
References
2. Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol. (2020) 17(7):414–29. doi: 10.1038/s41575-020-0275-y
3. Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. (2017) 18(3):336–46. doi: 10.1016/S1470-2045(17)30086-4
4. van Gijn W, Marijnen C, Nagtegaal I, Kranenbarg E, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. (2011) 12(6):575–82. doi: 10.1016/S1470-2045(11)70097-3
5. Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. (2009) 373(9666):811–20. doi: 10.1016/S0140-6736(09)60484-0
6. Collette L, Bosset J, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European organisation for research and treatment of cancer radiation oncology group. J Clin Oncol. (2007) 25(28):4379–86. doi: 10.1200/JCO.2007.11.9685
7. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. (2012) 30(16):1926–33. doi: 10.1200/JCO.2011.40.1836
8. Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CBM, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch colorectal cancer group (DCCG) randomized phase III trial. Ann Oncol. (2015) 26(4):696–701. doi: 10.1093/annonc/mdu560
9. Rouanet P, Rullier E, Lelong B, Maingon P, Tuech J-J, Pezet D, et al. Tailored treatment strategy for locally advanced rectal carcinoma based on the tumor response to induction chemotherapy: preliminary results of the French phase II multicenter GRECCAR4 trial. Dis Colon Rectum. (2017) 60(7):653–63. doi: 10.1097/DCR.0000000000000849
10. Bigness A, Imanirad I, Sahin I, Xie H, Frakes J, Hoffe S, et al. Locally advanced rectal adenocarcinoma: treatment sequences, intensification, and rectal organ preservation. CA Cancer J Clin. (2021) 71(3):198–208. doi: 10.3322/caac.21661
11. Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman A, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. (2006) 24(4):668–74. doi: 10.1200/JCO.2005.04.4875
12. Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: grupo cancer de recto 3 study. J Clin Oncol. (2010) 28(5):859–65. doi: 10.1200/JCO.2009.25.8541
13. Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer G, Fietkau R, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: cAO/ARO/AIO-12. J Clin Oncol. (2019) 37(34):3212–22. doi: 10.1200/JCO.19.00308
14. Cercek A, Roxburgh C, Strombom P, Smith J, Temple L, Nash G, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. (2018) 4(6):e180071. doi: 10.1001/jamaoncol.2018.0071
15. Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. (2020) 3(12):e2030097. doi: 10.1001/jamanetworkopen.2020.30097
16. Liu S, Jiang T, Xiao L, Yang S, Liu Q, Gao Y, et al. Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: a systematic review and meta-analysis. Oncologist. (2021) 26(9):e1555–66. doi: 10.1002/onco.13824
17. Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. (2020) 271(3):440–8. doi: 10.1097/SLA.0000000000003471
18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
20. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, et al. The cochrane Collaboration's Tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
23. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117
24. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM-K, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6
25. Bhatti ABH, Waheed A, Hafeez A, Akbar A, Syed AA, Khattak S, et al. Can induction chemotherapy before concurrent chemoradiation impact circumferential resection margin positivity and survival in low rectal cancers? Asian Pac J Cancer Prev. (2015) 16(7):2993–8. doi: 10.7314/APJCP.2015.16.7.2993
26. Borg C, André T, Mantion G, Boudghène F, Mornex F, Maingon P, et al. Pathological response and safety of two neoadjuvant strategies with bevacizumab in MRI-defined locally advanced T3 resectable rectal cancer: a randomized, noncomparative phase II study. Ann Oncol. (2014) 25(11):2205–10. doi: 10.1093/annonc/mdu377
27. Chakrabarti D, Rajan S, Akhtar N, Qayoom S, Gupta S, Verma M, et al. Short-course radiotherapy with consolidation chemotherapy versus conventionally fractionated long-course chemoradiotherapy for locally advanced rectal cancer: randomized clinical trial. Br J Surg. (2021) 108(5):511–20. doi: 10.1093/bjs/znab020
28. Conroy T, Bosset J-F, Etienne P-L, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22(5):702–15. doi: 10.1016/S1470-2045(21)00079-6
29. Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the spanish GCR-3 phase II randomized trial. Ann Oncol. (2015) 26(8):1722–8. doi: 10.1093/annonc/mdv223
30. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. (2015) 16(8):957–66. doi: 10.1016/S1470-2045(15)00004-2
31. Jin J, Tang Y, Hu C, Jiang L-M, Jiang J, Li N, et al. Multicenter, randomized, phase III trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol. (2022) 40(15):1681–92. doi: 10.1200/JCO.21.01667
32. Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 55 gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. (2016) 27(5):834–42. doi: 10.1093/annonc/mdw062
33. Kim SY, Joo J, Kim TW, Hong YS, Kim JE, Hwang IG, et al. A randomized phase 2 trial of consolidation chemotherapy after preoperative chemoradiation therapy versus chemoradiation therapy alone for locally advanced rectal cancer: kCSG CO 14-03. Int J Radiat Oncol Biol Phys. (2018) 101(4):889–99. doi: 10.1016/j.ijrobp.2018.04.013
34. Maréchal R, Vos B, Polus M, Delaunoit T, Peeters M, Demetter P, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. (2012) 23(6):1525–30. doi: 10.1093/annonc/mdr473
35. Moore J, Price T, Carruthers S, Selva-Nayagam S, Luck A, Thomas M, et al. Prospective randomized trial of neoadjuvant chemotherapy during the “wait period” following preoperative chemoradiotherapy for rectal cancer: results of the WAIT trial. Colorectal Dis. (2017) 19(11):973–9. doi: 10.1111/codi.13724
36. Liang H-Q, Dong Z-Y, Liu Z-J, Luo J, Zeng Q, Liao P-Y, et al. Efficacy and safety of consolidation chemotherapy during the resting period in patients with local advanced rectal cancer. Oncol Lett. (2019) 17(2):1655–63. doi: 10.3892/ol.2018.9804
37. Marco MR, Zhou L, Patil S, Marcet JE, Varma MG, Oommen S, et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum. (2018) 61(10):1146–55. doi: 10.1097/DCR.0000000000001207
38. Markovina S, Youssef F, Roy A, Aggarwal S, Khwaja S, DeWees T, et al. Improved metastasis- and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: results of a matched pair analysis. Int J Radiat Oncol Biol Phys. (2017) 99(2):417–26. doi: 10.1016/j.ijrobp.2017.05.048
39. Tuta M, Boc N, Brecelj E, Peternel M, Velenik V. Total neoadjuvant therapy standard therapy of locally advanced rectal cancer with high-risk factors for failure. World J Gastrointest Oncol. (2021) 13(2):119–30. doi: 10.4251/wjgo.v13.i2.119
40. Voogt ELK, Schaap DP, van den Berg K, Nieuwenhuijzen GAP, Bloemen JG, Creemers GJ, et al. Improved response rate in patients with prognostically poor locally advanced rectal cancer after treatment with induction chemotherapy and chemoradiotherapy when compared with chemoradiotherapy alone: a matched case-control study. Eur J Surg Oncol. (2021) 47(9):2429–35. doi: 10.1016/j.ejso.2021.05.017
41. Calvo FA, Sole CV, Serrano J, Del Valle E, Rodriguez M, Muñoz-Calero A, et al. Preoperative chemoradiation with or without induction oxaliplatin plus 5-fluorouracil in locally advanced rectal cancer. Long-term outcome analysis. Strahlenther Onkol. (2014) 190(2):149–57. doi: 10.1007/s00066-013-0469-0
42. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. (2004) 351(17):1731–40. doi: 10.1056/NEJMoa040694
43. Bosset J-F, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. (2006) 355(11):1114–23. doi: 10.1056/NEJMoa060829
44. Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: nSABP R-03. J Clin Oncol. (2009) 27(31):5124–30. doi: 10.1200/JCO.2009.22.0467
45. Azria D, Doyen J, Jarlier M, Martel-Lafay I, Hennequin C, Etienne P, et al. Late toxicities and clinical outcome at 5 years of the ACCORD 12/0405-PRODIGE 02 trial comparing two neoadjuvant chemoradiotherapy regimens for intermediate-risk rectal cancer. Ann Oncol. (2017) 28(10):2436–42. doi: 10.1093/annonc/mdx351
46. Omejc M, Potisek M. Prognostic significance of tumor regression in locally advanced rectal cancer after preoperative radiochemotherapy. Radiol Oncol. (2018) 52(1):30–5. doi: 10.1515/raon-2017-0059
47. Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. (2014) 32(15):1554–62. doi: 10.1200/JCO.2013.54.3769
48. Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. (2010) 467(7319):1114–7. doi: 10.1038/nature09515
49. Nassar D, Blanpain C. Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol. (2016) 11:47–76. doi: 10.1146/annurev-pathol-012615-044438
50. Rödel C, Liersch T, Hermann RM, Arnold D, Reese T, Hipp M, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol. (2007) 25(1):110–7. doi: 10.1200/JCO.2006.08.3675
51. Machiels JP, Duck L, Honhon B, Coster B, Coche JC, Scalliet P, et al. Phase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: the RadiOxCape study. Ann Oncol. (2005) 16(12):1898–905. doi: 10.1093/annonc/mdi406
52. Rahma OE, Yothers G, Hong TS, Russell MM, You YN, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol. (2021) 7(8):1225–30. doi: 10.1001/jamaoncol.2021.1683
53. Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol. (2012) 30(14):1620–7. doi: 10.1200/JCO.2011.39.6036
Keywords: total neoadjuvant therapy, standard chemoradiotherapy, locally advanced rectal cancer, prognosis, meta-analysis
Citation: Ma Z, Tan L, Liu Z and Xiao J (2022) Total neoadjuvant therapy or standard chemoradiotherapy for locally advanced rectal cancer: A systematic review and meta-analysis. Front. Surg. 9:911538. doi: 10.3389/fsurg.2022.911538
Received: 2 April 2022; Accepted: 10 August 2022;
Published: 26 August 2022.
Edited by:
Alexandra Zaborowski, St. Vincent's University Hospital, IrelandReviewed by:
Shenghui Huang, Fujian Medical University Union Hospital, ChinaPang Minghui, Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital, China
© 2022 Ma, Tan, Liu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang-wei Xiao eGlhb2ppYW5nd2VpMjAxOEAxNjMuY29t
†These authors share first authorship
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Zhou Ma1,2,†
Zhou Ma1,2,† Ling Tan
Ling Tan Jiang-wei Xiao
Jiang-wei Xiao